Research Article Open Access
Dopaminergic Positron Emission Tomography Study on Amyotrophic Lateral Sclerosis/Parkinsonism–Dementia Complex in Kii, Japan
Yasumasa Kokubo1*, Kenji Ishii2, Satoru Morimoto3, Maya Mimuro4, Ryogen Sasaki5, Shigeo Murayama3 and Shigeki Kuzuhara61Kii ALS/PDC Research Center, Mie University Graduate School of Regional Innovation Studies, Japan
2Department of Neuroimaging, Tokyo Metropolitan Institute of Gerontology, Japan
3Department of Neuropathology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology, Japan
4Institute for Medical Science of Aging, Aichi Medical University, Japan
5Department of Neurology, National Mie Hospital, Japan
6Department of Neurology and Medicine, Medical Welfare, Suzuka University of Medical Science, Japan
- *Corresponding Author:
- Yasumasa Kokubo
Kii ALS/PDC Research Center
Mie University, Graduate School of Regional Innovation Studies
Mie 514-8507, Japan
Tel: +81 59 231 5117
E-mail: ktyktykty@me.com
Received date: February 06, 2017; Accepted date: February 28, 2017; Published date: March 07, 2017
Citation: Kokubo Y, Ishii K, Morimoto S, Mimuro M, Sasaki R, et al. (2017) Dopaminergic Positron Emission Tomography Study on Amyotrophic Lateral Sclerosis/Parkinsonism–Dementia Complex in Kii, Japan. J Alzheimers Dis Parkinsonism 7:311. doi:10.4172/2161-0460.1000311
Copyright: © 2017 Kokubo Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: Amyotrophic lateral sclerosis and parkinsonism-dementia complex in the Kii peninsula, Japan (Kii ALS/PDC) is an endemic and rare neurodegenerative disease. We conducted positron emission tomography (PET) study using C-11 CFT (2-b-carbomethoxy-3b-(4-fluorophenyl) tropane) and C-11 Raclopride for two patients with Kii ALS/PDC. Objective and methods: Patient #1 was 64 years old male having 12 years’ duration of the illness. Patient #2 was 68 years old female having 7 years’ duration of the illness. Results: In patient #1, CFT-PET showed marked decreased uptake in the bilateral corpus striatum, almost no signal in the posterior part of the putamen. RAC-PET showed mild decrease in the bilateral caudate nucleus and slight increase in the posterior part of the putamen. In patient #2, CFT-PET showed severe decrease in the bilateral corpus striatum, marked decrease in the posterior part of the putamen and in the caudate nucleus. RACPET showed the mild decrease in the bilateral corpus striatum. Conclusion: These results were compatible with L-dopa resistant Parkinsonism and similar to those of PSP.
Keywords
Dopaminergic positron emission tomography; Amyotrophic lateral sclerosis; Parkinsonism-dementia complex; Kii peninsula; Guam.
Introduction
Amyotrophic lateral sclerosis and parkinsonism-dementia complex (ALS/PDC) is an endemic and rare neurodegenerative disease common in the Kii peninsula, Japan and the Guam Island [1-3]. ALS/PDC clinically shows combination of Parkinsonism, dementia and motor neuron symptoms and neuropathologically accumulation of tau, α-synuclein and transactivation responsive region (TAR)-DNA-binding protein of 43 kDa (TDP-43) in the central nervous system. Parkinsonism of ALS/ PDC is clinically indistinguishable from that of Parkinson’s disease (PD), except for L-DOPA resistance. Neuroradiological background regarding Parkinsonism of Kii ALS/PDC has not been investigated so far. We conducted a positron emission tomography (PET) study using 11C-2-b-carbomethoxy-3b-(4-fluorophenyl) tropane (CFT) and 11C-raclopride (RAC) in two patients with Kii ALS/PDC to evaluate preand post-synaptic dopaminergic function.
Objectives and Methods
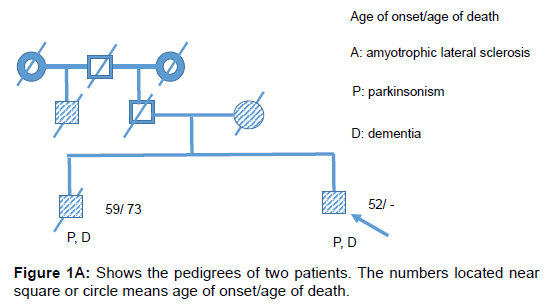
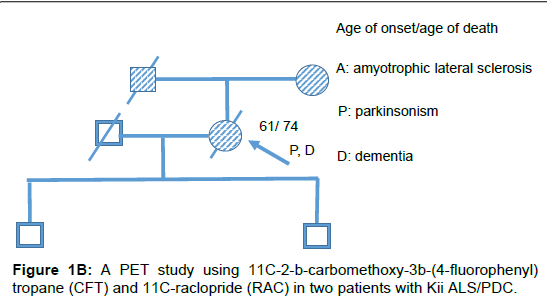
Two patients with Kii ALS/PDC participated in the study. The patients were diagnosed by the diagnostic criteria [4]. Patient #1 was a 64 year old male who had lived with the illness for 12 years. His mother and brother also had ALS/PDC (Figure 1A). He developed bradykinesia and dementia at age 53. During PET, the mini mental state examination (MMSE) score was unevaluable and he demonstrated L-DOPAresistant Parkinsonism of Hoehn-Yahr’s stage 4 and pyramidal tract signs. He was diagnosed with probable ALS/PDC. Gene analysis of this patient has not been done. Patient #2 was a 68 year old female who lived with the illness for 7 years. Her father and mother also had ALS/PDC (Figure 1B). She developed bradykinesia and poor memory at age 61. L-DOPA was effective only for 1 year after disease onset. During PET, the MMSE score was 28/30 and she demonstrated apathy, L-DOPAresistant Parkinsonism of Hoehn-Yahr’s stage 4.5 and pyramidal tract signs. Six years later, she was diagnosed with definite Kii ALS/PDC on neuropathological evaluation. Briefly, nerve cell loss and neurofibrillary tangles were chiefly observed in the nuclei of the brainstem, limbic system, medial temporal cortex and frontal cortex. α-synuclein accumulation was also observed in the nuclei of the brainstem, limbic system and neocortical lesion. Gene analysis of this patient excluded C9orf72 mutation [5]. CFT-PET and RAC-PET were conducted in both patients on dopaminergic medication to evaluate pre- and post-synaptic dopaminergic function as previously described [6]. Furthermore, MRI and fluorodeoxyglucose (FDG)-PET in both patients and Pittsburgh compound B (PiB)-PET study in patient #1 were conducted to evaluate brain atrophy, glucose metabolism and amyloid protein burden in the brain, respectively.
Results
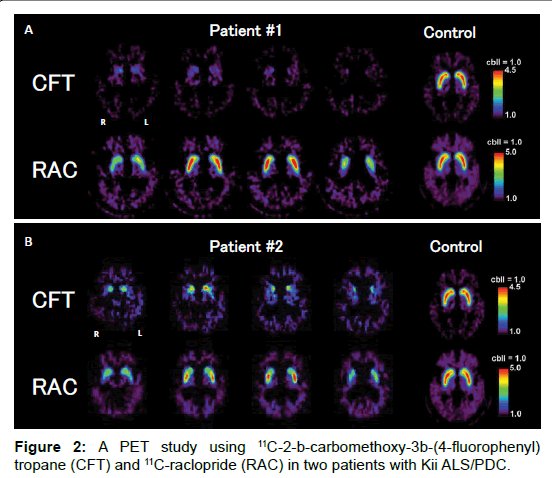
In patient #1, brain MRI revealed mild atrophy of the frontal and temporal lobes. FDG-PET showed glucose metabolism decrease in the bilateral temporal and parietal lobes. PiB-PET was negative. CFT-PET showed marked CFT decrease in the bilateral corpus striatum; notably, there was almost no signal of CFT in the posterior part of the putamen. RAC-PET showed mild RAC decrease in the bilateral caudate nucleus and slight RAC increase in the posterior part of the putamen (Figure 2A).
In patient #2, brain MRI revealed moderate dilatation of the lateral ventricle and mild atrophy of the temporal lobe. FDG-PET showed severe glucose metabolism decrease in the right temporal lobe and lower part of the right parietal lobe and a mild decrease in the left medial temporal lobe. CFT-PET showed severe CFT decrease in the bilateral corpus striatum; notably, there was marked CFT decrease in the posterior part of the putamen and caudate nucleus. RAC-PET showed mild RAC decrease in the bilateral corpus striatum (Figure 2B). Relatively mild RAC increase in the posterior part of the putamen in both patients reflected compensatory up-regulation and/or dopaminergic depletion. CFT-PET and RAC-PET showed no remarkable laterality in these two patients.
Discussion
CFT-PET study of both patients revealed marked pre-synaptic dopaminergic dysfunction, suggesting severe neuronal degeneration of the substantia nigra, consistent with the neuropathological findings of patient #2. The RAC-PET study also revealed mild post-synaptic dysfunction, suggesting neuronal degeneration within the corpus striatum. Furthermore, L-DOPA-resistant Parkinsonism after the short period of effectiveness may be related not only to the degeneration of the corpus striatum but also to that of the pallidum and thalamocortical pathway, consistent with the neuropathological findings of patient #2. These pre- and post-synaptic dysfunctions may be related to tau and α-synuclein pathology and neuronal cell loss in these lesions [7].
Snow et al. reported PET study using 18F-6fluorodopa on four Guamaninan ALS, eight Guamanian PDC and seven clinically normal Guamanians and observed severe pre-dopaminergic dysfunction in Guamaninan PDC, moderate in Guamaninan ALS and mild even in clinically normal Guamanians. They insisted that nigrostriatal dopaminergic lesion in Guamanian Parkinsonism is difficult to discriminate from that in idiopathic Parkinsonism [8]. Taken together with Snow’s report and our report, severe pre-synaptic dopaminergic dysfunction was revealed common feature between Kii ALS/PDC and Guam ALS/PDC. The post-synaptic dopaminergic function has not been examined in Guam ALS/PDC patients, therefore we could not comment on the utility to provide a differential diagnosis, based on dopaminergic PET imaging. There is no PET study regarding ALS/PDC in Papua state in Indonesia.
Regarding discrimination from other Parkinsonism, these patterns of pre- and post-synaptic dopaminergic dysfunction of Kii ALS/PDC were similar to those of progressive nuclear palsy (PSP), as shown by the decreased uptake of CFT-PET and relatively spared uptake of RACPET [9]. Thus, Kii ALS/PDC may be discriminated from PD, wherein preserved post-synaptic function and obvious laterality is observed, and multiple system atrophy, wherein severe post-synaptic dopaminergic dysfunction exists [8]. And to discriminate from PSP, other clinical findings and neuroimaging study, like preserved eye movement or decrease of iodine I 131 metaiodobenzylguanidine (MIBG) uptake in Kii ALS/PDC [10,11], are useful.
Conclusion
According to dopaminergic PET studies, Kii ALS/PDC showed severe pre-synaptic dopaminergic dysfunction, relatively spared postsynaptic dopaminergic dysfunction and no obvious laterality. These results were similar to those of PSP. Although this study is limited by the number of patients, dopaminergic PET study may be useful for differential diagnosis from PD and MSA. To reveal the timing of the defect in dopaminergic synaptic functions, time course observation would be of interest for future analysis.
Acknowledgement
The authors thank Ms. Junko Karita for clerical assistance. The authors would like to thank Enago (https://www.enago.jp/) for the English language review.
Disclosure
Drs. Kokubo, Ishii, Morimoto, Mimuro, Sasaki, Murayama and Kuzuhara report no disclosures. There is no ghost writing by anyone not named on the author list. This study was approved with the ethics committee of Mie University Hospital, Tsu, Mie, Japan and written informed consent was obtained from all patients and/or their families prior to the study.
Study Funding
This study was partly supported by grants-in-aid from the Mie Medical Fund (to SM, YK), Japan Foundation for Neuroscience and Mental Health (to YK), the Research Committee of CNS Degenerative Diseases (to YK) and the Research Committee of Muro disease (Kii ALS/PDC) (to YK 21210301), Ministry of Health, Labor and Welfare, Japan, by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture (to YK), and by a grant-in-aid of the Research Consortium of Kii ALS/PDC from the Japan Agency for Medical Research and Development, AMED (to YK), Japan.
References
- Shiraki H, Yase Y (1975) Amyotrophic lateral sclerosis in Japan. In: Vinken PJ, Bruyn GW, Klawans HL (eds): Handbook of Clinical Neurology. Amsterdam, North Holland Publishing Company 22: 353-419.
- Hirano A, Malamud N, Elizan TS, KurlandLT (1966) Amyotrophic lateral sclerosis and parkinsonism-dementia complex on Guam, further pathologic studies. Arch Neurol 15: 35-51.
- Kuzuhara S, Kokubo Y, Sasaki R, Narita Y, Yabana T, et al. (2001) Familial amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Kii peninsula of Japan: Clinical and neuropathological study and Tau analysis. Ann Neurol 49: 501-511.
- Kokubo Y (2015) Diagnostic criteria for amyotrophic lateral sclerosis/parkinsonism-dementia complex in the Kii peninsula, Japan. Brain Nerve 67: 961-966.
- Ishiura H, Takahashi Y, Mitsui J, Yoshida S, Kihira T, et al. (2012) C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch Neurol 69: 1154-1158.
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, et al. (2009) Regional analysis of age-related decline in dopamine transporters and dopamine D2-Like receptors in human striatum. Synapse 63: 282-290.
- Kokubo Y, Taniguchi A, Hasegawa M, Hayakawa Y, Morimoto S, et al. (2012) a-Synuclein pathology in the amyotrophic lateral sclerosis/parkinsonism dementia complex in the Kii peninsula, Japan. J Neuropathol Exp Neurol 71: 625-630.
- Snow BJ, Peppard RF, Guttman M, Okada J, Wayne Martin WR, et al. (1990) Positron emission tomographic scanning demonstrates a presynaptic dopaminergic lesion in Lytico-Bodig, The amyotrophic lateral sclerosis-parkinsonism-dementia complex of Guam. Arch Neurol 47: 870-874.
- Brooks DJ, Ibanez V, Sawle GV, Playford ED, Quinn N, et al. (1992) Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol 31: 184-192.
- Kokubo Y, Nomura Y, Morimoto S, Kuzuhara S (2012) Cardiac 123I-meta-iodobenzylguanidine scintigraphy in patients with ALS/PDC of the Kii peninsula, Japan. Parkinsonism Relat Disord 18: 306-308.
- Kokubo Y, Morimoto S, Shindo A, Hirokawa Y, Shiraishi T, et al. (2011) Cardiac 123I-meta-lodobenzylguanidine scintigraphy and Lewy body pathology in a patient with ALS and PDC of Kii, Japan. Movement Disorders 26: 2300-2301.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3656
- [From(publication date):
April-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 2736
- PDF downloads : 920