Does Trypanosoma brucei brucei have the Ability to Invade Human Microglial Cells?
Received: 02-May-2017 / Accepted Date: 08-Jun-2017 / Published Date: 15-Jun-2017
Abstract
African trypanosomes are flagellated extracellular parasites, causal agents of African trypanosomiasis. Trypanosoma brucei is the most frequent species for the majority of African trypanosomiasis. Two sub-species are pathogenic for humans: Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense while Trypanosoma brucei brucei is not.
Objective: The aim of this work was to study the infectivity of Trypanosoma brucei brucei, on human microglial cells (CMH-5).
Methods: Trypanosomes (Trypanosoma brucei brucei Antat 1.9) labeled with a fluorescent dye were co-cultured with CMH-5 cells for 3 h. The nucleus and cytoskeletal microtubules of the cells were then labeled with two fluorescent dyes and the stained cell layer was examined by confocal microscopy.
Results: Trypanosomes were observed within CMH-5 cells.
Conclusion: Trypanosoma brucei brucei, a non-human parasite and described as an extracellular parasite, appears to have the ability to enter in human microglial cells in vitro. This raises a number of issues concerning the behaviour of this parasite in the human CNS and the immune response initiated by microglial cells which corresponds to endogenous defenses of the human brain.
Keywords: African trypanosomiasis; Trypanosoma brucei brucei; Human microglial cell; Intra-cellular parasite; Confocal microscopy
66486Rapid Communication
African trypanosomiasis is an endemic disease localized in Sub- Saharan Africa. It is caused by different subspecies of an extracellular protozoan called Trypanosoma. One of these subspecies, Trypanosoma brucei , and more specifically Trypanosoma brucei (T.b.) gambiense and T.b. rhodesiense , is able to infect humans causing Human African Trypanosomiasis (HAT) or sleeping sickness, or T.b. brucei can also cause Animal African Trypanosomiasis (AAT) in wild or farm animals. T.b. brucei is not pathogenic for humans because it exists as a trypanolytic complex, in human serum, with a haptoglobulin and an apolipoprotein which lyse the parasites [1]. For this reason, T.b. brucei is the most commonly used trypanosome in research laboratories. These parasites are transmitted by an infected tsetse fly which bites the host during its blood meal. Trypanosomes are present in blood and lymph. This is the early stage. Later, the parasites are able to cross the blood brain barrier (BBB) to invade the Central Nervous System (CNS), and are involved in the development of neurological disorders. This is the meningoencephalitic stage and left untreated, African Trypanosomiasis is fatal [2]. Furthermore, therapy is stage-dependent, which limits treatment for patients when they are in stage 2. It is necessary to develop new therapies by studying the ability of trypanosomes to cross the BBB. At present, several publications report a correlation between the understanding of the crossing of the BBB by the trypanosome and the improvement of the stage 2 treatment [3]. Indeed, studies of the invasive pathways of the CNS by the trypanosome and its behavior during this phase could help to understand certain cases of relapses [4,5].
One question concerns how the trypanosome is able to cross the BBB which is a barrier that separates the blood circulation from the cerebral extracellular fluid in the central nervous system. It is composed of endothelial cells, tight junctions, pericytes around the capillaries and astrocyte endfeet [6]. The BBB protects the brain from pathogens and toxins, maintains cerebral homeostasis by the exchange control between blood and brain [4]. But, the BBB is not present in all brain regions such as ventricles and circumventricular organs (CVOs), and perhaps it is at those areas where trypanosomes cross into the brain. Trypanosomes can express enzymes including metalloproteases and cysteine protease, that facilitate tissue invasion [7,8]. Furthermore, the host’s immune system plays a key role in lymphocyte invasion and the action of IFN-γ which determines which sites penetration of T.b. brucei was observed in the brain [9].
Our objective was to demonstrate the ability of T.b. brucei Antat 1.9, which is not pathogenic for humans, to invade human microglial cells in vitro. Microglial cells are the resident immune cells of the CNS which normally respond to neuronal damage and remove damaged cells by phagocytosis. During HAT, an inflammatory process in the central nervous system is believed to play an important role in the pathway leading to neuronal cell death. The inflammatory response is mediated by the activated microglia, which could be implicated in the migration of protozoa.
To answer this question, we co-cultured T.b. brucei Antat 1.9 on Human Microglial Cell 5 (CMH-5).
Parasite Culture
Trypanosomes were cultured in Baltz Medium [10]: MEM (Eagle's Minimum Essential Medium, Gibco®) supplemented with 20% heat-inactivated horse serum (Gibco®), L-glutamine (2 mM, Gibco®), HEPES (25 mM, Gibco®), glucose (0.2%, Sigma-Aldrich®), sodium pyruvate (2 mM, Gibco®), penicillin (150 IU/L, Gibco®), thymidine (0.1 mM, Sigma-Aldrich®), β-mercaptoethanol (0.5 mM, Sigma-Aldrich®), L-cysteine (15 mM, Sigma-Aldrich®), and bathocuproïne sulfate (0.0001%, Sigma®). Parasites were cultured at 37°C in humidified air containing 5% CO2.
Cell culture
CMH-5 (kindly provided by Pr P. Vincendeau, Bordeaux, France) were grown in flasks in a Dulbecco's Modified Minimum Essential Medium (DMEM, Gibco®), containing L-cysteine (0.2 mM, Sigma- Aldrich®), L-glutamine (2 mM, Gibco®), sodium pyruvate (2 mM, Gibco®), streptomycin-penicillin (100 IU/L, Gibco®), HEPES (25 mM, Gibco®) and β-Mercaptoethanol (0.1 mM, Sigma-Aldrich®). This medium was supplemented with decomplemented 10% fetal calf serum (Gibco®). Flasks were incubated at 37°C in a 5% CO2 humidified atmosphere. Cells were seeded in 25 cm2 culture flasks at a density of 5 × 106 cells/mL and confluent cells were trypsinized in DMEM.
Co-culture and fluorescent immunostaining
Trypanosomes were stained with PKH26 Red (PKH26 Red Fluorescent Cell Linker Kits for general cell membrane labeling, Sigma-Aldrich®) and co-cultured (105/mL) on a CMH-5 layer in well culture chambers (Lab-tek, Sarstedt®) for 3 h at 37°C in a humidified atmosphere at 5% CO2. For each culture chamber, a well was seeded with cells only and served as the control for our analysis. The cell layer was washed twice with PBS (phosphate buffered saline) and fixed with 4% formaldehyde for 15 min. Following two washes with PBS, wells were saturated with PBS, 2% BSA (Bovine Serum Albumin, Gibco®) for 5 min. Cells were permeabilized with PBS, 0.1% triton X-100 (Gibco®) for 5 min. The CMH-5s were then stained simultaneously with Alexa Fluor phalloidin (Alexa Fluor 488 phalloidin, Molecular Probes®) and Hoechst according to manufacturer’s instructions (Hoechst fluorescent, Molecular Probes®). Excess stain was eliminated by two washes with PBS and one with sterile water.
Confocal microscopy
Fluorescent Z stack was acquired with a Zeiss LSM 510 META confocal microscope using a Plan-Apochromat 100x/1.4 lens over a range of 7.65 μm and with a z step of 0.450 μm. Orthogonal projection on the X and Y axes, using the LSM 5 Image Browser and ZEN2 software, was used to localize the trypanosome in the cell (Figure 1).
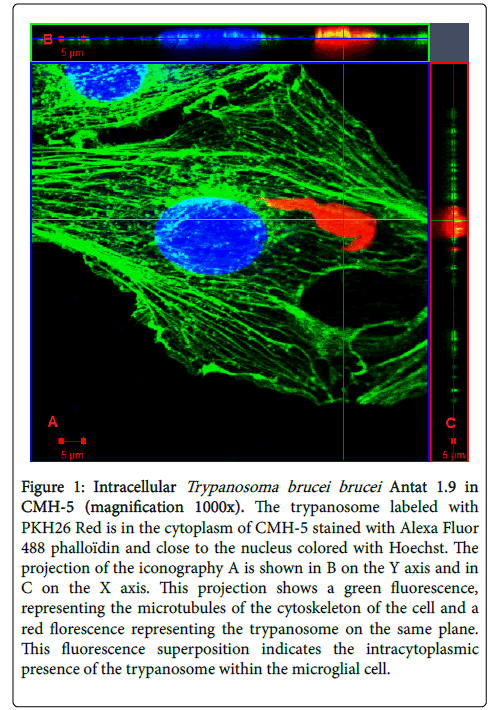
Figure 1: Intracellular Trypanosoma brucei brucei Antat 1.9 in CMH-5 (magnification 1000x). The trypanosome labeled with PKH26 Red is in the cytoplasm of CMH-5 stained with Alexa Fluor 488 phalloïdin and close to the nucleus colored with Hoechst. The projection of the iconography A is shown in B on the Y axis and in C on the X axis. This projection shows a green fluorescence, representing the microtubules of the cytoskeleton of the cell and a red florescence representing the trypanosome on the same plane. This fluorescence superposition indicates the intracytoplasmic presence of the trypanosome within the microglial cell.
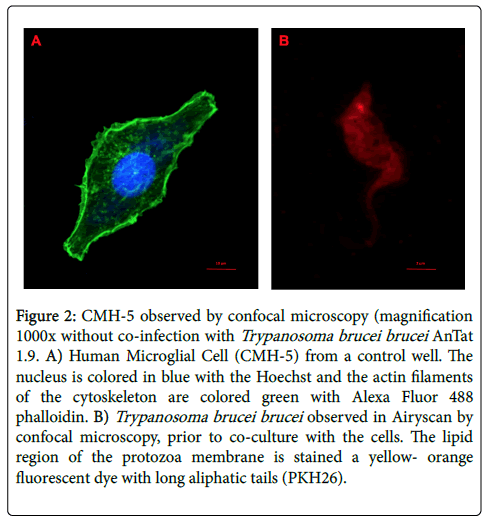
Controls were used to verify that cells were viable, that they grew well and that the stain did not modify their morphology (Figure 2A). Trypanosoma brucei brucei AnTat 1.9 was specifically observed with a Zeiss LSM880 confocal microscope with the airyscan module to enhance the resolution (Figure 2B).
Figure 2: CMH-5 observed by confocal microscopy (magnification 1000x without co-infection with Trypanosoma brucei brucei AnTat 1.9. A) Human Microglial Cell (CMH-5) from a control well. The nucleus is colored in blue with the Hoechst and the actin filaments of the cytoskeleton are colored green with Alexa Fluor 488 phalloidin. B) Trypanosoma brucei brucei observed in Airyscan by confocal microscopy, prior to co-culture with the cells. The lipid region of the protozoa membrane is stained a yellow- orange fluorescent dye with long aliphatic tails (PKH26).
Results and Discussion
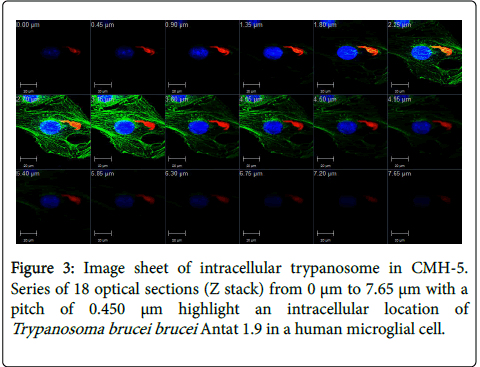
After co-incubation with labeled (red) T.b. brucei Antat 1.9 and CMH-5 during 3 h, CMH-5 actin cytoskeleton and nuclei were stained and a random screening of slides by confocal microscopy made it clear that some T.b. brucei were found present in CMH-5 even after several extensive washes. The present study demonstrated that T. brucei can infect microglial cells in the human CNS. The presence of trypanosomes inside cells, in an intra-cytoplasmic position, was confirmed by an overlay of trypanosome-labeled fluorescence and CMH-5 cytoskeletal microtubule labeled fluorescence (Figure 1). The optical series (Figure 3) also showed the presence of trypanosomes at each step and throughout the entire cell thickness. This image sheet confirms the intracellular presence of the trypanosome.
The preliminary results presented in this short communication did not allow us to define a frequency of cellular invasion by the parasite. However, the presence of the trypanosome in the intracellular position could be observed only a small number of times, so it appears that the entry of the trypanosome into the cell is a rare event.
Microglia represents the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors such as parasites [11]. Activation of microglia is a hallmark of brain pathology but it can be used by infectious agents as transporters [12,13]. Our study demonstrates that a strain of Trypanosoma can infect brain macrophages, which naturally play an essential role in the propagation of neuro-inflammation but also has migratory properties.
In a previous report, Grab et al. already demonstrated paracellular traversal of African trypanosomes (T.b. gambiense and brucei ) across the human BBB. The cell models used to identify this migration were human brain microvascular endothelial cells. This intracellular form has long been challenged in HAT research, although it is unclear at this time how important this intracellular form is in human CNS. Intracellular detection of trypanosomes (Figure 1) highlights the ability of this non-human parasite to cross over the human cell membrane. Our study should be completed to show that T.b. gambiense and rhodesiense have the same abilities as T.b. brucei to cross the BBB and to elucidate mechanisms used by trypanosomes to invade cells.
In 2006, Nikolskaia et al. showed that T.b. gambiense could cross the BBB [14] and, once in human BMEC, they are protected from drugs and host immune responses. Furthermore, T.b. gambiense is able to use paracellular and transcellular routes to cross the barrier during an in vitro study [3]. Before that, others studies have shown the presence of T.b. rhodesiense in the cells of choroid plexus epithelium [15]. This crossing might be possible because of the presence of an enzyme, serine oligopeptidase [16], which is similar to an enzyme in T. cruzi implicated in the calcium mediated intracellular penetration of host cells [17]. Another enzyme found in Leishmania called Leishmanial GP63 could be implicated also in this internalization phenomenon [18]. To understand the mechanisms used by this parasite to invade the CNS, in 2017, Namayanja et al. studied T.b. brucei with two different types of canine and human dermal cells (MDCK II and HDMEC) mimicking biological barriers. They found that T.b. brucei could use two mechanisms: paracellular and transcellular migration across biological host barriers [5]. Similarly, they showed the formation of structures called “possible cuplike structures” when the parasites are going through HDMEC. These structures play a key role in the transcellular route of migration. Moreover, the trypanosomes are able to modify their morphology and their flagella to pass across membrane barriers [5].
Other aspects of this study need to be addressed. One of them is the viability of trypanosomes when they are intracellular. We do not have information concerning their viability and their capacity to multiply if they leave the intracellular compartment. It is tempting to speculate that trypanosomes that have adapted to life in the bloodstream of mammals can adapt to a different environment such as the cell compartment. The intracellular trypanosome life inside human CNS cells could be a new mechanism to escape the immunological host response. The intracellular medium could represent a refuge for trypanosomes to escape from CSF lymphocytes and cytokines due to the immune response as well as the therapeutic trypanocidal molecules present in the CSF following stage 2 treatment. It would now be interesting to determine whether or not T.b. brucei is able to exit the cell. This may explain how, following appropriate neurological therapy, the parasite could re-infect different biological fluids and explain some relapse cases.
However, caution must be taken when extrapolating these results to in vivo situations during human disease which is certainly more complex. But we can now affirm that T.b. brucei Antat 1.9 can enter CMH-5. It is conceivable that trypanosomes can cross the human BBB via different cells types and not only via disruption of the endothelial tight junctions or anatomical weaknesses. Otherwise, these results need to be confirmed and extended, in particular by real-time observation of living cells and trypanosomes during co-culture. It will also be interesting to see the behaviour of trypanosomes in contact with a larger panel of human cells and for different duration of co-incubation.
Acknowledgements
This work was supported by grants from Conseil Régional du Limousin. The authors express thanks to Claire Carrion (BISCEm core facility, GEIST, Limoges, France) for her technical help in confocal microscopy imaging. The authors would like to thank J. Cook-Moreau for is technical and scientific assistance.
References
- Baral TN (2010) Immunobiology of African trypanosomes: need of alternative interventions. J Biomed Biotechnol 2010: 389153.
- Bacchi CJ (2009) Chemotherapy of human african trypanosomiasis. Interdiscip Perspect Infect Dis 2009: 195040.
- Grab DJ, Nikolskaia O, Kim YV, Lonsdale-Eccles JD, Ito S, et al. (2004) African trypanosome interactions with an in vitro model of the human blood-brain barrier. J Parasitol 90: 970-979.
- Mogk S, Boßelmann CM, Mudogo CN, Stein J, Wolburg H, et al. (2016) African trypanosomes and brain infection – the unsolved question. Biol Rev Camb Philos Soc 1.
- Namayanja M, Dai Y, Nerima B, Matovu E, Lun ZR, et al. (2017) Trypanosoma brucei brucei traverses different biological barriers differently and may modify the host plasma membrane in the process. Exp Parasitol 174: 31-41.
- Pham YT, Gimenez F (2001) Capture, efflux et modulation du transport des médicaments au niveau cérébral. J Pharm Clin 20: 52-63.
- Lonsdale-Eccles JD, Grab DJ (2002) Trypanosome hydrolases and the blood–brain barrier. Trends Parasitol 18: 17-19.
- Grandgenett PM, Otsu K, Wilson HR, Wilson ME, Donelson JE (2007) A function for a specific zinc metalloprotease of African trypanosomes. PLoS Pathog 3: 1432-1445.
- Masocha W, Robertson B, Rottenberg ME, Mhlanga J, Sorokin L, et al. (2004) Cerebral vessel laminins and IFN-gamma define Trypanosoma brucei brucei penetration of the blood-brain barrier. J Clin Invest 114: 689-694.
- Baltz T, Baltz D, Giroud C, Crockett J (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J 4: 1273-1277.
- Rustenhoven J, Park TIH, Schweder P, Scotter J, Correia J, et al. (2016) Isolation of highly enriched primary human microglia for functional studies. Sci Rep 6: 19371.
- Mammari N, Vignoles P, Halabi MA, Darde ML, Courtioux B (2014) In Vitro Infection of Human Nervous Cells by Two Strains of Toxoplasma gondii: A Kinetic Analysis of Immune Mediators and Parasite Multiplication. PLoS One. 9: e98491.
- Capuccini B, Lin J, Talavera-López C, Khan SM, Sodenkamp J, et al. (2016) Transcriptomic profiling of microglia reveals signatures of cell activation and immune response, during experimental cerebral malaria. Sci Rep 6: 39258.
- Nikolskaia OV, Kim YV, Kovbasnjuk O, Kim KJ, Grab DJ (2006) Entry of Trypanosoma brucei gambiense into microvascular endothelial cells of the human blood–brain barrier. Int J Parasitol 36: 513-519.
- Abolarin MO, Evans DA, Tovey DG, Ormerod WE. (1982) Cryptic stage of sleeping-sickness trypanosome developing in choroid plexus epithelial cells. Br Med J (Clin Res Ed) 285: 1380-1382.
- Morty RE, Lonsdale-Eccles JD, Mentele R, Auerswald EA, Coetzer TH (2001) Trypanosome-derived oligopeptidase B is released into the plasma of infected rodents, where it persists and retains full catalytic activity. Infect Immun 69: 2757-2761.
- Caler EV, Vaena de Avalos S, Haynes PA, Andrews NW, Burleigh BA (1998) Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J 17: 4975-4986.
- LaCount DJ, Gruszynski AE, Grandgenett PM, Bangs JD, Donelson JE (2003) Expression and function of the Trypanosoma brucei major surface protease (GP63) genes. J Biol Chem 278: 24658-24664.
Citation: Bonnet J, Boudot C, Courtioux B (2017) Does Trypanosoma brucei brucei have the Ability to Invade Human Microglial Cells?. Arch Parasitol 1: 108.
Copyright: © 2017 Bonnet J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 6201
- [From(publication date): 0-2017 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 5295
- PDF downloads: 906