Does a Three Week's Treatment with Microcurrents Improve the FunctionalLevel of People with Fibromyalgia Syndrome?
Received: 09-Jan-2017 / Accepted Date: 01-Feb-2016 / Published Date: 06-Feb-2017
Abstract
Objective: Microcurrent treatment is proposed to reduce fibromyalgia symptoms and improve the functional ability of those affected. The aim of this study was to evaluate how microcurrent therapy affects functional manifestations and pain in subjects with fibromyalgia after three weeks of treatment and one month after the end of treatment.
Methods: This study was a randomised, placebo-controlled, single-blind study. We recruited forty-three volunteers diagnosed with fibromyalgia (100% females), and thirty-four completed the follow-up analysis. Participants were randomly assigned to a microcurrent intervention group (18 patients, 100 microamperes, 30 to 40 Hz) or to a placebo group (18 patients, sham microcurrent). All subjects received two 30-minute sessions a week for three weeks.
The Spanish version of the Fibromyalgia Impact Questionnaire, (Cuestionario de Impacto de la Fibromialgia CIF), was used for assessment: at the baseline (CIF0), at the end of treatment (CIF1) and after one month (CIF2).
Results: Treatment group patients showed greater improvement than patients in the placebo group at the end of treatment and one month after the end of treatment, but there were no statistically significant differences between the microcurrent group and the placebo group. At the end of treatment, the average CIF0 - CIF1 values of the microcurrent group were 9.48 (13.99), and those of the placebo group were 4.18 (16.64) (p=0.31). One month after the end of treatment, the average CIF0 - CIF2 value of the microcurrent group was 9.92 (17.19), and that of the placebo group was 6.73 (15.34) (p=0.57).
Conclusion: This study has found no improvement in the functional level of fibromyalgia patients either following three weeks of microcurrent application or at one-month later.
Keywords: Fibromyalgia; Electric stimulation therapy; Physical therapy; Chronic pain; Adult; Middle age; Physical fitness; Female
4895Introduction
Fibromyalgia (FM) is a common medical condition characterised by chronic widespread pain and allodynia. Existing data suggest that disturbed central pain processing plays an important role in the pathogenesis of this syndrome [1,2].
Multimodal therapy, including exercise and pharmacological and behavioural treatments, has proven useful in treating patients with FM [3] and is consequently recommended [4]. Research into multidisciplinary programs has brought attention to treatments shown to be beneficial in treating the symptoms of FM [5]. One of these is microcurrent therapy, which is characterised by microamperage current that provides electrons at physiologic amperage in millions of an ampere 10-2–10-6 amperes [6]. It is applied in different ways: cranial electrotherapy stimulation [7-12], hand held probes [10,11], self-adhesive electrodes [11], and graphite conducting gloves [6,13].
Despite the difference in name, cranial electrotherapy stimulation, originally called electrosleep, is a type of microcurrent therapy that is administered across the head using ear clip electrodes. Some authors attribute to this technique a direct action on the brain although this theory is speculative yet [12]. Usually, treatments that are administered through hand held probes or self-adhesive electrodes are called microcurrent electrical therapy, microcurrent stimulation, or microcurrent electrical stimulation.
Frequency specific microcurrent treatment uses graphite conducting gloves or self-adhesive electrodes and frequencies specific to each tissue and pathology. McMakin et al. [13] found that the specific frequency for treating FM associated with cervical trauma pain ranged from 40 to 10 Hz.
According to our hypothesis, microcurrent treatment reduces FM symptoms and thus improves the functional ability of those affected. The aim of this study was to evaluate how microcurrent therapy affects functional manifestations and pain in subjects with FM after three weeks of treatment and one month after the end of treatment.
Materials And Methods
Research design
This study was a randomised, placebo-controlled, single-blind study. The Clinical Research Ethical Committee of the Hospital Sant Joan Reus approved the study protocol. Clinical trial number: ISRCTN04459421.
Subjects
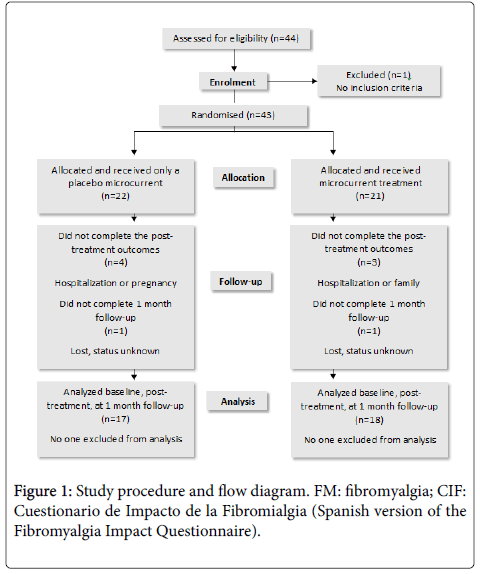
Forty-four subjects from the Catalan Association for People Affected by Fibromyalgia were recruited. This sample size was chosen based on a priori sample size calculations given that the results can be expected to reveal a 12.5 (8) reduction in the CIF values of the treated group [6] and an estimated 5 (8) reduction in the CIF values of the control group. To increase sample power and to lessen the effects of possible drop-outs, we increased the sample size from 24 to 44 subjects (power of 92%). To be included in the study, all subjects had to have been diagnosed with FM in accordance with the American College of Rheumatology’s criteria [14]. Patients were excluded from the study if they suffered from a multiple organ disease, were pregnant, or had demand-type cardiac pacemakers. Patients who were receiving physiotherapy treatment or had finished such treatment during the previous month were also excluded. These criteria led to one subject being excluded from the study (Figure 1).
All remaining subjects were interviewed individually to provide them with details about the nature of the study and to ask for their informed consent. All of them signed voluntary consent forms to participate in the study.
Intervention description
The study was carried out during the period from April 2006 to September 2006. The subjects were randomized into a microcurrent group and a placebo group. They all received two 30-minute sessions a week for three weeks. They were asked to attend all sessions but were able to leave the study at any time. Seven subjects left the study for various reasons: hospitalization, pregnancy, family, etc. (Figure 1).
Treatment involved applying self-adhesive electrodes to the two areas that the subject found most painful at that moment. The researcher chose the electrode position on the basis of these indicators and using the research team’s predefined protocol, which had standardized the electrode positions (Table 1). The subject was then placed in a prone, lateral, or supine position and made as comfortable as possible with supporting cushions.
| Location of pain | Location of positive electrode | Location of negative electrode |
|---|---|---|
| Cervical spine | Bilateral application on paraspinal muscles at the level of C6-C7. | |
| Dorsal spine | Bilateral application on paraspinal muscles at the level of T4-T5. | |
| Lumbar spine | Bilateral application on paraspinal muscles at the level of L4-L5. | |
| Head | Bilateral application on the suboccipital muscles. | |
| Arm | Unilateral application on the suboccipital muscles. | Ipsilateral application on the infraspinatus muscular belly. |
| Leg | Unilateral application on the paraspinal muscles at the level of L4-L5. | Ipsilateral application on the muscular belly of the gluteus medius, under iliac crest and immediately outside the gluteus maximus. |
Table 1: Standardised positioning of the electrodes according to the location of the pain in the patient.
The microcurrents were applied at an intensity of 100 microamperes and at a frequency ranging between 30 and 40 Hz. The equipment used was an Endomed 482u Enraf-Nonius, which allowed the current to be applied through two channels. The placebo group was led to believe that the equipment was being switched on and off so that they would think they were receiving the same treatment. However, the equipment remained off at all times, and the current was not applied. This simulation was possible because microcurrents are not sufficiently intense to stimulate the sensitive nerve fibres and thus are not perceived by patients [15].
Although infrequent, secondary effects have been described in the 24 h following the application of microcurrents. These include nausea, headache, fatigue, and increased pain. In the cases published, these effects decrease from the third or fourth session onwards. To prevent the potential side effects from occurring, patients should be given water to drink at the end of the session and during the three hours thereafter [16]. For these reasons, all the subjects were given a glass of water when the session finished and were told to drink another glass when they returned home.
When the last of the six sessions was over, the patients were reassessed, their daily drug diaries were recorded and they were told the dates of their third evaluations which were carried out individually a month later.
Main outcomes
Subjects were initially assessed using the Fibromyalgia Impact Questionnaire; (Cuestionario de Impacto de la Fibromialgia, CIF) [17], and a record was made of the medications habitually taken by each subject. In addition, each subject was given a diary to record any changes in their medication regimens. The CIF and the visual analog scale (VAS) were analysed to see if the treatment improved the symptoms. The CIF is a multidimensional questionnaire designed specifically to assess the functional capacity of FM patients [17]. It is designed so that it can be self-administered by the patient. The values of the CIF are between 0 and 100, 0 being the best state of health and 100 the worst. It takes five minutes to complete the CIF.
This questionnaire is a version of the different Spanish translations and validations, and it includes the modifications made to an up-todate version of the original FIQ [18]. The CIF, the primary outcome, and the VAS, the secondary outcome, were used for assessment at the start of treatment (CIF0, VAS0), at end of the treatment (CIF1, VAS1), and at one month after treatment (CIF2, VAS2).
Data analysis
The data were analysed using the Statistical Package for the Social Sciences 15.0 (SPSS). The homogeneity of the groups was assessed by means of a CIF0 t-test. The variables were compared at the beginning of treatment, at the end of treatment, and one month after the end of treatment. The averages were compared using a Student’s t-test. Before these tests were applied, the homogeneity of the variances was verified using Levene’s test. If there was no homogeneity, the Kruskal Wallis test was used. Proportions were analysed by chi square. A 5% type I error was accepted for the unilateral contrast of the hypothesis. We rejected the one-tailed null hypotheses when the p value was lower than 0.05.
Results
Sample characteristics
A total of 43 patients, 21 in the microcurrent group and 22 in the placebo group participated in the study. The subjects were all Caucasian females, with an average age of 57.07 (8.34). Almost half of them (44.3%) had only received the minimum compulsory education, and 7.2% had been to University. Half the population was currently working and 23.11% were on sick leave. The CIF0 of the placebo and treatment groups were similar because the lower and upper limits of the 95% confidence interval of their difference were -10.6 and 4.9, respectively. The characteristics of both groups can be seen in Table 2.
| N | Total | Intervention Group | Placebo Group | |
|---|---|---|---|---|
| 43 | 21 | 22 | ||
| Ages (years) | Mean (SD) | 57.07 (8.34) | 55.25 (8.88) | 58.20 (7.66) |
| Range | 33-74 | 33-71 | 38-74 | |
| CIF0 | Mean (SD) | 66.66 (12.52) | 65.19 (11.23) | 68.07 (13.75) |
| Range | 66.66-96.99 | 43.99-88.66 | 47.95-96.99 | |
| VAS0 | Mean (SD) | 7.44 (2.02) | 7.24 (2.04) | 7.64 (2.03) |
| Range | 2-10 | 2-10 | 3-10 | |
SD:Standard Deviation; CIF: Cuestionario de Impacto de la Fibromialgia (Spanish version of the Fibromyalgia Impact Questionnaire); VAS:Visual Analog Scale
Table 2: Description of the sample and basic data of both groups.
Effect of microcurrents
Thirty-four individuals-seventeen from each group-completed the analysis one month after treatment. While comparing CIF and VAS scores, no significant differences were observed. However, the baseline values were higher than the subsequent values, which indicated an improvement.
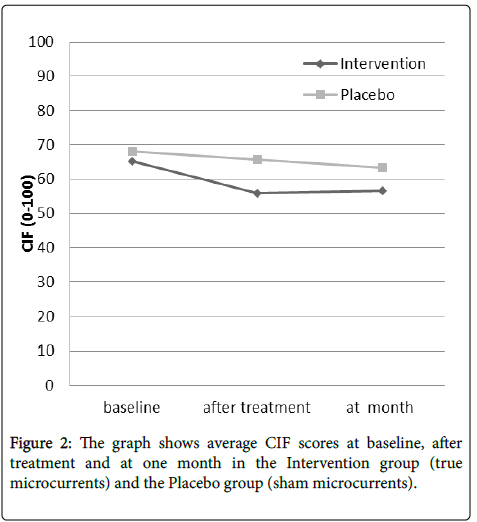
The initial mean (standard deviation) CIF values were better after treatment, from 65.19 (11.23) to 55.84 (18.34) for the Intervention group and 68.07 (13.75) to 65.65 (14.55) for the Placebo group. Those values were maintained at one month, 56.65 (24.76) and 63.40 (12.53) respectively (Figure 2).
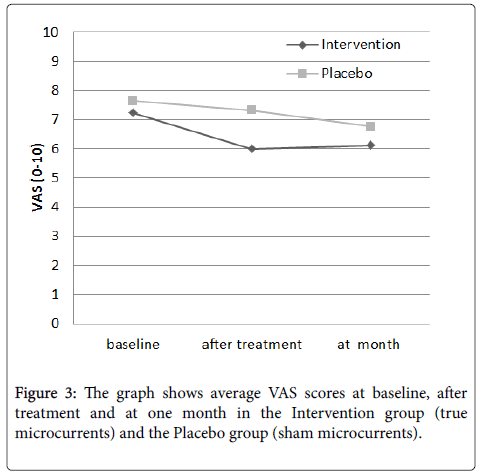
There were similar VAS values at the Intervention group, 7.24 (2.04), and the Placebo group, 7.64 (2.03). After treatment, values were lower for both groups, 6.0 (2.47) and 7.33 (2.06), respectively, and increased again for both groups, 6.12 (2.47) and 6.76 (2.31) respectively, at one month (Figure 3).
Although none of the results was statistically significant, the differences between the baseline values and the subsequent values were positive, as stated above, this was indicative of improvement (Table 3). The overall proportion of patients who reported dizziness after one of the sessions was 21.1%. Of these, 62.5% belonged to the treatment group and 37.5% to the placebo group, but this difference was not statistically significant (chi square, p=0.44).
| Parameters | N | Intervention Mean (SD) | Placebo Mean (SD) | 95% CI | p |
|---|---|---|---|---|---|
| CIF0- CIF1 | 18 | 9.48 (13.99) | 4.18 (16.64) | 15.71 to-5.12 | 0.31 |
| CIF0- CIF2 | 17 | 9.92 (17.19) | 6.73 (15.34) | 14.57to -8.19 | 0.57 |
| VAS0-VAS1 | 18 | 1.00 (2.38) | 0.39 (2.22) | 2.17 to -0.95 | 0.43 |
| VAS0-VAS2 | 17 | 1.18 (2.24) | 0.82 (2.24) | 1.92 to -1.25 | 0.65 |
SD, standard deviation; CI, confidence interval; CIF, Cuestionario de Impacto de la Fibromialgia (Spanish version of the Fibromyalgia Impact Questionnaire); VAS, visual analog scale.
Table 3: Differences between baseline and final values in the two groups at the end of treatment and one-month later.
Medication changes
During the treatment, most of the patients maintained their doses of analgesics (84.2%) and anti-inflammatories (73.7%). The small remaining number of patients decreased their medications, although this did not lead to any differences being observed between the treatment group and the placebo group.
Discussion
Our randomised placebo-controlled single-blind study of the efficacy of three weeks micro current therapy gave no effect on FM patients at the end of the treatment and one month later. These modest results contrast with those obtained in other similar studies [5-13]. These other studies reported that microcurrents had a very positive effect on the symptoms of FM patients, although, in contrast with the present study, none of them analysed whether these effects were maintained on time.
Of all these studies, only Lichtbroun et al. [7], Cork et al. [9] and Taylor et al. [12] compared the effects of microcurrent therapy with the effects of a sham microcurrent. Moreover, Taylor et al. [12] point out methodological errors on those previous studies: study design, nonobjective measures, statistical analysis and short period of data analysis. According to Taylor’s study we did an accurate design and analysis, except our assessment: despite FIQ is a subjective measure, it is a FM validated measure. Because Taylor assumed that 3 weeks treatment is a too short period for improvements to show up, he applied an 8 week treatment but without follow-up. In our opinion, the clue is not how long the treatment period is but how long the positive effects are maintained in time. Lichtbroun et al. [7] and Cork et al. [9] demonstrated positive effects after 3 weeks treatment. Like them, in the present study microcurrents were applied for 3 weeks and we reassess at month in order to demonstrate effects through time.
Besides of Taylor’s methodology observations, we will add one more, the use of the tender point score as one of their variables in Lichtbroun et al. [7] and Cork et al. [9] studies. They tested all patients on nine bilateral tender point and three bilateral control tender points (at the midpoint of the biceps brachii muscle, abdomen 5 cm to left/ right of umbilicus and gastrocnemius) and they subtracted any scores obtained at these points from the total tender point score. In Lichtbroun et al. study, the treated group had significant mean gains in the tender point score, whereas the placebo group experienced a slight improvement and the control group worsened. Cork et al. reported lower tender point score in the CES group than in the placebo group (p <0.01). The use of this measurement went against the current notion of control points. In fact, some investigators state that positive control points are a common feature in FM [19] and the consistently lower severity of tenderness at these points closely correlates with the severity and tenderness at the tender points [20].
In addition, other studies using microcurrents for different clinic circumstances agreed in describing this technique as effective. This was the case in chronic pain associated with spinal cord injury [21], cutaneous injuries [22], acute pain associated with post total knee arthroplasty [23], in treatment to increase the range of movement in a spastic ankle caused by cerebral palsy [24] and in acute myofascial pain syndrome [6].
Of all studies we looked at, only Tan et al. [25] reported poor results after using microcurrents when treating patients with chronic pain, as our study. They had a very high drop-out rate, 15 out of the original 28 patients. In our case, the drop-out rate was also high (9 out of 43 patients). In addition to this, a placebo group improvement had been registered in both studies. We want to reinforce that high drop-out rate and placebo group improvement are common research results in FM condition. So, we can doubt about research methodology studies that didn’t obtained them.
Besides these methodological concerns, to not achieve statistical differences between groups it could be explained by the low application time. Microcurrents were applied for 30 minutes in the present study, whereas most of the studies applied the microcurrents for less than 40 minutes. However, in most cases, these studies used portable equipment which does not require the patient to be stationary, as was the case with our study. When designing the study, we decided an application time of 30 minutes following the McMakin [6] protocol of microcurrent therapy. Since we have found better results in the group treated with microcurrents, it would be interesting to study whether a longer application improve these results. Another limitations of the present study is the small sample size. Besides of that, if our microcurrent treatment had the same effect that others studies [6,7,9,12] this sample should be enough to show group differences.
This is the first study analysing microcurrent applied to FM using self-adhesive electrodes. The portable equipment could be a very interesting option for treating this type of patients, because patients can worn them underneath the clothing, thus preventing the discomfort caused by ear clip electrodes. Other studies have all dealt with the application of CES, probes, or gloves. In Smith’s study [11], the patient was able to choose how the microcurrents were applied, adhesive electrodes being one of the options; however, the patients’ choices are not analysed by the author.
Although microcurrents have been used since the beginning of the 1900s, their application in the treatment of illnesses has been growing in recent years. This growth has been accompanied by research into their strengths and limits. For this growth in research to continue, first of all the nomenclature must be standardised. Therapists are confused by the number of keywords used by different authors to refer to microcurrent therapy. These include electrosleep [10], microcurrent therapy [6], microcurrent electrical therapy [10,8,16], ultra-low microcurrent therapy [22], micro-current skin patch [23], microcurrent stimulation [10,24,25], muscular electrical nerve stimulation [26], microcurrent electrical stimulation [27], or microcurrent electrical tissue stimulation [28]. These different terms make it difficult to compare studies and thus to conclude which parameters are the best. Although this study has not been able to demonstrate a functional improvement in FM patients after three weeks of microcurrent treatment and one month after it, this study can contribute to this growing body of research.
In conclusion, this study has found no improvement in the functional level of FM patients either following three weeks of microcurrent application or at one month later.
Acknowledgements
The authors gratefully acknowledge the Catalan Association of People Affected by Fibromyalgia. We would also like to thank Enraf- Nonius for loaning the treatment devices used in the study and Angela Torres for her collaboration in collecting the data.
References
- Russell IJ, Larson AA (2009) Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum Dis Clin North Am 35: 421-435.
- Kindler LL, Bennett RM, Jones KD (2011) Central Sensitivity Syndromes: Mounting Pathophysiologic Evidence to Link Fibromyalgia with Other Common Chronic Pain Disorders. Pain Management Nursing 12: 15-24
- Russell IJ (2008) Multidimensional Therapy for the Fibromyalgia Syndrome. J Musculoske Pain 16: 129-131.
- Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, et al. (2008) EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 67: 536-541.
- Anderson FJ, Winkler AE (2006). Benefits of Long-Term Fibromyalgia Syndrome Treatment with a Multidisciplinary Program. J Musculoske Pain 14: 11-25.
- McMakin CR (2004) Microcurrent therapy: a novel treatment method for chronic low back myofascial pain. J Bodyw Mov Ther 8: 143-153.
- Lichtbroun AS, Raicer MM, Smith RB (2001) The treatment of fibromyalgia with cranial electrotherapy stimulation. J Clin Rheumatol 7:72-78.
- Gilula MF (2007) Cranial stimulation and fibromyalgia. Expert Rev Med Devices 4: 489-495.
- Cork RC, Wood P, Ming N, Shepherd C, Eddy J, et al. (2004). The effect of Cranial Electrotherapy Stimulation (CES) on pain associated with Fibromyalgia.The Internet J Anesthesiol 8: 1-7.
- Kulkarni AD, Smith RB (2001) The use of microcurrent electrical therapy and cranial electrotherapy stimulation in Pain Control. Clin Pract Alternative Med 2: 99-102.
- Smith RB (2001) Is Microcurrent stimulation effective in Pain management? An additional perspective. AJPM 11: 64-68.
- Taylor AG, Anderson JG, Riedel SL, Lewis JE, Kinser P, et al. (2013) Cranial Electrical Stimulation Improves Symptoms and Functional Status in Individuals with Fibromyalgia. Pain Manag Nurs 14: 327-335.
- McMakin CR, Gregory WM, Phillips TM (2005) Cytokine changes with microcurrent treatment of fibromyalgia associated with cervical spine trauma. J Bodyw Mov Ther 9: 169-176.
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum 33: 160-172.
- Mercola JM, Kirsch DL (1995) The basis for microcurrent electrical therapy in conventional medical practice. Journal of Advancement in Medicine 8: 107-120.
- Chaitow L (2003) Fibromyalgia Syndrome: A practitioner’s guide to treatment. (2th ed), Churchill Livingstone, London.
- Esteve-Vives J, Rivera J, Salvat I, de Gracia M, Alegre C (2007) Propuesta de una versión de consenso del Fibromyalgia Impact Questionnaire (FIQ). Reumatol Clin 3: 21-24.
- Bennet R (2005) The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 23 Suppl 39: S154-162.
- Dadabhoy D, Crofford LJ, Spaeth M, Russell J, Clauw DJ (2008) Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res. Ther. 10: 211-229.
- Hart M, Nielson WR (2007) The Fibromyalgia Tender Points: Use Them or Lose Them? A Brief Review of the Controversy. J Rheumatol 34: 914-922.
- Tan G, Rintala DH, Thonby JI, Yang J, Wade W, et al. (2006) Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. J Rehabil Res Dev 43: 461-474.
- Lee BY, Wendell K, Al-Waili N, Butler G (2007) Ultra-low microcurrent therapy: a novel approach for treatment of chronic resistant wounds. Adv Ther 24:1202-1209.
- El-Husseini T, El-Kawy S, Shalaby H, El-Sebai M (2007) Microcurrent skin patches for postoperative pain control in total knee arthroplasty: a pilot study. Int Orthop (SICOT) 31: 229-233
- Mäenpää H, Jaakkola R, Sandström M, von Wendt L (2004) Does microcurrent stimulation increase the range of movement of ankle dorsiflexion in children with cerebral palsy? Disabil Rehabil 26: 669-677.
- Tan G, Monga T, Thornby J (2000) Efficacy of microcurrent electrical stimulation on pain severity, psychological distress, and disability. Am J Pain Manag 10: 35-44.
- Wieder DL(1991) Microcurrent therapy; wave of the future? Rehab Manag 4: 34-35.
- Bertolucci LE, Di Dario B (1995) Clinical comparative study of microcurrent electrical stimulation to mid-laser and placebo treatment in degenerative joint disease of the temporomandibular joint. Cranio 13:116-120.
- Lin YL, Moolenaar H, van Weeren PR, van de Lest CH (2006) Effect of microcurrent electrical tissue stimulation on equine tenocytes in culture. Am J Vet Res 67: 271-276
Citation: Salvat I, Monterde S, Miralles I, Montull S, Fernandez-Ballart J (2017) Does a Three Weeks’ Treatment with Microcurrents Improve the Functional Level of People with Fibromyalgia Syndrome? Fibrom Open Access 2: 113.
Copyright: © 2017 Salvat I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4783
- [From(publication date): 0-2017 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 3807
- PDF downloads: 976