Research Article Open Access
Does a History of Non Specific Low Back Pain Influence Electromyographic Activity of the Erector Spinae Muscle Group during Functional Movements?
| Nicolas Mazis* | |
| Queen Margaret University, Edinburgh, UK | |
| Corresponding Author : | Nicolas Mazis Queen Margaret University, School of Health Sciences Department of Physiotherapy, Edinburgh, UK Tel: +30 210 6540051 E-mail: n.mazis@yahoo.gr |
| Received July 15, 2014; Accepted September 26, 2014; Published September 28, 2014 | |
| Citation: Mazis N (2014) Does a History of Non Specific Low Back Pain Influence Electromyographic Activity of the Erector Spinae Muscle Group during Functional Movements? J Nov Physiother 4:226. doi:10.4172/2165-7025.1000226 | |
| Copyright: © 2014 Mazis N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Purpose: The present study aimed to identify potential muscular activity differences of the erector spinae muscle group between NSLBP individuals and healthy controls.
Methods: 37 Queen Margaret University (QMU) students were recruited to serve as subjects. Based on LBP information obtained by the Nordic Questionnaire, they were assigned to one of the three experimental groups: subjects who had experienced NSLBP within the past 12 months (W12), subjects who had had NSLBP anytime in the past but not within the last 12 months (A12) and healthy individuals who had never had an episode of NSLBP (C). Subjects performed a trunk flexion-extension protocol and the amplitude of bilateral EMG signals was recorded during different movement phases.
Results: Significant differences were revealed on the EMG values during standing, flexion, full flexion, flexionrelaxation ration (FRR) as well as on the side-to-side differences of these variables between the three experimental groups (p<0.0005). The side-to-side EMG signal difference during extension, varied significantly between the three groups (p=0.002). The control group varied significantly from the back pain groups at the variables of full flexion, FRR and standing (p<0.05). In flexion the control group differed significantly only with group W12 (p<0.0001) however, both back pain groups exhibited a greater side-to-side imbalance during this movement (p<0.005). The discriminant analysis clearly differentiated the control from the back pain groups.
Conclusions: The findings of the present investigation indicate that NSLBP can result in an increased and asymmetric activity of the erector spinae muscle group. No significant differences were detected between the two NSLBP groups, challenging the certainty of the results of previous investigations which characterize as healthy subjects, individuals who were asymptomatic of LBP for 6-12 months. The EMG activity in full flexion and the FRR as well as the side-to-side difference of these variables can be used as a criterion of identification of NSLBP individuals from controls.
| Keywords |
| Low back pain; Electromyography; Erector spinae |
| Introduction |
| Low back pain (LBP) is a common and disabling health problem of western societies which costs billions in health care and lost productivity [1]. LBP has a lifetime prevalence of 60-85%. At any one time, about 45% of adults will develop LBP [2]. In spite of extensive research efforts, the causes of LBP are still elusive and management effects are unsatisfactory [3]. Furthermore, despite receiving treatment and becoming pain-free, up to 70% of the patients report recurrence of LBP within a year and 8-30% report chronic pain [4]. Regardless initial return to function and recovery, some people continue to have recurrent episodic LBP [1]. Unfortunately, it is not known why LBP becomes recurrent in some people and not in others. A major challenge is the identification of modifiable factors that contribute to recurrence or persistence of symptoms [4]. |
| Changes in muscular control of the spine constitute a biologically plausible mechanism responsible for LBP recurrence [5]. The lumbar back muscles and particularly the erector spinae contribute to the control of spinal motion and stability and are critical for spinal function and health [6]. In the assessment of patients with lumbar complaints, measuring the electromyographic (EMG) activity of the trunk musculature is used by health care professionals as an objective method to assess the function of the lumbar spine [7]. It has been documented that EMG differences exist between patients with LBP and healthy individuals during various protocols measuring back muscle activity in static [8], dynamic [9] and isometric tasks [10]. Furthermore, a sideto- side EMG activity imbalance in back muscles has been observed in people with LBP, suggesting greater level of asymmetry in the muscle activation of patients comparing to healthy controls [11,12]. |
| The majority of the relevant studies mentioned above, have examined the EMG back muscle activity of chronic LBP (CLBP) patients who have already established some significant level of functional disability and pain provocation during functional movements. Nevertheless, there is a large number of individuals who have experienced a single or recurrent episodes of nonspecific LBP (NSLBP), which usually resolve within a short period [13,14]. To date, there is limited research, examining the effects of one or recurrent NSLBP episodes in respect to paravertebral muscle activity of currently asymptomatic individuals presenting no pain or functional impairment. The examination of the erector spinae muscle, in particular, could be considered critical since the specific muscle group plays a major role in the stability and control of the trunk [12,15]. |
| In addition, the control groups utilized by the studies investigating CLBP patients versus a “control” group defined the “healthy control” subjects as those individuals who had not experienced back pain in the preceding 6 or 12 months. However the rationale for the selection of the specific time frame has not been justified. It is unknown if patients who have not had an episode of NSLBP within the past 12 months have the same spinal muscular performance as individuals who have never experienced LBP. This methodological weakness might have had an impact on the results of the investigations comparing LBP patients and “healthy” control subjects. The range between consecutive back pain incidences has been found to vary and in some cases exceeded the 12 month period [16], which has been used by various studies [17-20] to define “healthy” control subjects. The back muscle activity during this time has not been previously examined, addressing the necessity for further investigation. |
| Therefore, the purpose of the present study was primarily to investigate the erector spinae electromyography activity of NSLBP individuals who have experienced back pain during the last 12 months, NSLBP individuals who have experienced back pain in the past but not the preceding 12 months and healthy control subjects (those who have never had LBP). Secondarily, the objective of the present research was to investigate the utility of the EMG data to discriminate NSLBP individuals from healthy controls. |
| Methodology |
| Participants |
| A total of 59 Queen Margaret University (QMU) students volunteered to participate in the present investigation via a moderator message. All individuals participated voluntarily and were informed of the purpose and potential risks of the research. A written consent form was obtained prior to the initiation of the research. The study was approved by The Physiotherapy Ethics Panel of QMU and completed in accordance with the Declaration of Helsinki for studies involving human subjects. |
| Back pain prevalence and data were obtained using the Nordic Low Back Questionnaire [21] the validity of which has been previously determined [22]. Based on the information obtained from this questionnaire subjects were assigned to one of three experimental groups. Group W12 comprised of asymptomatic (ie pain free) healthy subjects who had experienced one or more episodes of NSLBP within the past 12 months, Group A12 comprised of asymptomatic healthy subjects who had had one or more episodes of NSLBP anytime in the past but not within the last 12 months prior to data collection. Group C consisted of healthy individuals who had never had an episode of NSLBP. |
| The inclusion criterion for the back pain groups was any episode of pain in the low back region (i.e. any point at the back below the ribs and above the gluteal fold lines) in the time frames specified above with no functional impairment. The issue of functional impairment was assessed through the Nordic questionnaire, by the subject’s selfreport that he/she is healthy with no known current or past injury/ illness which could possibly affect their function. The control group consisted of individuals asymptomatic of LBP, who had never had an episode of LBP. Exclusion criteria included pregnant women a history of previous back surgery, signs of underlying nerve root pain and spinal pathology (i.e. pain radiating below the knee, numbness/paraesthesia, thoracic pain etc.), back pain during the last 7 days prior to testing, neurologic deficit, malignancy, diabetes, and symptoms of vertigo or dizziness. Individuals with current ankle, knee, or hip pain or scoliosis were also excluded. |
| Instrumentation |
| A two-channel portable microcomputer muscle tester ME-3000 (Mega Electronics Ltd., Kuopia, Finland) was used to record the EMG from the erector spinae muscles. After the skin had been cleaned with alcohol, a pre-gelled disposable pair of 10-mm diameter silver chloride surface disc electrode pairs (Medi-Trace, Tyco Healthcare, Hampshire, UK) were applied at the L3-L4 level over the left and the right erector spinae musculature (about 3 cm lateral from the midline). Electrodes were placed parallel to the underlying muscle fibres in a bipolar configuration. Centre to centre electrode distance was 2.5 cm. Two reference electrodes (one for each pair) were placed on the anterior superior iliac spines (ASIS) [23]. |
| The muscle tester ME-3000 records, amplifies, and digitally stores on memory cards the electrical signal as raw EMG and integrated electromyography. The sensitivity of the EMG preamplifier was 1 μV with a metering band for EMG of 20–500 Hz. The microcomputer converted the raw EMG signals into digital ones, which were then transformed into absolute values (full-wave rectification). The absolute EMG values (μV) were integrated every 0.1 second. The EMG unit was connected to a laptop computer (Toshiba Satellite Pro SP6100) and data were transferred and analyzed with the software Mega Win version 2.3 (Mega electronics Ltd, Kuopio, Finland). |
| Protocol |
| The subjects were advised of the nature of the test and were told their right to withdraw from the study at any time. All the testing took place in the Human Performance Laboratory of QMU with a constant temperature of 24°C. The selected protocol of the present investigation was adopted by other relevant studies examining the back muscle EMG activity of LBP patients [23,24]. The subjects were required to remove their clothing down to their underwear. Electrode sites were identified and placed as described above. Following the placement of the electrodes the subjects were allowed to rest for 5 min prior to data collection while, testing procedure was explained to them and a demonstration of the technique was performed by the investigator. The subjects stood upright with their backs positioned close to a wall. Their hips and knees were stabilized in an extended position by firmly strapping the pelvis and the thighs to the wall. The patients stood with their arms by their side and their feet shoulder width apart. The position of the feet was marked for consistency between trials. During standing the subjects were required to keep their eyes fixed on a mark on the wall set at eye level to reduce any artefact caused by alteration of head position. Each subject was allowed to practice the movement required prior to testing to ensure that they could completed the task within the time epoch. When an adequate movement technique and signal were ensured the following task was then conducted and analyzed for surface EMG activity in the following four phases. Subjects were asked to stand upright for 2 seconds (phase 1), then bend as far forward as they could to the count of two (phase 2). During phase 2 patients were asked to tuck their chin into their chest as they bent forward. This positioning was used because head position has been suggested to influence EMG activity during this body movement [24]. During phase 3, the patients were asked to stay in full flexion for 2 seconds and then return to standing over a 2 second period (phase 4). Data collection was performed during a period of 10 seconds to assure that all the data were captured. The average from three technically acceptable trials was recorded. To control for differences in amplification, factors such as body mass index (BMI), body weight and height which might influence the surface EMG signal, were taken into account. Therefore, the data were analysed as normalized (by dividing the data for a phase by the average EMG during phase 1) or as raw depending on the existence of significant differences on BMI between the experimental groups. This method of EMG data normalization has been suggested by other studies [24,25]. EMG signals from the left and right sides were averaged. Results for each subject were then divided into the four phases as described above and the following readings were taken based on the calculated root mean square (RMS) of the raw signal [23]. |
| As suggested by other studies [24] the flexion-relaxation response was computed by dividing the maximum EMG for 1 second during flexion by the maximum EMG for 1 second in full flexion. |
| Statistical analysis |
| The intra-rater reliability of the tests was assessed on a group of 7 participants (3 from W12, 1 from A12 and 3 from C group). Pearson’s product-moment correlations were computed for EMG data on the same participant at two separate points in time one week apart. Descriptive statistics were used to characterize the sample. Variables were analysed by the Shapiro-Wilk method to assess normality of distribution and the measures of central tendency were expressed as means (SD) or as medians with interquartile, ranges as appropriate. Multivariate analysis of variance (MANOVA) was conducted to evaluate the EMG values as well as the demographic characteristics between the three groups. Sheffe post hoc tests were performed in the presence of significant differences. The utility of the EMG data to discriminate the back pain groups from the control was investigated with discriminant function analysis (DFA). The alpha level for all statistical tests was set at 0.05. Statistical analysis was carried out using SPSS statistical software, version 16.0 (SPSS Inc, Chicago, Illinois, USA). |
| Results |
| Participants |
| From the initial 59 QMU students who initially volunteered to participate in the study 22 of them met the exclusion criteria of participation and 37 (16 males and 21 females) were finally recruited to serve as subjects. The demographic features of the three experimental groups are presented in Table 1. |
| Intra-rater reliability of EMG data |
| Table 2 presents the findings on the EMG reliability measurements. The between session correlations demonstrate a very good level of reliability for all measures since all the reliability coefficients were at or above 0.78 and significance p values were less than 0.001. |
| Analysis of demographic characteristics |
| MANOVA (3×4) was conducted to identify significant differences on the demographic characteristics such as age, body weight, height and BMI, between the three experimental groups. The analysis revealed that there were no significant group differences (p>0.05). BMI, height and body weight did not varied significantly between groups and consequently, there was no need to control for differences in amplification as described previously (methodology section). |
| EMG group differences: standing, flexion, full flexion and extension |
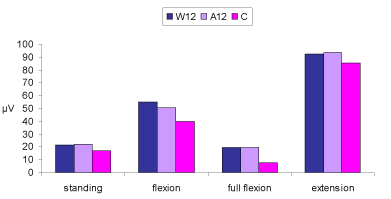
| The MANOVA (3×4) revealed that there were significant differences on the combined dependent variable of EMG activity during standing, flexion, full flexion and extension (p<0.0005). Analysis of each individual dependent variable using a Bonferroni adjusted alpha level showed that the three groups differed significantly in terms of EMG during standing (p<0.0005), flexion (p=0.010), as well as full flexion (p<0.0005). No significant differences were observed on EMG values during extension (p=0.86. Table 3 presents the EMG data of the different movement phases for each group. |
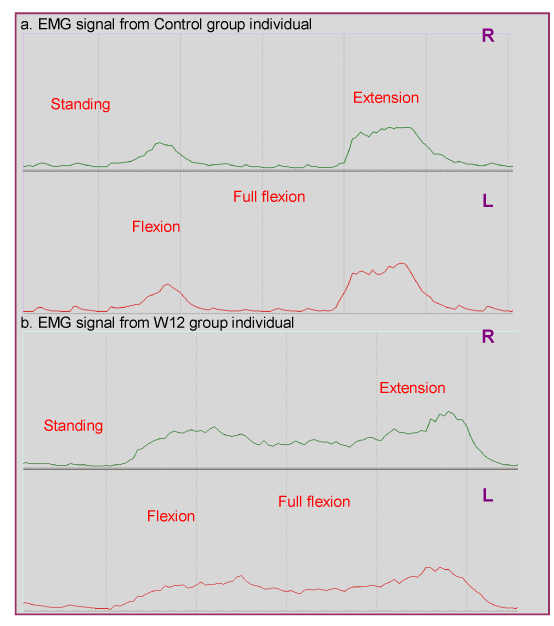
| Employing the Sheffe post hoc test, significant differences were found between the control group and groups W12 and A12 for the dependent variables of full flexion and standing (p<0.005). No significant differences were observed between W12 and A12 groups (p>0.05). In flexion, the control group differed significantly only with group W12 (p<0.001) with no further differences between back pain groups (p>0.05). Figure 1 presents the EMG signal during trunk flexion and extension from 2 participants assigned to the control and W12 groups. Figure 2 provides a graphic representation of the EMG data of the different movement phases for each group. |
| Side-to-side EMG activity differences |
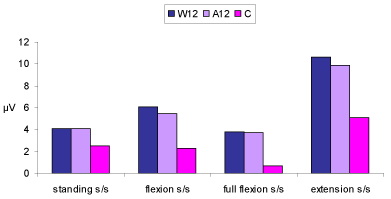
| The MANOVA (3×4) revealed that there were significant differences on the combined dependent variable of EMG side-to-side activity difference during standing, flexion, full flexion and extension between the three groups (p<0.0005). Analysis of each individual dependent variable, using a Bonferroni adjusted alpha level showed that the three groups differed significantly in EMG side-to-side activity difference during standing (p<0.0005), flexion (p<0.0005), full flexion (p<0.0005) and extension (p=0.002). Table 4 presents the data of the EMG side-to-side activity differences during the various movement phases for each group. |
| Employing the Sheffe post hoc test, significant differences were found between the control and groups W12 and A12 for the dependent variable of EMG side-to-side activity difference during flexion and full flexion (both p<0.0005). No differences were detected for the same variables between groups W12 and A12 (p=0.7 and p=0.9 for flexion and full flexion respectively). In standing the EMG side-to-side activity difference was significantly greater in groups W12 and A12 compared to the control group (p=0.001). The level of asymmetry in standing between the two back pain groups (W12 and A12) was not significant (p=1). During extension, the EMG side-to-side activity difference was greater in group W12 and A12 compared to the control group (p=0.004, p=0.001 for group W12 and A12 respectively). The level of asymmetry during extension between the two back pain groups was not significant (p=0.9). A graphic representation of EMG side-to-side activity differences during the various movement phases for each group is provided in Figure 3. |
| FRR and FRR side-to-side differences |
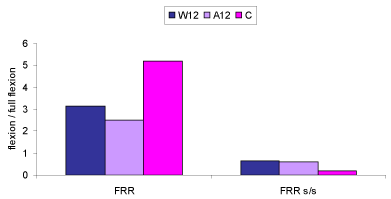
| The MANOVA (3×2) revealed that there were significant differences on the combined dependent variable of FRR and FRR sideto- side difference between the three groups (p<0.0005). Analysis of each individual dependent variable using a Bonferroni adjusted alpha level showed that the three groups differed significantly in FRR (p<0.0005) and FRR side-to-side difference (p<0.0005). Table 5 presents the FRR and FRR side-to-side difference data for each group. |
| Employing the Sheffe post hoc test, significant differences were found between the control and groups W12 and A12 for both the dependent variables (both p<0.0005). No differences were detected between back pain groups for neither FRR (p=0.4) nor FRR side-toside difference (p=0.8). Figure 4 provides a graphic representation of FRR and FRR side-to-side difference values. |
| Multiple discriminant function analysis: standing, flexion, full flexion, extension, FRR |
| DFA was conducted to determine the dimensions along which the three groups differ using as independent variables the EMG activity during the different phases of trunk flexion and extension as well as the FRR. Only one significant function emerged (p<0.0005; Table 6) accounting for most of the variance (99.2%). |
| Structure matrix (Table 7) showed that function 1 was mostly explained by EMG activity in full flexion. FRR was negatively correlated with the discriminant function value. |
| The results of discriminant classification (Table 8) show that 78.4% of subjects were correctly classified. The healthy control individuals were the most accurately classified with 100% of the cases correct. The A12 were next (72.7%) followed by the W12 (58.3%). 27.4% (3 cases) of A12 were incorrectly classified as W12 whereas 41.7% (5 cases) of W12 were incorrectly classified as A12. |
| Multiple discriminant function analysis: Side-to-side difference in standing, flexion, full flexion, extension, FRR |
| DFA was conducted to determine the dimensions along which the three groups differ using as independent variables the EMG sideto- side activity differences during the phases of trunk flexion and extension as well as the FRR side-to-side difference values. Again, only one significant function emerged (p<0.0005; Table 9) accounting for nearly all of the variance (99.7) |
| Structure matrix (Table 10) showed that function 1 was mostly explained by the EMG side-to-side activity difference in full flexion as well as the FRR side-to-side difference. |
| The results of discriminant classification (Table 11) show that 78.4% of subjects were correctly classified. The healthy control individuals were the most accurately classified with 100% of the cases correct. The W12 were next (66.7%) followed by the A12 (63.6%). 36.4% (4 cases) of A12 were incorrectly classified as W12 whereas 33.3% (4 cases) of W12 were incorrectly classified as A12. |
| Overall, the DFA data indicate a clear distinction between the control and the back pain groups however, the discrimination between W12 and A12 was not obvious. |
| Discussion |
| Methodological issues |
| Before discussing the findings of the present investigation, some methodological issues should be addressed. This study employed a convenience sample which was derived from QMU students eligible to participate. Overall the participants had a significant lower mean of age, body weight and BMI compared to similar studies investigating the EMG activity of back musculature. The back pain participants involved were asymptomatic and without any functional impairment making the findings more difficult to compare with those from other studies involving chronic LBP patients with considerable level of pain and impairment. |
| LBP prevalence was obtained using the Nordic LBP questionnaire, which does not stipulate variables such as pain severity and symptom duration, so is probable to yield a different evaluation of LBP prevalence than other questionnaires which may take into account these factors [22]. Any specific spinal pathology was excluded by the participants’ self-report and/or by the principal investigator’s observation (i.e. scoliosis). |
| It has been postulated that the comparison of EMG signals between different studies can be problematic since factors such as equipment, type of hardware and software may lead to different absolute signals [23]. In addition, although normalizing EMG data is still debatable, various prior studies examining the erector spinae activity have selected to normalise their EMG values in order to control for factors that may influence EMG signals such as obesity, body weight and BMI. This method has been supported to detect more accurately EMG group differences between CLBP patients and healthy subjects. Back pain individuals exhibit significantly greater BMI and body weight due to the deconditioning effect of CLBP. Nonetheless, all the groups studied in the present investigation consisted of young students who did not differ significantly in their demographic characteristics and therefore, data were not normalised. |
| Additionally, the raw EMG signals reported in relevant studies have shown a considerable degree of variation. However, the EMG signals collected in the present study are very similar with those reported by others [7,26]. Furthermore, the present investigation demonstrates that the EMG signals recorded, can by reliably measured over time. The findings on the intra-rater reliability are similar to those reported in other studies [27] suggesting that the protocol used to assess the EMG activity of the erector spinae is of an acceptable level of repeatability. |
| EMG group differences: standing, flexion, full flexion, extension |
| The findings confirm previous studies suggesting an increased activation profile of the erector spinae muscle for the LBP patients compared to healthy control subjects [28]. Notably, no significant differences were revealed between the two back pain groups, W12 and A12, in any of the variables tested. This result is of particular importance, challenging the certainty of the findings of previous investigations comparing the EMG activity of back muscles between LBP patients and controls. The control group of these studies was defined as individuals who had not experienced back pain 6 or 12 months prior to testing [20,29,30]. However, the present investigation clearly demonstrated that the subjects of A12 group, who had an incidence of back pain sometime in the past but not within the last 12 months, exhibited significantly different EMG values compared to the control subjects (those who had never experienced back pain). Moreover, while the discriminant analysis clearly differentiated the control from the back pain groups, the distinction between the latter was not obvious; that is a great percentage of W12 subjects could be incorrectly identified as A12 and vice versa. Our findings suggest that future studies, when examining the EMG activity of LBP patients compared to controls, should recruit healthy subjects never having experienced a LBP incidence. Otherwise, as demonstrated form the present investigation, certain methodological issues may arise. This however, might make research protocols difficult to design, when large sample sizes are required, as only a small percentage of the general adult population manages to remain back pain free [31]. Consequently, the recruitment of ‘healthy-control’ subjects may be challenging. Alternatively, further research should aim to identify alterations in back muscle EMG activity classified on different levels of pain intensity instead of pain-free periods. It has been documented that the existence of various erector spinae disorders is correlated with pain thresholds [32] and hence, it can be assumed that the EMG activity changes will be sensitive to pain status. However, no definite conclusions can be drawn from the data of our study, since the intensity of pain among LBP pain individuals was not recorded. |
| The hyperactivity of the erector spinae muscle group in back pain individuals has been explained as a way of providing additional muscular stabilisation and control to the spine [33]. Various reasons that have been suggested to trigger the particular muscle group to change activity patterns may explain the results of the present study. |
| In line with the pain-spasm-pain model, it has been proposed that the increased activation pattern observed in LBP patients can be attributed to adaptations required to compensate for osseoligamentous changes form injury to prevent further pain or injury [4]. In LBP patient groups, the hyperactive superficial muscles are considered to represent an adaptive strategy aiming for postural adjustments as well as control the perturbation to the spine [34]. There is strong evidence to suggest that the hyperactive muscular behaviour serves to stiffen and protect the spine [35]. Nevertheless, if maintained long term, this adaptive muscle activity pattern can be problematic because as the superficial trunk muscles (erector spinae) stiffen the spine via sustained and augmented compression, a continuous stimulation of nociceptors in spinal structures may predispose and result in further injury [4]. |
| In relation to the mechanical behaviour and stability of the spine, injuries to the ligaments or discs resulted from mechanical overloading can generate an increase of the neutral zone (i.e. part of the movement trajectory where stiffness is minimal), a significant decrease of stiffness outside the neutral zone as well as an increase of the ROM [36]. The consequence of these alterations will be a deficiency in spinal stability of LBP individuals as the stiffness of the affected/injured spinal motion segments is decreased and the range of motion increased [37]. In addition, spino-ligamentous injuries are considered to cause a disturbance of the control of trunk equilibrium, reduced proprioception and consequently impaired postural control, since ligaments are considered highly important in sensory function and feedback control of joint position [38]. This function is also compromised in the presence of injuries in the annulus fibrosus, due to the rich supply of these structures with mechanoreceptors [3]. There is evidence to suggest that a reflexive coupling exists between damage to the annulus and ligaments on one hand and the activity of the back muscles on the other [33]. |
| These alterations may trigger muscular corrections and consequently, adaptations of muscular activation might be required to compensate for the reduced segmental stiffness and postural control. Back superficial extensor muscles and specifically the lumbar part of the erector spinae may increase their activity and contraction rate patterns to adapt in the new load sharing and stability requirements [4]. Hence, the increased erector spinae activation found in back pain groups compared to the control during flexion (W12) and full flexion (both W12 and A12) could be possibly explained as a response of the particular muscle group to limit the segmental range of motion, restricting the excursion of the vertebrae with respect to each other where the passive stiffness is insufficient. Moreover, the observed differences in standing could be attributed to the reduced postural control and proprioception, due to impaired sensory information (as a consequence of ligamentous/disc injury), triggering the contraction of the erector spinae. |
| Nevertheless, this notion is challenged by several studies suggesting that trunk muscle activity increases during experimental LBP (injection of hypertonic saline) when spinal structures are uninjured [39]. In addition, in the present study ligamentous or/and disc injury could be only suspected for the participants of the back pain groups since, as mentioned before, the information concerning the LBP prevalence were assessed only by the Nordic questionnaire and the subject’s self-report. These methods could not identify any possible underlying pathology or injury and therefore other additional reasons should be accounted for in order to explain the observed hyperactive back muscles. |
| It has been proposed that back pain individuals would need additional muscular stability of the spine, due to the decreased back muscle force and consequent reduced capacity to correct perturbations. This need is met by an increased activity pattern of their lumbar superficial muscles [40]. Numerous studies have demonstrated that LBP patients exhibit reduced trunk muscle force [41] due to deep extensor muscle mass wasting [42] as well as reduced endurance of the trunk extensor muscles [10]. These findings suggest that patients with LBP are possibly less capable of developing rapidly trunk muscle force. As a result their capacity to correct perturbations of the trunk is impaired. Therefore, as a response the erector spinae muscle becomes hyperactive in order to overcome this weakness and prevent spinal instability [3]. Back muscle biopsy studies of LBP patients can accurately support this theory. More specifically, it has been documented that while paraspinal muscles of healthy individuals show a large proportion of Type I (slow-twitch) fibres, LBP patients demonstrate a significantly higher proportion of Type II (fast-twitch) fibres accounting for lower endurance levels in test protocols [43]. Moreover, it has been observed that deep back muscles (multifidus) of LBP individuals are less active and have an atrophic profile compared to healthy controls [1,43]. Reflex inhibition due to sensory stimuli (i.e. pain) can possibly explain the hypo-activity and the subsequent fibre atrophy of the multifidus muscle of back pain individuals [44]. As a strategy to enhance spinal control the erector spinae becomes more active and a selective fibre Type II hypertrophy occurs acting in response to the decreased proportion of Type I fibres and the consequent compromise of the endurance capacity of the muscle [45]. Nevertheless, because muscle biopsy is an invasive investigation, most studies of muscle histology relied on specimens obtained during surgery and involve chronic LBP patients with specific spinal pathology [43,46]. With regard to NSLBP no investigation on the muscle fibre characteristics has been carried out and therefore the information discussed above cannot precisely explain the increased EMG values observed in the present study. Furthermore, it is believed that the alteration in muscle fibre characteristics, is a long term process and hence it is unlikely that the subjects of the W12 and A12 groups have their fibre characteristics modified at the extent of the chronic LBP patients. It should be also noted that it is questionable whether the typical fibre type characteristics observed in LBP patients are a result of the disease process or inherited genetically and function as a predisposition factor for LBP development [47]. |
| Interestingly, various other explanations have been given accounting for the altered muscle activity pattern observed in LBP patients even when they are pain free. It has been postulated that these changes can be attributed solely at the anticipation of pain which can disrupt cortical processing, alter motor output associated with voluntary movements and activate cortical motor networks. Furthermore, the anticipation of pain has been considered responsible for the development of chronic unremittent pain syndromes, and especially when it is associated with fear, it is believed to result in even more disabling conditions than pain itself [39]. Nevertheless, as the subjects of both back pain groups of the present study were currently asymptomatic and pain-free, the anticipation of pain can only partially explain the muscle hyperactivity observed in the W12 group. However, it is unlikely that pain anticipation can explain the increased activity patterns for the A12 group due to the sufficient pain-free period of 12 months. In contrast with pain anticipation and fear, another theory based on pain ignorance has been proposed. Accordingly, it has been suggested that a subgroup of LBP patients have a tendency to cope with pain using alternative strategies. As previously reported [48] it could be hypothesized that this subgroup of LBP individuals appears to ignore their pain and overload their muscles. Again, the back muscle overuse in the long term can lead to muscular hyperactivity. Nevertheless, whether this hypothesis can explain the increased activity of the erector spinae of back pain groups, particularly A12, due to the pain ignorance theory cannot be confirmed based on our present data. However, an interesting concern is introduced since, if this hypothesis can be established, the particular group of back pain individuals would not benefit from rehabilitation programmes which target the increase of physical fitness as this may overload their back muscles even more [49]. Therefore, further research is required to verify the pain ignorance model and possibly classify such LBP individuals in subgroups according to their back pain behaviour. |
| Another alternative hypothesis accounting for the hyperactive back musculature of the back pain subjects is that these changes may be tuned to the individual problem probably through learning strategies caused by motor cortex reorganisation after the first incidence of pain [50,51]. Various alterations including delayed abdominal and back muscle activation as well as increased activity of superficial trunk muscles in LBP patients persist after the resolution of symptoms (i.e. pain) and have been attributed to the re-organisation of control of these responses in the motor system. The motor cortex is believed to contribute significantly to postural control as human studies have demonstrated that inhibition of the motor cortex decreases the postural activity of the trunk muscles [34]. It is suggested that specific areas of the brain such as the motor and sensory cortices have an enormous potential, to become subject of an organizational change which in the past thought to be possible only during early human development [52]. For example the motor cortex is extensively reorganised following stroke [53], in conditions such as phantom limb pain and in complex regional pain syndrome [54] where the CNS remains largely intact. The plasticity of the sensorimotor cortex in LBP individuals has been reported previously [55] documenting an expansion and shift in the representation of the lower back in the somatosensory cortex. Additionally, it has been suggested that LBP patients require higher thresholds to evoke facilitation of inhibition of responses of the erector spinae muscle over the motor cortex compared with healthy control subjects resulting in altered muscle activity patterns [12,56]. Nevertheless, the association of the changes in motor cortical representation with the deficits in postural control triggering the activity of trunk muscles has not been accurately explained and various theories have been proposed. One possibility is that reorganisation in motor cortical map of trunk muscles in LBP patients could distort their coordination [51]. Additionally, it has been documented that the trunk muscles receive multiple projections from other supraspinal and spinal centres. For example the reticulospinal and vestibulospinal neurons, indirectly involved in postural control, have descending projections to the abdominal motorneurons. Changes in the excitability and organisation of these regions of the CNS have been considered to contribute to changes in postural control of the trunk muscles, altering their activity patterns [50]. Hence, it may be postulated that the increased erector spinae activity of the individuals in the present investigation can be attributed to motor cortex reorganisation after the first incidence of back pain. These changes may have caused back muscle hyperactivity, indicating the development of new strategies for postural control, and possibly remained active even after the LBP resolution. The observed increased EMG activity may reflect an adjusted pattern of spinal stability and control, secondary to past back pain incidence, through the reorganisation of motor cortex. This possible mechanism for LBP to affect motor control of the trunk muscles and particularly erector spinae, is of remarkable importance since as it has been documented, proper treatment and motor learning can reorganise motor cortex over again and consequently modify muscular alterations towards a more healthy profile [51,52]. |
| EMG side-to-side muscle activity differences |
| The results revealed that the back pain groups had a greater level of left/right asymmetry of the erector spinae compared to the control group, indicating a significant level of muscle activation imbalance. No significant differences were detected between the two back pain groups. The DFA could clearly discriminate the control from the A12 and W12 groups but none of the predictor variables could differentiate the last two groups. |
| Various studies have advocated that side-to-side muscle recruitment patterns in LBP patients differ from healthy control subjects [11,19]. This observation, accounts for the increased shear forces developed on the lumbar region of back pain individuals, since besides the effects of trunk muscle increased activity, it is also possible that muscle activation imbalance may further increase lumbar shear forces [57]. This is supported by the hypothesis that contralateral imbalances, prior or secondary to back pain, produce mechanically induced back pain by loading the spine incorrectly [15]. Therefore, some authors have promoted the idea of scanning EMG contralateral differences for clinical purposes, suggesting that left/right differences in EMG activation level are indicative of pathology and can be viewed as indicators of a biomechanical lesion [58]. |
| In contrast, several authors have supported that dominant to non-dominant strength imbalances are normal to some extent and an alteration of muscle physical properties due to training or handedness may result in asymmetric postures and activity patterns [15]. The latter can explain the side-to-side differences observed not only in LBP individuals but also in the healthy control subjects of our investigation. Not every neuromuscular imbalance can be interpreted as a sign of underlying pathological abnormality since unilateral muscular and neuronal adjustments are often significant preconditions for athletic performance [59]. It is documented that alterations of muscle recruitment patterns can be triggered by training or handedness since right-handed athletes showed significant lower EMG measures on the left side of erector spinae, and vice versa [60]. Therefore, asymmetric trunk loading stimulates neuromuscular imbalances resulting in reduced EMG activity patterns on the non-dominant body side. The question whether these neuromuscular imbalances are a result or cause of LBP cannot be answered with certainty due to the lack of relevant research. Nevertheless, based on the significant greater degree of asymmetry observed in the back pain groups of our study compared to the control, we can assume that some additional factors might have contributed to the imbalanced activity patterns of LBP individuals. |
| It is believed that in a chronic state of LBP injury there are known effects of both central and peripheral factors inducing EMG imbalance. Central sensitization after prolonged nociceptor activity from tissue injury sites is associated with hyperalgesia and sustained pain sensation, causing muscular imbalances [19]. However, the back pain group individuals of the present study were asymptomatic and especially the subjects of the A12 group were back pain free for at least 12 months. Therefore, it is not possible to explain the results of the present study as part of the pain related physiological mechanisms. In addition, there is evidence to support that activation imbalance of the erector spinae is not associated with low back injury [57]. |
| Hence, the observed EMG contralateral imbalances should be attributed to other factors unrelated to current back pain per se. As discussed earlier, motor cortex reorganisation after the first pain incidence might occur as CNS alters the muscle recruitment pattern in order to enhance stability. Additionally, the pain avoidance behaviour displayed by many back pain individuals may lead to specific muscle activation and development of selective copping strategies [60]. Added together, these changes may constitute the variables of a motor learning technique directing an asymmetric erector spinae activity even after the resolution of back pain symptoms [19]. Coupled with this, the reduced back muscle endurance and strength seen in LBP individuals may result in insufficient control of lateral bending and/or axial rotation efforts causing further imbalanced back muscle activity which in long term may lead to specific spinal injury [47]. In the present investigation certain variables such as erector spinae strength and endurance were not tested. However, due to the significant larger asymmetrical activity patterns of the back pain groups compared to the control, we can support the hypothesis of the development of a motor learning technique among LBP individuals which encourages an imbalanced side-to-side muscular activity. |
| Flexion-Relaxation phenomenon |
| The findings on EMG activity in full flexion as well as on FRR of the present investigation are in agreement with those reported before [27,61]. An increased activity of the erector spinae at full trunk flexion and a decreased FRR has been documented in studies examining the EMG activity of LBP individuals compared to healthy control subjects. Patients with LBP typically show an inability to fully relax the back muscles in the posture of full flexion [62]. Moreover, as verified in the present study, the values obtained in full flexion and FRR can accurately differentiate back pain group subjects from controls [7]. The results clearly demonstrated that when FRR and EMG values in full flexion were used as predictor variables, a clear discrimination was revealed between the control and the back pain groups. However, no obvious differentiation was detected between W12 and A12 participants. |
| In healthy asymptomatic individuals, the EMG silence of the erector spinae at full trunk flexion is thought to be invoked by a stretch inhibition reflex [63]. As the trunk bends forward, the posterior elements of the spine and hip joints undergo further elongation which enhances their passive tension. This tension is continuously monitored by the CNS via the sensory afferents mediated by mechanoreceptors within all these tissues. It seems that when this perceived passive tension reaches a threshold, the CNS decides to deactivate the erector spinae, which is the active controlling element of the movement, in order to conserve energy, since it assumes that the passive element is capable of controlling the movement independently. This is when the flexion-relaxation phenomenon is observed [6]. The increased EMG activity demonstrated at full trunk flexion and the absence of flexion-relaxation in LBP patients seems to be due to changes in the sensitivity of lumbar afferents as a result of an injury [23]. Spinal ligaments and intervertebral discs are supplied with mechanoreceptors and nociceptive afferents in order to detect joint loads, motion, and the presence of injury/inflammation. Subsequently, ligament and muscular reflexes are triggered between the spinal ligaments, discs and paravertebral muscles, which operate to modify the load imposed on the passive elements through active contraction [63]. A sequence of neuromuscular changes associated with increasing the sensitivity of the afferent receptors occurs in response to static loading of the spine. Muscle pain or spasm generates disturbances in proprioception, stiffness regulation, and motor control by altering stretch sensitivity and the discharge of spindle afferents via gamma (γ) fusimotor neurons [33]. The result of increased activity of the afferent receptors in response to an injury in the lumbopelvic region would be to increase the muscle stiffness to maintain the stability of the spine in a position where potentially injured passive structures are mechanically compromised [23]. Nevertheless, given the available data of the present study, the likelihood of a spinal injury/inflammation on A12 and W12 group participants cannot be assumed with certainty. Therefore, the changes in the sensitivity of lumbar afferents should be attributed to other factors as well. |
| It has been suggested that an increased activity of the extrapyramidal motor pathways due to increased perceptions of pain or fear of pain, evident even after the resolution of symptoms, can also lead to increased activity of the alpha (α) and gamma (γ) spindle systems [23]. This increased local output due to pain is believed to manifest in the FRR as increased activity at full trunk flexion. In contrast, other studies suggest that the FRR is not a direct correlate to pain [24]. This is in agreement with our study since the participants of A12 and W12 groups were currently asymptomatic. However, pain was not assessed during the task performance, which might otherwise have assisted in the interpretation of the full flexion and FRR data. As stated previously [24] the fact that people report no pain in daily activity does not mean that they do not feel any pain during trunk full flexion. Although no functional impairment was recorded through the Nordic questionnaire, subjects may unconsciously avoid flexion during daily activities, and the test requirements of trunk flexion may have provoked an amount of pain, responsible for voluntary and/or involuntary protection, resulting in the observed absence of flexion-relaxation. |
| Collectively, a past injury and pain stimuli and the consequent changes in the sensitivity of lumbar afferents can be considered as factors responsible for the reorganisation of motor cortex and motor learning techniques [27]. As discussed earlier, these strategies developed to cope with LBP may direct muscle activity patterns even after the resolution of back pain explaining the increased EMG activity of A12 and W12 groups in full flexion. |
| Furthermore, in explaining the absence of flexion relaxation phenomenon, it should be noted that LBP has been considered to alter the physiological properties of the hamstring muscle group resulting in stiffness and decreased flexibility [64]. Numerous studies have documented a decreased hamstring muscle flexibility of patients with LBP compared to healthy controls [65]. During a trunk flexion task, hamstring stiffness, has been suggested to result in erector spinae increased activity as a defence mechanism encountered by the CNS to balance the stabilisation of the spine [6]. Therefore, although we did not assess hamstring muscle flexibility, an increased stiffness of the particular muscles, which constitutes a general observation in LBP individuals [66], can be suspected to explain the increased erector spinae activity of the back pain groups. |
| Conclusion and clinical implications |
| The findings of the present investigation indicate that NSLBP can result in an increased activity of the erector spinae muscle group. Overall the back pain groups demonstrated a hyperactive and asymmetric back muscle pattern. No significant differences were detected between the two NSLBP groups in any of the variables tested, challenging the certainty of the results of previous investigations which characterize as healthy subjects, individuals who were asymptomatic of LBP for 6-12 months. Moreover, the present research demonstrates that the EMG activity in full flexion and the FRR as well as the side-to-side difference of these variables can be used as a criterion of identification of NSLBP individuals from controls. The persistent activation of the erector spinae musculature among NSLBP individuals may represent the body’s attempt to stabilize and protect the spinal structures. Different factors such as mechanical overloading, spinal injuries, pain and/or fear of pain, decreased muscle force, motor cortex reorganisation, which are working independently or synergistically, can be attributed to the development of an alternative strategy by which the CNS controls the spine. The subsequent effect on the erector spinae activity is associated with an increased loading in spinal structures and predisposition to injury if maintained long term. Therefore, the clinical implications of the current study are that the EMG data of the erector spinae activity, especially during full flexion and the FRR can be used as an objective method of discriminating between NSLBP and healthy individuals as well as an objective outcome measure following physiotherapy rehabilitation. The alteration of postural strategy observed in NSLBP individuals may be caused by a variety of factors which predispose the individual to back problems. However, they can be possibly trained by proper treatment, such as skilled motor training. Future research should aim to identify altered back muscle activity patterns in relation to LBP pain intensity and possibly classify the LBP individuals accordingly. |
| Acknowledgement |
| Special Thanks to Dr Stella Howden. Her leadership, knowledge and integrity as a clinician, teacher and researcher are highly appreciated. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
| Table 5 | Table 6 | Table 7 | Table 8 |
| Table 9 | Table 10 | Table 11 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 16824
- [From(publication date):
September-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12143
- PDF downloads : 4681
