Research Article Open Access
Docosahexaenoic Acid Protects against 1-Bromopropane Induced Cognitive Deficits in Rats involving in GSK-3β Activation and Oxidative Stress Inhibition
Junlin Yang1, Hua Yuan2, Lulu Jiang1, Ying Guo1, Zengjin Wang1, Keqin Xie1 and Xiulan Zhao1*1Institute of Toxicology, School of Public Health, Shandong University, Jinan, Shandong Province, 250012, China
2Shouguang People’s Hospital, Shouguagn, Shandong Province, 262700, China
- *Corresponding Author:
- Xiulan Zhao
Institute of Toxicology, School of Public Health
Shandong University, Jinan, Shandong Province, 250012, China
Tel: +86 531 88382132
E-mail: zhao.xl@sdu.edu.cn
Received date: September 23, 2016; Accepted date: November 01, 2016; Published date: November 08, 2016
Citation: Yang J, Yuan H, Jiang L, Guo Y, Wang Z, et al. (2016) Docosahexaenoic Acid Protects against 1-Bromopropane Induced Cognitive Deficits in Rats involving in GSK-3β Activation and Oxidative Stress Inhibition. J Alzheimers Dis Parkinsonism 6:282. doi:10.4172/2161-0460.1000282
Copyright: © 2016 Yang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
A number of organic solvent are known to be neurotoxic substance, which can cause neurotoxicological effects in humans. 1-Bromopropane (1-BP) is an alternative to ozone-depleting solvent that widely used in industrial production. Occupational exposure to 1-BP becomes a major health concern due to its neurotoxicity displayed in animals and humans. Docosahexaenoic acid (DHA), long chain n-3 polyunsaturated fatty acids (PUFA) and the main component of fish oil, is essential for normal neurological development and displays potent neuroprotective capacity. Here we investigate the protective effects and underlying mechanisms of DHA against 1-BP-induced deficits of spatial learning and memory ability in rats. Cognitive performance was assessed by Morris Water Maze test (MWM). Neuronal injury was determined by Nissl staining and TUNEL staining. The apoptosis-related proteins, including cleaved caspase-3, Bcl-2 and Bax, and proteins modified by 4-hydroxy-2-nonenal (4-HNE) or acrolein, in the brain were determined by Western blot. The inactive glycogen synthase kinase-3β (GSK-3β) in the brain of rats was also detected by specific antibody. Exposure to 1-BP resulted in learning deficits and memory loss of rats, neuronal apoptosis in the hippocampus cornu ammonis 3 (CA3) and prefrontal cortex, accompanied with significant GSK-3β inhibition by phosphorylation. Importantly, we found that pre-treatment with DHA significantly improved MWM performances of rats intoxicated with 1-BP, as well as the abrogation of neuron loss, alleviation of redox unbalance and GSK-3β activation in the brain. Our findings suggested that DHA supplementation would be a promising intervention for the central neurotoxicity of 1-BP, which might be correlated with oxidative stress and GSK-3β inhibition.
Keywords
Docosahexaenoic acid; Neurotoxicant; Apoptosis; Glycogen synthase kinase-3β; 4-hydroxy-2-nonenal; Acrolein
Abbreviations
1-BP: 1-Bromopropane; DHA: Docosahexaenoic Acid; PUFA: Polyunsaturated Fatty Acid; CNS: Central Nervous System; AD: Alzheimer’s Disease; PD: Parkinson’s Disease; ALS: Amyotrophic Lateral Sclerosis; GSH: Glutathione; GSK-3β: Glycogen Synthase Kinase-3β; Akt: Protein Kinase B; 4-HNE: 4-Hydroxy-2- Nonenal; MWM: Morris Water Maze; CA3: Cornu Ammonis 3
Introduction
A number of organic solvent are known to be neurotoxic substance, which can cause neurotoxicological effects in humans. 1-Bromopropane (1-BP) has been widely used in the workplace as cleaning agent for metal, electronics, optical instruments since the ozone layer depleting solvent chlorofluorocarbons and 1,1,1-trichoroethane have been prohibited [1,2]. Upon exposed to 1-BP, human and laboratory animals displayed both peripheral and central nervous system (CNS) poisoning symptoms, such as sensory and motor deficits and depression, anxiety as well as cognitive deficit [3-6]. Compared with other neurotoxic organic solvent such as hexane intoxicated cases, human with 1-BP intoxication were more likely to exhibit clinical symptoms of CNS [7]. The hyperreflexia displayed by human cases was also pointed to the lesions in the CNS [8]. It is well known that the brain is rather susceptible to oxidative stress due to enrichment in polyunsaturated fatty acids (PUFA) and high oxygen consumption. Oxidative stress is regarded to readily elicit neuronal apoptosis and the mitochondrial pathway is generally believed to be essential in mediating this event [9,10]. Growing evidence has supported that oxidative stress and mitochondria dysfunction are the dominant players in triggering neuronal apoptosis in multiple neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) [11-13]. Our previous studies demonstrated that 1-BP exposure in rats disturbed the redox status of brain evidenced by glutathione (GSH) depletion, lipid peroxidation and reactive aldehydes modified proteins elevation, which were closely correlated with the poor neurobehavioral performances [14]. Additionally, the significant dose-dependent loss of neurons was also found in layer 5 of prefrontal cortex in 1-BP-treated rats [15].
Recently, the serine/threonine kinase glycogen synthase kinase-3β (GSK-3β) has been reported to regulate apoptosis under wide range of neurotoxic conditions [16,17]. GSK-3β is unique since it is constitutively active in resting conditions of cells and can phosphorylate an array of substrates including cytoskeletal proteins, transcription factors, and metabolic regulators. Phosphorylation of GSK-3β on a serine residue of N terminus (ser 9) by the activated protein kinase B (Akt) (phosphorylated-Akt) results in its inactivation [18]. Growing evidence suggest that dysregulation of GSK-3β is implicated in the etiology of neurodegenerative diseases, and gained prominence as a potential therapeutic and preventive target [19,20]. Therefore, we hypothesized that the GSK-3β might be related to the 1-BP-induced neuronal loss and related CNS damage.
Docosahexaenoic acid (DHA, 22:6n-3), a dominant long chain n-3 PUFA in the brain, is essential for normal mammalian development [21]. Studies showed that pre-administration of DHA attenuated the cognitive deficit in an animal model of AD [22].while DHA deficiency was associated with a loss of discriminative learning and memory ability [23]. Previous studies had demonstrated that dietary DHA supplementation could significantly increase DHA levels in both plasma and brain of rats, which was associated with the mitigation of memory defects induced by amyloid-β (Aβ) peptide [24]. DHA cannot be synthesized de novo in vertebrate tissue. The brain concentration of DHA depends on dietary content and on hepatic conversion from α-linolenic acid (18:3n-3), as well as circulating eicosapentaenoic acid (EPA, 20:5n-3) [25]. It is generally accepted that the DHA enters the brain from the circulation [26]. It has been demonstrated that plasma non-esterified DHA is the major plasma pool supplying the brain with DHA in adult rats [27]. The fatty acids could rapidly cross the blood– brain barrier (BBB), and emerging data suggest that both membrane and cytosolic localized proteins potentially facilitate brain fatty acid uptake [28]. The most recent study from Bazinet group demonstrated the effect of dietary DHA on tissues and plasma DHA levels, which showed that fish oil could increase the brain DHA concentrations by 26%-43% in different brain regions of rats, as well as the liver levels by 7-10 folds, compared with olive oil-fed controls [29]. The increased levels in liver might indirectly contribute to the brain DHA contents. Evidence suggests that DHA exhibited potent anti-oxidative and antiinflammatory effects [30-32]. We therefore hypothesized that DHA may ameliorate the impairment of neurotoxicity induced by 1-BP exposure. The neurobehavioral performance of rats and neuronal injury in the brain were evaluated. As the reliable marker of oxidative stress [33,34], the 4-hydroxy-2-nonenal (4-HNE) and acrolein modified proteins in the brain were determined, as well as the apoptosis-related proteins and GSK-3β. Thus, the data in the current study might provide further insights into the protective effects of DHA, the mechanisms of 1-BP induced CNS neurotoxicity, and possible preventive strategies.
Materials and Methods
Materials
1-BP was purchased from Sinopharm Chemical Reagent Co (Shanghai, CN). DHA was purchased from Rongcheng Baihe BioTechnology Co (Rongcheng, CN). The thionin and β-actin were purchased from Sigma Aldrich(St. Louis, MO). The TUNEL detection kits for apoptosis were purchased from Beyotime Institute of Biotechnology (Beijing, CN). Anti-4-HNE and anti-acrolein polyclonal antibody (pAb) were purchased from JaICA (Nikken SEIL Co., Shizuoka). Anti-cleaved caspase-3 mouse monoclonal antibody (mAb), anti-phosphorylation of AKT (p-AKT) (ser 473) rabbit pAb, anti-p-GSK-3β (ser 9) rabbit pAb, anti-AKT rabbit pAb and anti-GSK- 3β rabbit pAb were all obtained from Cell Signaling Technology, Inc. (Danvers, MA). Anti-Bcl-2 pAb, anti-Bax pAb, goat anti-mouse or rabbit horseradish peroxidase (HRP)-conjugated IgG were all supplied by Santa Cruz Biotech (Santa Cruz, CA). Anti-caspase-3 was purchased from Enzo Life Sciences (Farmingdale, NY). BCATM Protein assay kit was bought from Pierce (Rochford, IL). Polyvinylidenedifluoride (PVDF) membrane was purchased from Millipore (Bedford, MA). Chemiluminescence detection kit was purchased from Biological Industries Israel Beit Haemek LTD (Kibbutz, Beit-HaEmek). All other chemicals were of highest quality commercially available.
Animals and experimental design
Sixty male Wistar rats weighed 180-200 g were purchased from Vital River Laboratories (Beijing, China). They were housed in a room set on 12 h light/dark cycle, constant temperature (22 ± 2°C) and stable relative humidity (60%). The rats have access to water and special pathogen free (SPF) rodent standard rat chow [Bejing keao xieli feed CO., LTD., containing 4.2% crude fat comprised of 21.7% saturated fatty acid, 21.5% monounsaturated fatty acid and 56.8% polyunsaturated fatty acid (18:2n-6 52.2%, 18:3n-3 4.6%), without DHA]. The study of Zugno et al. [32] demonstrated that the n-3 fatty acid exhibited potent protective effects against ketamine-induced schizophrenia in Wistar rat, but has no effect on the neurobehavioral and oxidative parameter in controls. In another study, fish oil exerted little effects on the redox status and inflammatory parameters of brain in control rats, however, DHA ameliorated the learning deficit of diabetic rats [35]. The organotypic hippocampal-entorhino cortical slices cultures from adult rat also proved DHA per se exerted little effects on the neuroinflammatory pathways [36]. In our study, control, 1-BP group, 1-BP+low dose DHA group and 1-BP+high dose DHA group, with 15 rats in each group were used to determine the DHA protective effect on 1-BP induced neurodamage. After acclimation to the new environment for one week, 800 mg/kg.bw 1-BP dissolved in the corn oil was orally administered to rats once a day for consecutive 13 days. The rats in control group were received equal volume of corn oil accordingly. The gavage volume was 0.1 ml/100 g·bw. This dose of 1-BP induced paralysis of hind limbs in rats with long time exposure (17 weeks, 5 days per week), while short time exposure (2 weeks) led to the CNS deficits, which were similar to the neurotoxic symptoms of 1-BP intoxicated human cases [14,35,37]. DHA diluted in corn oil was given to rats by gavage (0.1 ml/100 g·bw) once daily for 7 days prior to 1-BP exposure, with low dose 250 mg/kg bw or high dose 500 mg/kg bw for 20 days. The dose of DHA was chosen based on the earlier studies and our pilot study, in which significant learning improvement and anti-oxidative effects had been demonstrated [22,38]. DHA was treated to rats 4h prior to 1-BP treatment. Rats in control group and 1-BP group received equal volume of corn oil. After 7 days of 1-BP exposure, the rats were subjected to Morris Water Maze (MWM). All animal procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Animal Experimentation Committee of Shandong University. All efforts were made to minimize animal suffering during experiment.
Behavioral test by Morris water maze
The spatial learning and memory ability of rats were assessed by the MWM test in a quiet room. The equipment (Huaibei Zhenghua biological instrument equipment Co., Ltd., China) consists of a black circular pool (diameter 180 cm, height 50 cm) filled to a depth of 35 cm with water (20 ± 2)°C. The water maze was divided into 4 equal quadrants (I–IV). There was a platform placed 1-2 cm below the water surface in the center of quadrant III. The MWM test was completed in 6 days, including the first day for training, four consecutive days for place navigation and the last day or the 6th day for probe test. Before the formal experiment, rats were trained for one day in advance and then rats were given a place navigation test for four consecutive days. On each day of place navigation, rats were placed in the water surface faced the wall of the pool at one entry point for four trials, the escape latency and swimming distance (swimming time and distance to locate the hidden platform) that rats searched for the hidden platform in a maximum of 120 s were recorded for each trial. If the rat failed to find the platform within 120 s, it was guided to the platform and allowed to stay for 30 s and the escape latency was recorded as 120 s. After four trials, the rat was dried and put it back to its cage. The escape latency and total swimming distance were used to assess the performance of learning ability of the animals. At the 6th day, the probe test was performed in the absence of the platform within 120 s and the number of crossing original platform region was recorded to evaluate the spatial memory of rats. At the end of behavioral test, 4 rats from each group were randomly selected for perfusion, fixation and followed by morphological examination. The remaining rats were sacrificed and the brains were removed rapidly for Western blot analysis.
Nissl staining and TUNEL staining
The chosen rats were deeply anesthetized with urethane (800 mg/ kg, i.p.) and perfused transcardially with 200 ml of PBS at 4°C, followed by 300 ml of ice-cold 4% paraformaldehyde in 0.1M PBS (pH7.4). Perfusion was performed using RWD double channel syringe pump (RWD Life Science Co. Ltd. Shenzhen, CN), the flow rates were set 40 ml/min for PBS and 20 ml/min for fixative. Serial sections (6 μm) of brain were cut coronally after being embedded in paraffin. For the Nissl staining, histological sections were processed by dewaxing, rehydration and staining with 1% (m/v) thionin for 30 min in 37°C incubator. After rinsing with distilled water, slides were photographed under light microscope (Olympus Corp., Japan). TUNEL staining was performed according to the instruction of detecting kit. Sections were dewaxed and rehydrated, followed by incubation with a TUNEL detection mixture containing 2 μl enzyme solution and 48 μl fluorescent label solution for 60 min in a light avoiding humidified box in 37°C incubator. After washing with PBS, tissue slices were observed under a fluorescence microscope (Oympus Corp., Japan). The prefrontal cortex and hippocampus are critical participants in the neural circuit of CNS, and the anatomical relationships and functional interactions between them are essentially required for learning and memory [39]. Thus, the areas of interest to be evaluate for the neuronal damages included anterior cingulated cortex (AC) of the prefrontal cortex and the corner region of CA3 in the hippocampus, which was anatomically defined according to the atlas of Paxinos and Watson [40].
Western blot analysis
After behavioral test, eleven rats of each group were anesthetized with urethane (800 mg/kg, i.p.). The hippocampus and prefrontal cortex were manually dissected out on ice and homogenized in icecold homogenization buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.5% NP-40, 1% sodium deoxycholate, 0.25% SDS, 1mM PMSF, 0.1% NaN3, 1% protease inhibitor cocktail, 10 mmol/l NaF, 1% Triton X-100, and 2 mmol/l Na3VO4, using a Bioprep-24 bead-based tissue homogenizer (Hangzhou allsheng instrument CO., Ltd., Hangzhou, China). The homogenate was kept on ice for 30 min, followed by centrifugation at 10,000 g for 10 min at 4°C, then the supernatant was collected and the protein concentration was determined using BCA assay kit. For Western blot, an equal amount of protein samples were boiled in buffer (50mM Tris-HCl, pH 6.8, 2% SDS, 0.14M 2-mercaptoethanol, 10% glycerol, 0.001% bromophenol blue), then separated through SDS-polyacrylamide gel electrophoresis and electrically transferred to a PVDF membrane. After blocking with 4% non-fat milk, the membranes were washed with Tris-buffered saline containing 0.05% Tween-20, then probed with specific antibodies respectively overnight such as Bcl-2, Bax, cleaved caspase-3, caspase-3, 4-HNE, acrolein, p-Akt (ser 473), Akt, p-GSK-3β (ser 9) and GSK-3β). Subsequently, the membranes were washed with TBST and incubated with HRP-conjugated anti-rabbit or anti-mouse IgG antibody for 1 h at room temperature. These target proteins were revealed by chemiluminescence using an ECL plus kit. Images were scanned and quantified by Kodak Imaging Program and Image-Pro-Plus software (Eastman Kodak Company, New Haven, CT, USA). The cleaved caspase-3 was normalized to caspase-3, p-Akt to Akt, p-GSK-3β to GSK-3β and other proteins to β-actin, which served as loading controls.
Statistical Analysis
Data were shown as the mean ± SEM and analyzed using the SPSS 17.0 software. In the MWM test, escape latency and total swimming distance were analyzed with repeated measures analysis of variance. Mauchly’s test of sphericity for the within-subject covariance matrices was done first and Geisser-Greenhouse adjustment for the degree of freedom and probability levels on the within subject F-tests was used when the sphericity assumption was violated. Dunnett method was used for post-hoc tests among four groups. The crossing times obtained from the MWM test and Western blot analyses were analyzed using one-way ANOVA. Values of p<0.05 were considered to be statistically significant.
Results
DHA rescued impaired neurobehavioral performances of rats induced by 1-BP
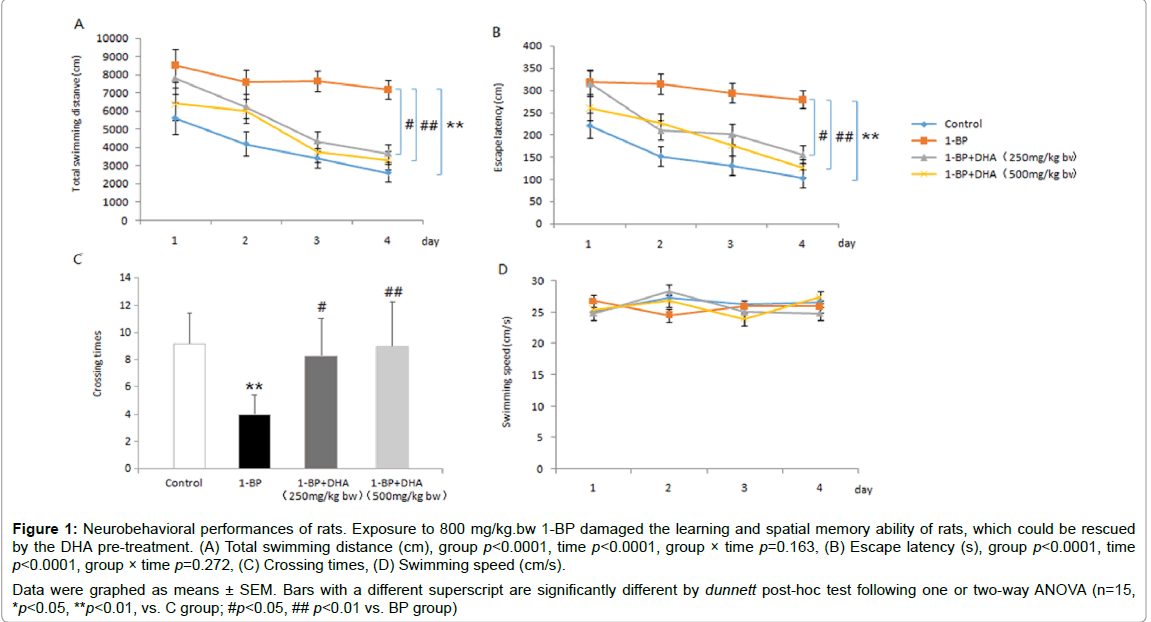
As shown in Figure 1 all rats swam at the similar speed without impairment of mobility (p>0.05). The rats in all groups had tendency of reduced latency and distance to find the hidden platform as training progressed and no significant difference in the decreasing trends among the four groups was observed after analysis by twoway ANOVA (p>0.05). The 1-BP-treated rats spent longer period of time and swimming distance in finding the platform than the control rats (p<0.05). Moreover, in the spatial probe test, compared with the control rats, 1-BP-treated rats crossed through the original platform region fewer times in given time (p<0.01). Results also revealed that 1-BP-treated rats had significant cognitive impairment. However, rats pre-treatment with DHA displayed improved performances in MWM test in a dose-dependent manner, as shown by decrease of latency and distance to find the submerged platform and increase of counts in crossing target region.
DHA reversed neuronal loss induced by 1-BP
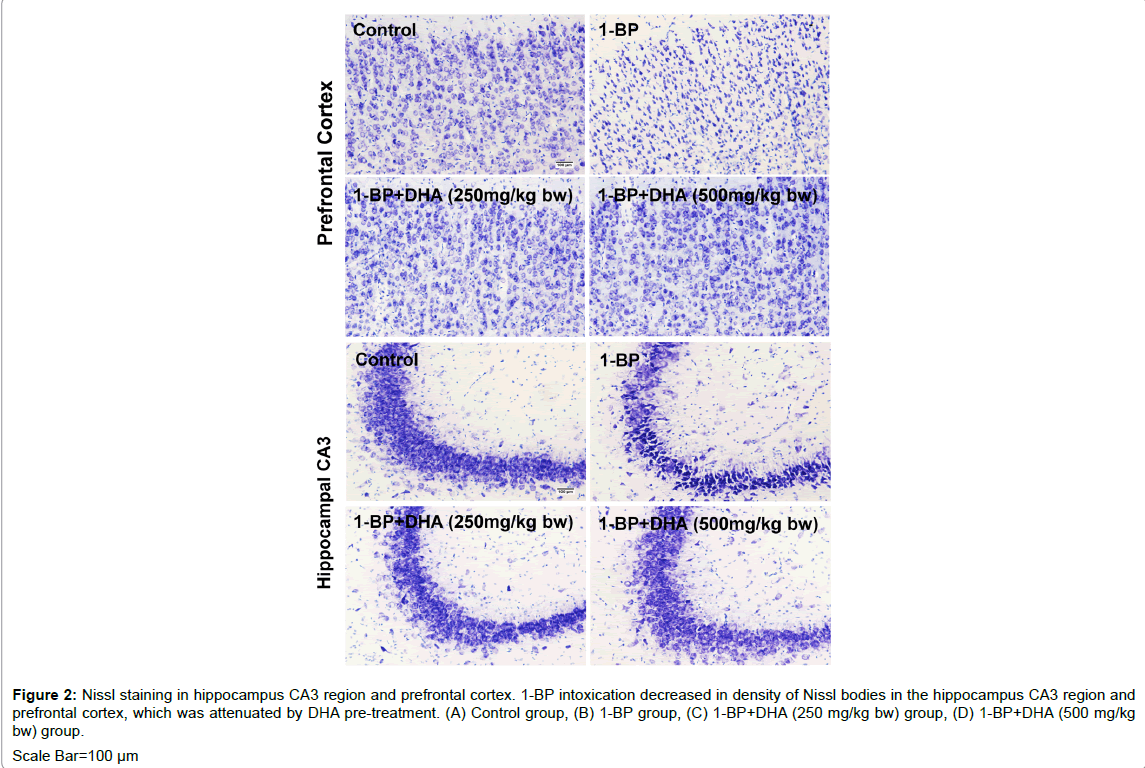
The results of MWM informed us that DHA might confer potential protection against 1-BP induced neurotoxicity in rats. The thionin was employed to stain the Nissl bodies in neurons, the reduced density of Nissl bodies was considered as an indicator for evaluate the neuronal damages [41]. As shown in Figure 2, 1-BP intoxication resulted in decrease in density of Nissl bodies and cytoplasmic vacuolation of pyramidal cells in the hippocampus CA3 region and prefrontal cortex, which was attenuated by DHA pre-treatment.
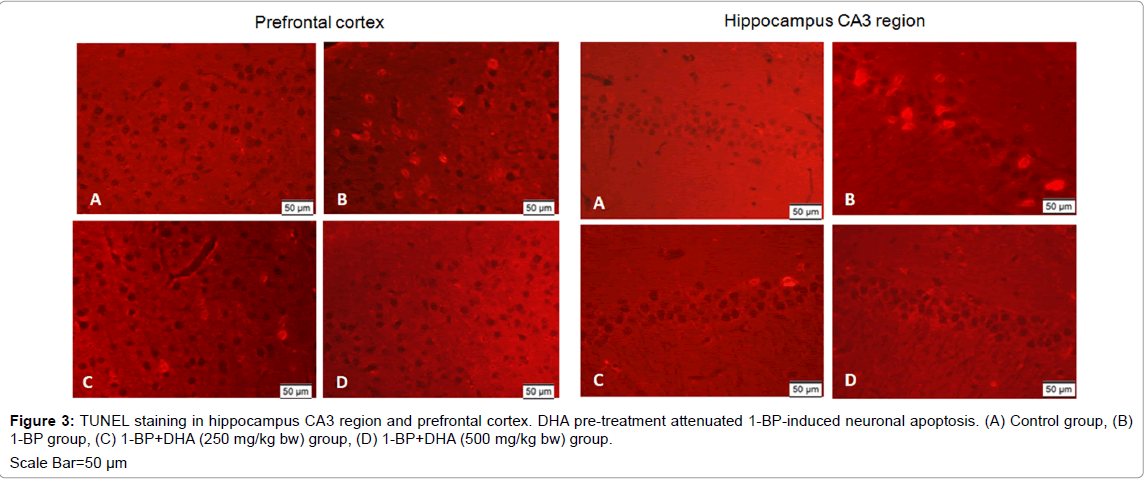
To characterize the underlying mechanisms of neuronal loss induced by 1-BP, TUNEL staining was employed to test the neuronal apoptosis. As shown in Figure 3, almost no TUNEL-positive cell appeared in the brain of control rats, whereas after exposure to 1-BP, TUNEL-positive cells were observed in the hippocampus CA3 region and prefrontal cortex. DHA pre-treatment attenuated 1-BP-induced neuronal apoptosis as shown by reduced number of TUNEL-positive cells compared with 1-BP intoxication alone group.
Figure 1: Neurobehavioral performances of rats. Exposure to 800 mg/kg.bw 1-BP damaged the learning and spatial memory ability of rats, which could be rescued by the DHA pre-treatment. (A) Total swimming distance (cm), group p<0.0001, time p<0.0001, group × time p=0.163, (B) Escape latency (s), group p<0.0001, time p<0.0001, group × time p=0.272, (C) Crossing times, (D) Swimming speed (cm/s). Data were graphed as means ± SEM. Bars with a different superscript are significantly different by dunnett post-hoc test following one or two-way ANOVA (n=15, *p<0.05, **p<0.01, vs. C group; #p<0.05, ## p<0.01 vs. BP group)
Figure 2: Nissl staining in hippocampus CA3 region and prefrontal cortex. 1-BP intoxication decreased in density of Nissl bodies in the hippocampus CA3 region and prefrontal cortex, which was attenuated by DHA pre-treatment. (A) Control group, (B) 1-BP group, (C) 1-BP+DHA (250 mg/kg bw) group, (D) 1-BP+DHA (500 mg/kg bw) group. Scale Bar=100 µm.
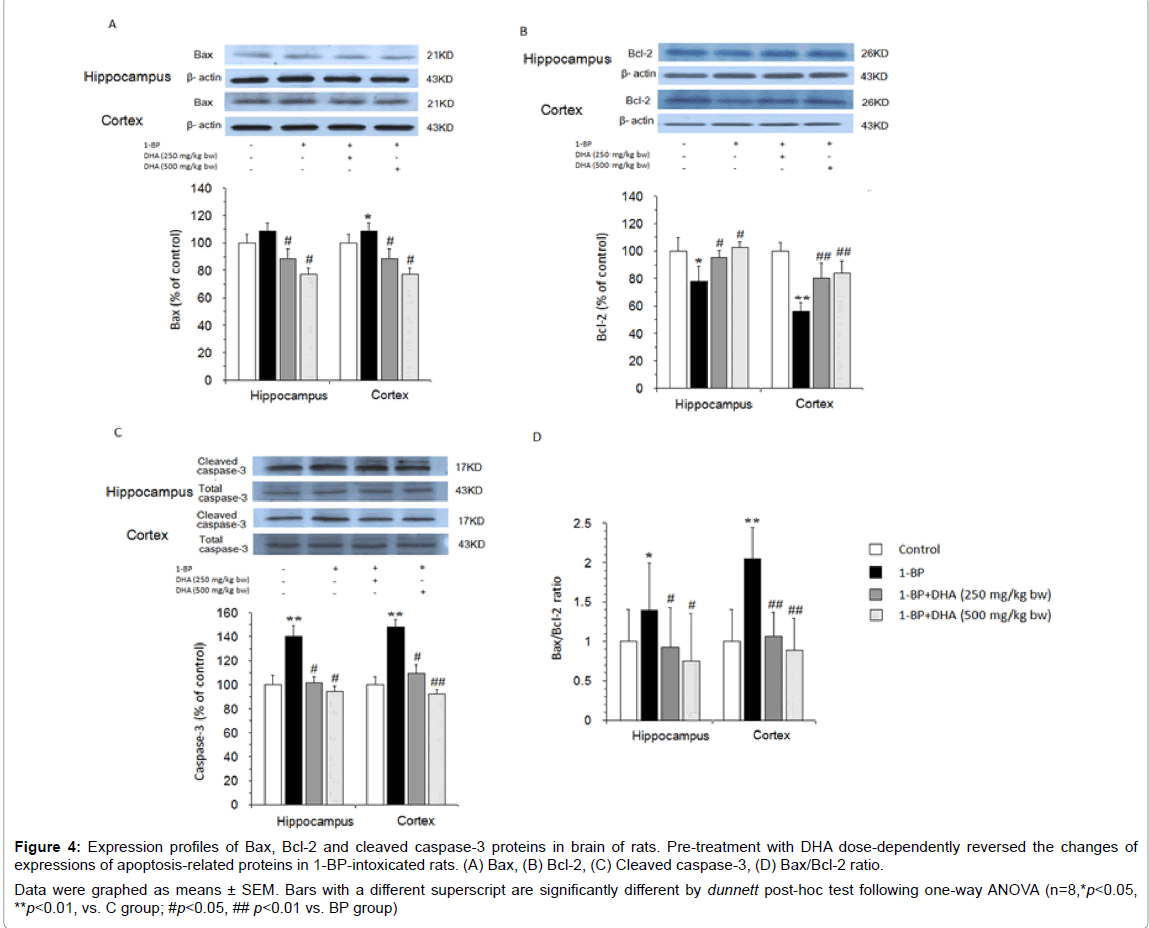
Figure 4: Expression profiles of Bax, BclÔ??2 and cleaved caspaseÔ??3 proteins in brain of rats. Pre-treatment with DHA dose-dependently reversed the changes of expressions of apoptosis-related proteins in 1-BP-intoxicated rats. (A) Bax, (B) Bcl-2, (C) Cleaved caspase-3, (D) Bax/Bcl-2 ratio. Data were graphed as means ± SEM. Bars with a different superscript are significantly different by dunnett post-hoc test following one-way ANOVA (n=8,*p<0.05, **p<0.01, vs. C group; #p<0.05, ## p<0.01 vs. BP group)
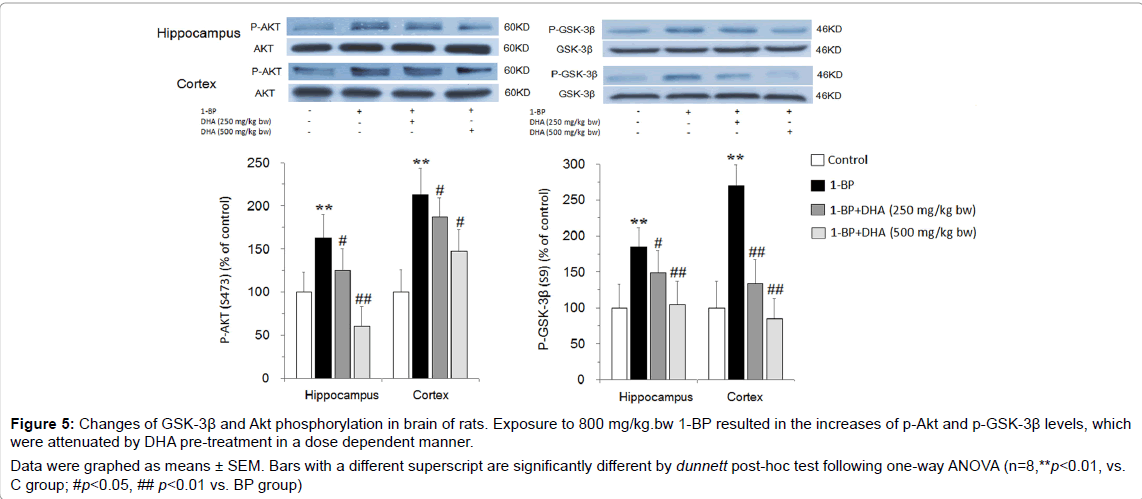
Figure 5: Changes of GSK-3β and Akt phosphorylation in brain of rats. Exposure to 800 mg/kg.bw 1-BP resulted in the increases of p-Akt and p-GSK-3β levels, which were attenuated by DHA pre-treatment in a dose dependent manner. Data were graphed as means ± SEM. Bars with a different superscript are significantly different by dunnett post-hoc test following one-way ANOVA (n=8,**p<0.01, vs. C group; #p<0.05, ## p<0.01 vs. BP group).
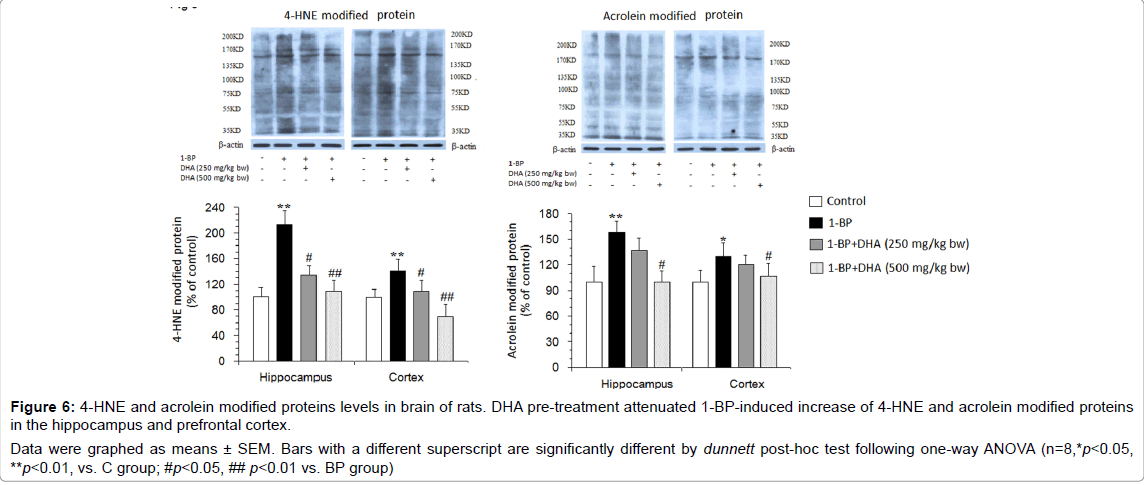
Figure 6: 4-HNE and acrolein modified proteins levels in brain of rats. DHA pre-treatment attenuated 1-BP-induced increase of 4-HNE and acrolein modified proteins in the hippocampus and prefrontal cortex.
Data were graphed as means ± SEM. Bars with a different superscript are significantly different by dunnett post-hoc test following one-way ANOVA (n=8,*p<0.05, **p<0.01, vs. C group; #p<0.05, ## p<0.01 vs. BP group).
DHA attenuated neuronal apoptosis through regulation of expression profiles of Bax, BclÔ??2 and cleaved caspaseÔ??3 proteins in brain of rats
To further conf´╗┐´╗┐irm the neuronal apoptosis after 1-BP exposure, we determined the expression profiles of apoptosis-related proteins in the brain of 1-BP-intoxicated rats. As shown in Figure 4, after 1-BP treatment, the expression of BclÔ??2 was significantly down-regulated, while Bax, cleaved caspase-3 expressions and Bax/Bcl-2 ratio were up-regulated in the hippocampus and prefrontal cortex. Consistent with reducing TUNEL-positive cells, pre-treatment with DHA dosedependently reversed the changes of expressions of apoptosis-related proteins in 1-BP-intoxicated rats.
DHA reduced AKT mediated GSK-3β phosphorylation in brain of 1-BP treated rats
To investigate whether GSK-3β is implicated in the 1-BP-elicited neurotoxicity, we detected the activities of GSK-3β by measuring p-GSK-3β (ser 9) and p-Akt (ser 473) changes in the hippocampus and prefrontal cortex of the brains. As shown in Figure 5, in the hippocampus and prefrontal cortex, the levels of p-Akt and p-GSK- 3β in 1-BP-treated rats were significantly elevated compared with that of controls. The increases of p-Akt and p-GSK-3β levels induced by 1-BP exposure were greatly attenuated by DHA pre-treatment in a dose dependent manner. These results suggested that the protective effects of DHA against 1-BP neurotoxicity might be related to the reversal of GSK-3β inactivation.
DHA mitigated the accumulation of 4-HNE and acrolein modified proteins levels in brain of rats
The reactive electrophiles possess the ability to modify the cellular proteins, which is thought to be a trigger event in neuronal death. As shown in Figure 6, compared with the controls, 1-BP-treated rats displayed an increased level of 4-HNE and acrolein modified proteins in the hippocampus and prefrontal cortex, which was significantly mitigated by DHA pre-treatment.
Discussion
As well known, cell death generally occurs by necrosis or apoptosis, and the latter is a predominant form in many chronic neurodegenerative diseases including AD, PD and ALS. Thus, many therapeutic and preventive strategies have been laid on preventing apoptotic process [42]. In the present study, apoptotic neurons were observed in the hippocampus CA3 region and prefrontal cortex of 1-BP treated rats. It is accepted that the hippocampus and the prefrontal cortex are crucial areas related to the cognitive function. Gonzalez et al. [43] reported that protein synthesis in the prefrontal cortex was necessary for retrieval of recent and remote memories in a rapid learning system. The pyramidal neurons of hippocampus CA3 region played a vital role in memory acquisition and consolidation [44] and was regarded as the key element in the neural circuit that ensure the brain function [45]. Furthermore, studies have delineated that a neurotransmitter projection extends from hippocampus to prefrontal cortex [46] and the prefrontal cortexhippocampus circuit were involved in the spatial working memory [47]. The histopathological changes of brain, as well as the poor MWM performances of 1-BP treated rats presented in this study suggested the damages of normal anatomical network between the prefrontal cortex and hippocampus.
Studies have demonstrated that environmental toxicants induced apoptosis mainly via the intrinsic mitochondria-dependent apoptotic pathway that involves Bcl-2 family alteration, cty c release and caspase-3 activation [48,49]. As a marker of apoptosis, the alteration of Bax/Bcl-2 ratio is more sensitive and might determine the susceptibility of cell apoptosis [50]. Our results revealed that the expression of Bcl-2 family could be changed by 1-BP intoxication. 1-BP-induced neuronal apoptosis in the brain of rats was accompanied with a significant upregulation of Bax/Bcl-2 ratio. Previous study demonstrated that DHA supplementation markedly reduced neuronal apoptosis in offspring of valproic acid-treated rats, which was mediated by the up-regulation of the Bcl-2 [51].
As early as 1996, the pivotal role of oxidative stress in apoptosis has been recognized [52]. As stated above, the brain enriched with PUFA, which is easily attacked by ROS, generates cytotoxic electrophilic aldehydes. Among them, 4-HNE, the most abundant aldehydes and acrolein, the most active aldehydes, are readily conjugated with protein residues. Once some crucial proteins were modified by these active delehydes, they would be detrimental to cellular homeostasis [12]. Contrary to the short half-life of ROS, these active aldehydes could readily diffuse across membranes, mediate and amplify the toxic effects of free radicals [53,54]. As the data showed, the 1-BP treatment resulted in the overproduction of aldehydes protein adducts, suggesting the redox imbalance in rat brain. It has been documented that the oxidative stress in brain could disturb the calcium homeostasis and cause the mitochondrial dysfunction, which would trigger the apoptosis cascade reaction and finally lead to the neuronal loss [55,56]. The present data showed that DHA pre-treatment could modulate the redox status and the cascade of caspase in brain, which hampered neuronal demise elicited by 1-BP exposure. Our finding is in line with the previous studies, in which DHA effectively prevented against the hippocampus neuronal apoptosis induced by high-glucose exposure [57]. Although, the exact mechanisms by which DHA exerts neuroprotective effects was not fully defined, the first proposed cellular mechanism had been identified as it enhanced free radical scavenging capacity, which is evidenced by increase of the GSH reductase activity, decrease of the accumulation of oxidized proteins and clearance of the free radicals against noxious stimuli [58]. Recently, it was reported that DHA could be converted to neuroprotectin D1 (NPD1), which play an important role in the attenuation of neuroinflammatory response elicited by detrimental factors [59]. The NPD1 was proved to potently inhibit nuclear factor kappa B (NF-κB) activation and control several inflammatory cytokines gene induction [60]. The NF-κB is a major participant in signaling pathways governing cellular responses to environmental oxidative stresses, and the neurodegenerative disorders had been documented to be associated with an increase of NF-κB activity [61]. Previous studies have demonstrated that the activation of NF-κB contributed to neurotoxicants-induced neuronal dysfunction and apoptosis [62,63]. Moreover, 1-BP had been reported to dosedependently increase the cyclooxygenase-2 (COX-2) protein and mRNA levels via NF-κB activation in murine RAW 264.7 macrophages [64]. The study in vivo also showed that 1-BP could significantly activate microglia in the cerebellum of rats [65]. Thus, these observations suggested that the pro-inflammatory effects might be involved in the neurotoxicity of 1-BP. DHA could inhibit the release of nitric oxide and tumor necrosis factor-α by a shift in microglial polarization toward the beneficial M2 phenotype both in vitro and in vivo [66].
The present data also showed that 1-BP induced inactivation of GSK-3β, which put forth the possibility that inhibition of GSK-3β mediated by Akt promoted the neuronal death under 1-BP intoxication. Recent evidence revealed the GSK-3β to be a crucial regulator of brain function and cell fate within the CNS, especially, a key mediator in the neuronal apoptosis induced by redox imbalance [16,67].Study showed that GSK-3β could abrogate the NF-κB activation and COX-2 expression in primary astrocytes [16]. Inhibition of GSK-3β resulted in the translocation of NF-κB into the nucleus and enhanced expression of matrix metalloproteinase-9 (MMP-9) [68]. NF-κB activation facilitated the breakdown of the collagen in basal membranes and destruction of the blood brain barrier integrity, as well as the secretion of proinflammatory mediators, and eventually led to apoptotic cell death of neurons [69]. In vivo study revealed that sustained inhibition of GSK- 3β activity by specific inhibitor lithium [70] or transgenic technique in Tet/DN-GSK-3 mice with conditional expression of dominantnegative- GSK-3β [71] resulted in neuronal apoptosis in various brain regions, with the impairment of motor coordination. Moreover, the neuronal apoptosis and motor-deficit phenotype were susceptible to revert upon restoration of normal GSK-3β activity by cessation of transgene expression in symptomatic Tet/DN-GSK-3 mice [71]. Altogether, our results demonstrated that the simultaneous treatment with DHA in 1-BP-exposed rats could bring the GSK-3β activity back, which was accompanied with the improvement of functional outcomes.
Conclusion
In conclusion, 1-BP exposure resulted in cognitive deficits and neuronal apoptosis in the hippocampus and prefrontal cortex, accompanied with obvious elevation of oxidative modified proteins and significant GSK-3β inhibition. Pre-treatment with DHA significantly alleviated the 1-BP induced central neurotoxicity owe to its antioxidative and anti-inflammatory properties.
Acknowledgement
This research was supported by National Natural Science Foundation of China (81172708) and the interdisciplinary breeding project of Shandong University (2016JC020).
References
- Yu X, Ichihara G, Kitoh J, Xie Z, Shibata E, et al. (2001) Neurotoxicity of 2-bromopropane and 1-bromopropane, alternative solvents for chlorofluorocarbons. Environ Res 85: 48-52.
- Fueta Y, Fukunaga K, Ishidao T, Hori H (2002) Hyperexcitability and changes in activities of Ca2+/calmodulin-dependent kinase II and mitogen-activated protein kinase in the hippocampus of rats exposed to 1-bromopropane. Life Sci 72: 521-529
- Ichihara G, Kitoh J, Yu X, Asaeda N, Iwai H, et al. (2000) 1-Bromopropane, an alternative to ozone layer depleting solvents, is dose-dependently neurotoxic to rats in long-term inhalation exposure. Toxicological Sciences : An Official Journal of the Society of Toxicology 55: 116-123.
- Ishidao T, Kunugita N, Fueta Y, Arashidani K, Hori H (2002) Effects of inhaled 1-bromopropane vapor on rat metabolism. Toxicol Lett 134: 237-243.
- Ichihara G, Li W, Ding X, Peng S, Yu X, et al. (2004) A survey on exposure level, health status, and biomarkers in workers exposed to 1-bromopropane. American Journal of Industrial Medicine 45: 63-75.
- Majersik JJ, Caravati EM, Steffens JD (2007) Severe neurotoxicity associated with exposure to the solvent 1-bromopropane (n-propyl bromide). Clin Toxicol (Phila) 45: 270-276.
- Ichihara G, Kitoh J, Li W, Ding X, Ichihara S, et al. (2012) Neurotoxicity of 1-bromopropane: Evidence from animal experiments and human studies. Journal of Advanced Research 3: 91-98.
- Meyer-Baron M, Kim EA, Nuwayhid I, Ichihara G, Kang SK (2012) Occupational exposure to neurotoxic substances in Asian countries - challenges and approaches. Neurotoxicology 33: 853-861.
- Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7: 65-74.
- Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF, et al. (2014) Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. International Journal of Oncology 45: 245-254.
- Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 348: 1365-1375.
- Sultana R, Perluigi M, Butterfield DA (2013) Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radical Bio Med 62: 157-169.
- Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7: 153-163.
- Zhong ZX, Zeng T, Xie KQ, Zhang CL, Chen JJ, et al. (2013) Elevation of 4-hydroxynonenal and malondialdehyde modified protein levels in cerebral cortex with cognitive dysfunction in rats exposed to 1-bromopropane. Toxicology 306: 16-23.
- Guo Y, Yuan H, Jiang L, Yang J, Zeng T, et al. (2015) Involvement of decreased neuroglobin protein level in cognitive dysfunction induced by 1-bromopropane in rats. Brain Res 1600: 1-16.
- Sanchez JF, Sniderhan LF, Williamson AL, Fan S, Chakraborty-Sett S, et al. (2003) Glycogen synthase kinase 3-beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling. Molecular and cellular biology 23: 4649-4662.
- Gómez-Sintes R, Lucas JJ (2013) Neuronal apoptosis and motor deficits in mice with genetic inhibition of GSK-3 are Fas-dependent. PLoS One 8: e70952.
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, et al. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955-968.
- Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29: 95-102.
- Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN (2010) GSK3beta: Role in therapeutic landscape and development of modulators. Br J Pharmacol 160: 1-19.
- Green P, Yavin E (1998) Mechanisms of docosahexaenoic acid accretion in the fetal brain. J Neurosci Res 52: 129-136.
- Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, et al. (2002) Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats. J Neurochem 81: 1084-1091.
- Neuringer M, Connor WE, Lin DS, Barstad L, Luck S (1986) Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America 83: 4021-4025.
- Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, et al. (2005) Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. The Journal of nutrition 135: 549-555.
- Rapoport SI (2013) Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins Leukot Essent Fatty Acids 88: 79-85.
- Rapoport SI, Ramadan E, Basselin M (2011) Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins & Other Lipid Mediators 96: 109-113.
- Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, et al. (2015) Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep 5: 15791.
- Murphy EJ (2015) Blood-brain barrier and brain fatty acid uptake: Role of arachidonic acid and PGE2. J Neurochem 135: 845-848.
- Kitson AP, Metherel AH, Chen CT, Domenichiello AF, Trépanier MO, et al. (2016) Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J Nutr Biochem 33: 91-102.
- Lonergan PE, Martin DS, Horrobin DF, Lynch MA (2002) Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to gamma-irradiation. The Journal of Biological Chemistry 277: 20804-20811.
- Taha AY, Jeffrey MA, Taha NMY, Bala S, Burnham WM (2010) Acute administration of docosahexaenoic acid increases resistance to pentylenetetrazol-induced seizures in rats. Epilepsy Behav 17: 336-343.
- Zugno AI, Chipindo HL, Volpato AM, Budni J, Steckert AV, et al. (2014) Omega-3 prevents behavior response and brain oxidative damage in the ketamine model of schizophrenia. Neuroscience 259: 223-231.
- Lovell MA, Xie C, Markesbery WR (2000) Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radical Biology & Medicine 29: 714-720.
- Nishikawa A, Furukawa F, Kasahara K, Ikezaki S, Itoh T, et al. (2000) Trans-4-hydroxy-2-nonenal, an aldehydic lipid peroxidation product, lacks genotoxicity in lacI transgenic mice. Cancer Letters 148: 81-86.
- Jia D, Heng LJ, Yang RH, Gao GD (2014) Fish oil improves learning impairments of diabetic rats by blocking PI3K/AKT/nuclear factor-kappaB-mediated inflammatory pathways. Neuroscience 258: 228-237.
- Tajuddin N, Moon KH, Marshall SA, Nixon K, Neafsey EJ, et al. (2014) Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS One 9: e101223.
- Wang QH, Zhong ZX, Chen JJ, Xie KQ, Zhao XL (2012) Development of peripheral neuropathy rat model induced by 1-bromopropane. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 30: 751-755.
- Takayama F, Nakamoto K, Totani N, Yamanushi T, Kabuto H, et al. (2010) Effects of docosahexaenoic acid in an experimental rat model of non-alcoholic steatohepatitis. J Oleo Sci 59: 407-414.
- McDonald RJ, King AL, Foong N, Rizos Z, Hong NS (2008) Neurotoxic lesions of the medial prefrontal cortex or medial striatum impair multiple-location place learning in the water task: Evidence for neural structures with complementary roles in behavioural flexibility. Experimental Brain Research 187: 419-427.
- Miller JW (2007) Screening children for developmental behavioral problems: Principles for the practitioner. Prim Care 34: 177-201.
- Ye L, Wang F, Yang RH (2011) Diabetes impair learning performance and affects the mitochondrial function of hippocampal pyramidal neurons. Brain Res 1411: 57-64.
- Roth KA, Shacka JJ (2009) Apoptosis in neurodegenerative disease. In: Encyclopedia of Neuroscience. Squire LR (edr.), Academic Press, Oxford.
- Gonzalez C, Kramar C, Garagoli F, Rossato JI, Weisstaub N, et al. (2013) Medial prefrontal cortex is a crucial node of a rapid learning system that retrieves recent and remote memories. Neurobiology of Learning and Memory 103: 19-25.
- Stupien G, Florian C, Roullet P (2003) Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem 80: 32-41.
- Bennett MR, Gibson WG, Robinson J (1994) Dynamics of the CA3 pyramidal neuron auto-associative memory network in the hippocampus. Philos Trans R Soc Lond B Biol Sci 343: 167-187.
- Swanson LW (1981) A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res 217: 150-154.
- Wang GW, Cai JX (2006) Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res 175: 329-336.
- Zou CS, Kou RR, Gao Y, Xie KQ, Song FY (2013) Activation of mitochondria-mediated apoptotic pathway in tri-ortho-cresyl phosphate-induced delayed neuropathy. Neurochemistry International 62: 965-972.
- Bossy-Wetzel E, Green DR (1999) Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. Journal of Biological Chemistry 274: 17484-17490.
- Hou XQ, Wu DW, Zhang CX, Yan R, Yang C, et al. (2014) BushenYizhi formula ameliorates cognition deficits and attenuates oxidative stress related neuronal apoptosis in scopolamineinduced senescence in mice. Int J Mol Med 34: 429-439.
- Gao J, Wang X, Sun H, Cao Y, Liang S, et al. (2016) Neuroprotective effects of docosahexaenoic acid on hippocampal cell death and learning and memory impairments in a valproic acid-induced rat autism model. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience 49: 67-78.
- Jacobson MD (1996) Reactive oxygen species and programmed cell death. Trends Biochem Sci 21: 83-86.
- Srivastava S, Chandra A, Wang LF, Seifert WE, DaGue BB, et al. (1998) Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. Journal of Biological Chemistry 273: 10893-10900.
- Witz G (1989) Biological interactions of alpha, beta-unsaturated aldehydes. Free Radic Biol Med 7: 333-349.
- Kamat PK, Tota S, Shukla R, Ali S, Najmi AK, et al. (2011) Mitochondrial dysfunction: A crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacology, Biochemistry and Behavior 100: 311-319.
- Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58: 39-46.
- Yang RH, Lin J, Hou XH, Cao R, Yu F, et al. (2014) Effect of docosahexaenoic acid on hippocampal neurons in high-glucose condition: Involvement of PI3K/AKT/nuclear factor-kappaB-mediated inflammatory pathways. Neuroscience 274: 218-228.
- Calon F, Cole G (2007) Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: Evidence from animal studies. Prostag Leukotr Ess 77: 287-293.
- Murphy EJ (2013) A lipid neurochemist's siren: docosahexaenoic acid and its elusive function in the central nervous system. J Neurochem 127: 299-302.
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG (2004) Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 101: 8491-8496.
- Mattson MP (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem Res 30: 883-893.
- Pizzi M, Goffi F, Boroni F, Benarese M, Perkins SE, et al. (2002) Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J Biol Chem 277: 20717-20723.
- Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, et al. (1999) Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 19: 4023-4033.
- Han EH, Yang JH, Kim HK, Choi JH, Khanal T, et al. (2012) 1-Bromopropane up-regulates cyclooxygenase-2 expression via NF-kappaB and C/EBP activation in murine macrophages. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 50: 1616-1622.
- Subramanian K, Mohideen SS, Suzumura A, Asai N, Murakumo Y, et al. (2012) Exposure to 1-bromopropane induces microglial changes and oxidative stress in the rat cerebellum. Toxicology 302: 18-24.
- Chen S, Zhang H, Pu H, Wang G, Li W, et al. (2014) n-3 PUFA supplementation benefits microglial responses to myelin pathology. Scientific reports 4: 7458.
- Marchetti B, L'Episcopo F, Morale MC, Tirolo C, Testa N, et al. (2013) Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson's disease: The Wnt/beta-catenin signaling cascade as the common final pathway for neuroprotection and self-repair. The European Journal of Neuroscience 37: 1550-1563.
- Kim SD, Yang SI, Kim HC, Shin CY, Ko KH (2007) Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res 1186: 12-20.
- Kamat PK, Swarnkar S, Rai S, Kumar V, Tyagi N (2014) Astrocyte mediated MMP-9 activation in the synapse dysfunction: An implication in Alzheimer disease. Ther Targets Neurol Dis 1: e243.
- Gomez-Sintes R, Lucas JJ (2010) NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. The Journal of clinical investigation 120: 2432-2445
- Gomez-Sintes R, Hernandez F, Bortolozzi A, Artigas F, Avila J, et al. (2007) Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. The EMBO journal 26: 2743-2754.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 5048
- [From(publication date):
November-2016 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 4068
- PDF downloads : 980