DNA Mismatch Repair Deficiency in Colorectal Adenocarcinoma and its Association with Clinicopathological Features
Received: 03-Feb-2015 / Accepted Date: 01-Apr-2015 / Published Date: 05-Apr-2015 DOI: 10.4172/2161-0681.1000220
Abstract
Background: Evaluation of mismatch repair (MMR) status in colorectal cancer (CRC) could be used as the guidance for postsurgical treatment. Defective MMR status is associated with distinct clinicopathological features. This study aims to evaluate associations between MMR status and clinicopathological features in a retrospective cohort of consecutive Chinese CRC patients.
Methods: Clinicopathological information was collected from 481 consecutive individuals who underwent curative surgery for CRC. MMR status was determined by Immunohistochemistry (IHC) staining, which was used to examine expression of MLH1, PMS2, MSH2 and MSH6 on paraffin embedded tissues containing adenocarcinoma.
Results: IHC results showed that 33 (of 481, 6.9%) CRC cases presented loss of MMR proteins expression, including 26 (of 205, 12.7%) from colon and 5 (of 262, 1.9%) from rectum. Tumors with deficient DNA mismatch repair (dMMR) status were significantly related to younger patients, larger tumor size, mucinous-focal or signet-ring histological phenotype, and preferred right-sided location (P<0.001). And dMMR tumors also implied poor differentiation and more occurrence in stage CRC patients. No significant association was found between MMR status and gender, invasion depth and lymph node metastasis.
Conclusions: We concluded that the prevalence of abnormal MMR protein expression in this cohort of Chinese CRC patients was comparable with the West. Meanwhile, tumors with dMMR status were associated with distinct clinicopathological characteristics of CRC patients.
Keywords: MMR protein; Colorectal cancer; Immunohistochemistry; Microstallite instability; Clinicopathological features
312707Introduction
Colorectal cancer (CRC) is the third most common cancer and the fifth most frequent cause of cancer death in China [1]. Two different genetic pathways are generally accepted for CRC carcinogenesis, which include chromosomal instability (CIN) and microsatellite instability (MSI). CIN occurs in about 85% patients with sporadic CRC and familial adenomatous polyposis (FAP) and is characterized by aneuploidy, multiple chromosomal rearrangements and an accumulation of somatic mutations in oncogenes and tumor suppressor genes [2]. However, MSI is more likely found in hereditary nonpolyposis colorectal cancer (HNPCC), which often accompany with changes of mutator pathway for diploid cancer [3].
A deficient DNA mismatch repair (dMMR) system is the main cause of MSI, which leads to accelerated accumulation of single nucleotide mutations and alterations in the length of simple, repetitive microsatellite sequences [4]. A germline mutation in one of the MMR genes, including MLH1, MSH2, MSH6 or PMS2, is the cause of dMMR in patients with HNPCC, which is an inherited disorder that increases the risk of occurring colorectal cancer [5]. On the other hand, dMMR is also observed in sporadic colorectal cancer, of which the majority of dMMR tumors are due to hypermethylation of MLH1 gene promoter, with MSH2 and MSH6 accounting for a smaller percentage [6].
Previous studies have revealed distinct clinicopathologic features of dMMR tumors, such as poor differentiation, abundant mucin secretion, marked lymphocytic infiltration, preferential location in the proximal colon and association with a favorable prognosis [7-12]. Until now, data on the role of MMR status in Chinese patients with CRC are scarce and the relationship between MMR status and clinicopathologic features is also uncertain. The current study was to evaluate the role of MMR status in relation to clinicopathologic features in patients with CRC.
Materials and Methods
Patient cohort
A total of 555 consecutive primary CRC patients were prospectively enrolled. All patients underwent initial curative surgical resection between January 2012 and June 2012 at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS, Beijing, China) with a histologically-confirmed diagnosis of a colorectal adenocarcinoma. Patients who had a history of preoperative radiochemotherapy or gastrointestinal surgical resection were excluded. Finally, 481 cases were taken into our analysis. The study was approved by the Institute Review Board of the Cancer Hospital, CICAMS.
Pathological analysis
Pathological records of 481 cases were reviewed. Information was obtained on patient’s demographics, tumor sites, histopathological features and TNM stages. Tumor site was defined as “right-side colon” for the location from cecum to the splenic flexure, and “left-side colon” from the splenic flexure to the sigmoid colon. The size of each tumor was evaluated by measuring its maximum diameter. Grading was determined according to the 2010 WHO histological classification. Histopathological type contains non-specific adenocarcinoma (no mucin production, NOS), mucin secretion group and ring-cell carcinoma. Mucin secretion was categorized as focal extracellular (<50%) and mucinous cancers, while mucinous cancers corresponded to carcinomas with areas of extracellular mucin >50%. The TNM stage system of the 7th edition AJCC cancer staging was used. Evaluating of M stage was mainly according to confirmed pathological results and/or radiological data.
Immunohistochemistry Study
A panel of four-antibody of MMR proteins was performed as a routine practice in the Department of Pathology, containing MLH1, PMS2, MSH2 and MSH6. All 481 samples were stained in an autostainer (Autostainer Link 48, Dako, Denmark). Four μm thick tissue sections were deparaffinized in xylene, rehydrated in graded alcohol, and washed in distilled water. Then slides were stained in the autostainer. Slides were incubated with the following primary mouse monoclonal antibodies: MLH1 antibody (ES05, Dako, Denmark), MSH2 antibody (FE11, Dako, Denmark); primary rabbit monoclonal antibodies: MSH6 antibody (EP49, Dako, Denmark) and PMS2 antibody (EP51, Dako, Denmark). With regard to synchronous cancers, which mainly refer to multiple primary cancers, the larger one was evaluated. Carcinomas were considered as dMMR when there was a completely absent staining of a detectable nuclear signal in neoplastic cells for at least one protein. While the adjacent normal mucosa or stromal/lymphoid cells that showed presence of nuclear staining are regarded as internal positive control.
BRAF mutation assay
Genomic DNA was extracted from formalin fixed paraffin embedded tissues using the QIAamp® DNA Mini kit (Qiagen) following the manufacturer's instructions. The BRAF V600E mutation was examined by human BRAF mutation qualitative detection kit (Beijing ACCB Biotech Ltd., Beijing, China). The assay was carried out according to the manufacturer’s protocol using MX3000P real-time PCR system (Stratagene, La Jolla, USA). The qualitative result (positive or negative) was shown from the amplification plot without calculating ΔCt.
Statistical Analysis
Pearson’s χ2 -test or Fischer’s exact test, as appropriate, was used to evaluate associations between MMR status and clinicopathological features. Statistical tests were two-sided, and P<0.05 was considered significant. Statistics were carried out using SPSS software (version 16.0 of SPSS, Chicago, IL, USA).
Results
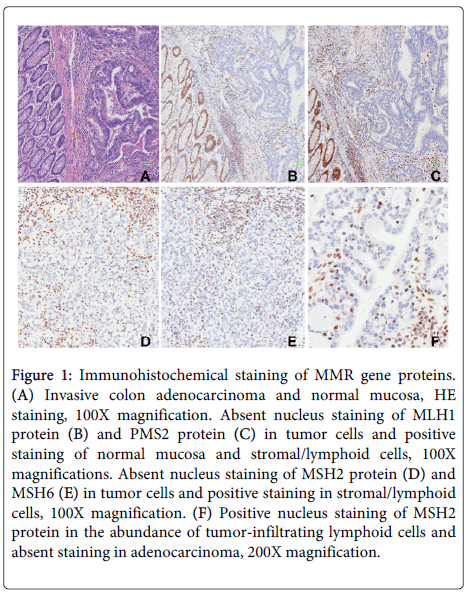
Among the 481 consecutive CRC samples, IHC analysis identified dMMR status in 33 patients (6.9%), which included 26 cases (26/205, 12.7%) in colon, 5 cases (5/262, 1.9%) in rectum and 2 (2/14, 14.3%) of them were synchronous cancers. Among 33 patients with dMMR, 26 (of 33, 78.8%) patients were due to lack of MLH1/PMS2 proteins expression, while 5 (of 33, 15.2%) patients showed absence of staining for the couple of MSH2/MSH6 proteins. No tumor showed loss of staining with MSH6/MSH2/MLH1 protein alone but 2 cases (6.1%) were identified isolated loss of PMS2 protein. Representative images of MMR defects were shown in Figure 1.
Figure 1: Immunohistochemical staining of MMR gene proteins. (A) Invasive colon adenocarcinoma and normal mucosa, HE staining, 100X magnification. Absent nucleus staining of MLH1 protein (B) and PMS2 protein (C) in tumor cells and positive staining of normal mucosa and stromal/lymphoid cells, 100X magnifications. Absent nucleus staining of MSH2 protein (D) and MSH6 (E) in tumor cells and positive staining in stromal/lymphoid cells, 100X magnification. (F) Positive nucleus staining of MSH2 protein in the abundance of tumor-infiltrating lymphoid cells and absent staining in adenocarcinoma, 200X magnification.
A total of 282 males and 199 females (male:female ratio of 1.42:1) were enrolled. There was no gender preponderance with MMR status (P=0.181). The patient ages ranged from 21 to 87 years with a median age of 58 years. The dMMR patients tended to be younger (<50 years) than pMMR patients (P=0.001). Moreover, dMMR cases accounted for 14.3% (6/42) in patients under age of 40 (Table 1).
| Characteristics | Total | dMMR | pMMR | P-value |
|---|---|---|---|---|
| n=481 | n=33(%) | n=449(%) | ||
| Sex | 0.181 | |||
| Male | 282 | 23(8.2) | 259(91.8) | |
| Female | 199 | 10(5.0) | 189(95.0) | |
| Age,years(Median±SD) | 58±11.3 | 0.001 | ||
| <50 | 115 | 16(13.9) | 99(86.1) | |
| ≤50 | 366 | 17(4.6) | 349(95.4) | |
| Site | 0.000 | |||
| Right-sided colon | 89 | 17(19.1) | 72(80.9) | |
| Left-sided colon | 116 | 9(7.8) | 107(92.2) | |
| Rectum | 262 | 5(1.9) | 257(98.1) | |
| Synchronous | 14 | 2(14.3) | 12(85.7) | |
| Size | 0.000 | |||
| <6cm | 366 | 16(4.4) | 350(95.6) | |
| ≥6cm | 115 | 17(14.8) | 98(85.2) | |
| Histological type | 0.001 | |||
| NOS | 360 | 15(4.2) | 345(95.8) | |
| Mucinous-focal | 94 | 14(14.9) | 80(85.1) | |
| Mucinous | 23 | 2(8.7) | 21(81.3) | |
| Signet-ring | 4 | 2(50.0) | 2(50.0) | |
| Tumour differentiation | 0.036 | |||
| Well | 16 | 1(6.2) | 15(93.8) | |
| Moderately | 366 | 19(5.2) | 347(94.8) | |
| Poorly | 99 | 13(13.1) | 86(86.9) | |
| pT | 0.571 | |||
| T1 | 20 | 1(5.0) | 19(95.0) | |
| T2 | 71 | 3(4.2) | 68(95.8) | |
| T3 | 376 | 27(7.2) | 349(92.8) | |
| T4 | 14 | 2(14.3) | 12(85.7) | |
| pN | 0.175 | |||
| N0 | 246 | 22(8.9) | 224(91.1) | |
| N1 | 139 | 6(4.3) | 133(95.7) | |
| N2 | 96 | 5(5.2) | 91(94.8) | |
| pTNM | 0.039 | |||
| Stage Ⅰ | 77 | 4(5.2) | 73(94.8) | |
| Stage Ⅱ | 170 | 19(11.2) | 151(88.8) | |
| Stage Ⅲ | 223 | 10(4.5) | 213(95.5) | |
| Stage Ⅳ | 11 | 0(0.0) | 11(100.0) |
Table1: Clinicopathological features according to MMR status.
Tumors with dMMR status were significantly associated with rightsided location and larger tumor size (long diameter ≥ 6cm) (P<0.001) (Table 1). Among the pMMR tumors, rectum was the most common location (262/481, 54.5%), followed by left-sided colon (116/481,24.1%) and right-sided colon (89/481, 18.5%). There were 17(17/89, 19.1%) dMMR cases located in the right-sided colon, while only 5 cases (5/262, 1.9%) was in rectum. Moreover, synchronous tumors were observed in 14 cases, and 2 of them were dMMR. Three subjects had two carcinomas arising in the right-sided colon, and one patient had absent expression of MMR proteins among them. Three patients had synchronous tumors in right-sided colon and rectum, and one of them was dMMR. Meanwhile, three patients had multiple primary cancers in sigmoid colon and three in rectum, and all of them were pMMR. In the last two pMMR subjects, one had two cancers in rightsided colon and sigmoid, the other in rectum and sigmoid.
There was significant association between abnormal MMR protein expression and histological type of cancer (P=0.001). The majority of carcinomas were classical adenocarcionoma (360/481, 74.8%). The others were constituted of 94 cases of mucinous-focal carcinomas, 23 mucinous carcinomas and 4 signet-ring carcinomas. 2 of 4 (50%) signet-ring carcinomas, 14 of 94 (14.9%) mucinous-focal carcinomas and 2 of 23 (8.7%) mucinous carcinomas showed dMMR, while 4.2% cases (15/360) of classical adenocarcinomas demonstrated dMMR (Table 1). Tumors with dMMR was also significantly associated with histological differentiation (P=0.036). Among them, 13.1% (13/99) of poorly differentiated cancers showed negative MMR protein expression, while 6.2% (1/16) of well differentiated and 5.2% (19/366) of moderately differentiated were dMMR (Table 1).
No differences were seen between the two groups regarding to invasion depth (pT) or lymph node metastasis (pN). Nevertheless, dMMR status was more likely to be in stage cancers (P=0.039) (Table 1).
A total of 434 cases were tested for BRAF V600E mutation, including 27 dMMR cases and 407 pMMR cases. Results showed that 11 (of 434, 2.5%) cases were mutated. Among them, one (of 27, 3.7%) was dMMR patients, while 10 (of 407, 2.7%) were pMMR patients.
Discussion
Our study presents the role of tumor MMR status in respect to prevalence in a cohort of consecutive Chinese CRC patients. We found that the prevalence of dMMR status (6.9%) in Chinese population was comparable with that of Caucasian population. Furthermore, tumors with dMMR status had distinct clinicopathological features with pMMR tumors.
Evaluation of MMR status in CRC could be used as the guidance for postsurgical treatment, as patients with dMMR tumors could not receive benefits in DFS with postoperative FU treatment compared with those who underwent surgery alone [13]. IHC analysis of these four MMR gene proteins is a widely-accepted method for identifying MMR defects.Tumors with dMMR status accounted for 6.9% in total patients, which were consisted of 26 cases (26/205, 12.7%) in colon, 5 cases (5/262, 1.9%) in rectum and 2 (2/14, 14.3%) in synchronous cancers. Actually, difference on the prevalence of dMMR status was reported in the literature due to several reasons. Firstly, a strict criterion was established and used in this study. Tumors that showed entire absence of nuclear staining while adjacent normal mucosa or stromal/lymphoid cells showed presence of nuclear staining were scored ‘‘negative’’ for expression of the protein. And then the specificity of IHC in predicting MSI-H would reach 100% like what the previous reports published [14]. When distinct nuclear staining of more than 10% of all nuclei was defined as positive staining, the sensitivity was 63.1%, and the specificity was 95.7% [8]. Secondly, in our study, rectum cancer (262/481, 54.5%) accounted for more than half of all cases’ onsets and only 5 of them (5/262, 1.9%) had defective MMR protein expression. Samowitz et al. also reported a low prevalent ratio (2.2%) of MMR defects in rectum cancer, though a higher prevalence in colon onsets [15]. In contrast, 26 samples of 205 (12.7%) colon cancers were dMMR in this study, which is roughly similar to that of incident rate of western populations [16,17]. Finally, an alternative possibility was due to racial differences between the Asian and the Western populations. In Korea, IHC identified 10.2% tumor samples with a loss of either MLH1 or MSH2 expression and MSI analysis identified 10% tumor samples with high-frequency in 2028 patients [8]. However, in America and Europe, approximately 15% of CRCs exhibit MSI-H [9,12,13,18,19].
IHC has several advantages over MSI testing as a first-line screening tool for identifying HNPCC. It is easily available and inexpensive as part of the routine services in general department of pathology. IHC is an effective method to identify the gene mutation, and thus could be regarded as a promising addition to MSI testing for defection of MMR-deficient tumors, especially MSH6-mutant patient [20]. Meanwhile, testing of four antibodies simultaneously could increase the sensitivity of IHC up to 92% [7].
The present research was composed of a mixture of sporadic CRC and HNPCC. Indeed, approximately 10–15% of sporadic CRCs exhibit MSI-H due to somatic hMLH1 promoter methylation, resulting in transcriptional silencing and absent MMR protein expression [6]. HNPCC is caused by germline mutations in DNA MMR genes, which includes hMLH1 or hMSH2 predominantly, hMSH6 less frequently and rarely hPMS2. Among 33 dMMR cases in our study, 16 cases were diagnosed at <50 years of age, and 8 patients who fulfilled the criterion of HNPCC-related tumors were diagnosed at <50 years in at least one first-degree relative. 4 cases had synchronous or metachronous colorectal cancers regardless of age. Eight patients were diagnosed at <60 years with poor-differentiation. Totally, 21 patients were identified at risk for HNPCC, who fulfilled at least one criterion of the Revised Bethesda Guidelines [21]. Due to BRAF mutation was rarely associated with unambiguous MMR gene germline mutation [22], we tested BRAF gene mutation in our cohort, and only one of 27 (3.7%) showed BRAF mutation. Additional tumor testing for MLH1 promoter methylation was needed in differentiating sporadic cancers versus HNPCC.
According to our results, patients with dMMR status had distinct clinicopathologic features, including younger age, larger tumor size, more likely right-colon location, mucinous-focal or signet-ring histological phenotype, poor differentiation and more common occurrence in stage . These findings generally agreed with previous reports [8,12]. Whereas, we did not demonstrate that there was significant association between dMMR status and mucinous carcinomas, of which only 2 cases (2/23, 8.7%) showed MMR deficiency. Cord Langner et al. suggested that mucinous differentiation was significantly associated with MMR deficiency [23]. The possible reasons for this difference might lie on racial differences and relatively small samples of mucinous cancers (23/481, 4.8%) in our study.
In conclusion, the prevalence of abnormal MMR protein expression in this cohort of Chinese CRC patients was similar with the recently published data in another Chinese cohort [24], and was comparable with the West, both for colon and rectal cancer patients. MMRdeficient patients had specific clinicopathological parameters. The IHC analysis was very efficient and useful in determining MMR status as an initial screening tool. Further studies are required to address the underlying molecular defects in these patients with mismatch repair deficiency.
References
- Wanqing C, Zheng R, Zhang S, Zhao P, Zeng H, et al. (2014). Annual report on status of cancer in China, 2010. Chin J Cancer Res 26: 48–58.
- Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, et al. (2002) Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. ProcNatlAcadSci U S A 99: 9433-9438.
- Pino MS, Chung DC (2011) Microsatellite instability in the management of colorectal cancer. Expert Rev GastroenterolHepatol 5: 385-399.
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, et al. (1998) A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58: 5248-5257.
- Zhang J, Lindroos A, Ollila S, Russell A, Marra G, et al. (2006) Gene conversion is a frequent mechanism of inactivation of the wild-type allele in cancers from MLH1/MSH2 deletion carriers. Cancer Res 66: 659-664.
- Imai K, Yamamoto H (2008) Carcinogenesis and microsatellite instability: the interrelationship between genetics and epigenetics. Carcinogenesis 29: 673-680.
- Shia J, Ellis NA, Klimstra DS (2004) The utility of immunohistochemical detection of DNA mismatch repair gene proteins. Virchows Arch 445: 431-441.
- Yoon YS, Yu CS, Kim TW, Kim JH, Jang SJ, et al. (2011) Mismatch repair status in sporadic colorectal cancer: immunohistochemistry and microsatellite instability analyses. J GastroenterolHepatol 26: 1733-1739.
- Kim H, Jen J, Vogelstein B, Hamilton SR (1994) Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 145: 148-156.
- Risio M, Reato G, di Celle PF, Fizzotti M, Rossini FP, et al. (1996) Microsatellite instability is associated with the histological features of the tumor in nonfamilial colorectal cancer. Cancer Res 56: 5470-5474.
- Ward R, Meagher A, Tomlinson I, O'Connor T, Norrie M, et al. (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48: 821-829.
- Kaur G, Masoud A, Raihan N, Radzi M, Khamizar W, et al. (2011) Mismatch repair genes expression defects & association with clinicopathological characteristics in colorectal carcinoma. Indian J Med Res 134:186-192.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, et al. (2010) Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J ClinOncol 28: 3219-3226.
- Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, et al. (2005) Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J SurgPathol 29: 96-104.
- Samowitz WS, Curtin K, Wolff RK, Tripp SR, Caan BJ, et al. (2009) Microsatellite instability and survival in rectal cancer. Cancer Causes Control 20: 1763-1768.
- Jover R, Zapater P, Castells A, Llor X, Andreu M, et al. (2009) The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer 45: 365-373.
- Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, et al. (2005) Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 65: 6063-6069.
- Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer 46: 2788-2798.
- Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, et al. (2002) Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J ClinOncol 20: 1043-1048.
- Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. the utility of immunohistochemistry. J MolDiagn 10: 293-300.
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96: 261-268.
- Bessa X, Ballesté B, Andreu M, Castells A, Bellosillo B, et al. (2008) A prospective, multicenter, population-based study of BRAF mutational analysis for Lynch syndrome screening. ClinGastroenterolHepatol 6: 206-214.
- Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, et al. (2012) Mucinous differentiation in colorectal cancer--indicator of poor prognosis? Histopathology 60: 1060-1072.
- Ye JX, Liu Y, Qin Y, Zhong HH, Yi WN, et al. (2015) KRAS and BRAF gene mutations and DNA mismatch repair status in Chinese colorectal carcinoma patients. World J Gastroenterol 21: 1595-1605.
Citation: Zhi W, Ying J, Zhang Y, Li W, Zhao H, et al. (2015) DNA Mismatch Repair Deficiency in Colorectal Adenocarcinoma and its Association with Clinicopathological Features. J Clin Exp Pathol 5:220. DOI: 10.4172/2161-0681.1000220
Copyright: © 2015 Zhi W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15818
- [From(publication date): 4-2015 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 11205
- PDF downloads: 4613