Research Article Open Access
Distribution of Wheat Stem Rust (Puccinia Graminis F. Sp. Tritici) in West and Southwest Shewa Zones and Identification of its Phsiological Races
| Alemayehu Hailu1*, Getaneh Woldeab1, Woubit Dawit2 and Endale Hailu1,2 | |
| 1Ethiopian Institute of Agricultural Research, Plant Protection Research Center P.O.Box 37, Ambo, Ethiopia | |
| 2Ambo University, P.O.Box 19, Ambo, Ethiopia | |
| Corresponding Author : | Alemayehu Hailu Ethiopian Institute of Agricultural Research Plant Protection Research Center P.O.Box 37, Ambo, Ethiopia E-mail: alemayehuhailu65@yahoo.com. |
| Received August 04, 2015; Accepted September 19, 2015; Published September 26, 2015 | |
| Citation: Hailu A, Woldeab G, Dawit W, Hailu E (2015) Distribution of Wheat Stem Rust (Puccinia Graminis F. Sp. Tritici) in West and Southwest Shewa Zones and Identification of its Phsiological Races. Adv Crop Sci Tech 3:189. doi:10.4172/2329-8863.1000189 | |
| Copyright: © 2015 Borlagdan PC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Stem rust (black rust) caused by Puccinia graminis f.sp.tritici is one of the most important air borne diseases of wheat (Triticum aestivum) in the central high lands of Ethiopia, including west and southwest Shewa zones. The pathogen is capable to produce new physiological races that attack resistant varieties and develop epidemic under favorable environmental conditions which results in a serious yield loss. However, information on the status of stem rust distribution and races in west and southwest Shewa zones is lacking. Therefore, the present studies were based on stem rust survey to compute the prevalence and intensity of disease; race analysis via inoculation of stem rust isolates and multiplication of single-pustule of the pathogen and race designation by inoculating on wheat differential lines. Eighty six wheat fields were assessed in 12 districts of west and south west Shewa zones with altitude ranges between1925-2915 m.a.s.l. Seventy five (87.2%) wheat fields infected with stem rust had the overall mean of 33% incidence and 10.8% severity. The mean prevalence of stem rust was 96.3% in southwest and 83.1% in west Shewa zones, whereas, the mean incidence was 34.7% and 31.2% in west and southwest Shewa zones, respectively. Similarly, mean severity was 14.5% in west and 7.1% in southwest Shewa zones. Forty five stem rust samples collected during the survey were analyzed on the twenty standard stem rust differentials and resulted in identification of 5 races (TTTTH, TTKSK, TKTTF, HKPPF & HKNTF). Of these, 88.4% of the isolates were TKTTF (Digalu race) followed by 4.7% of the isolates by TTKSK (Ug99). Among the five races, the most virulent, which made 18 Sr genes non-effective was TTTTH. TKTTF and TTKSK races were virulent on 85% of Sr genes. Differential host carrying Sr24 was an effective gene which confers resistance to all of the races identified in the area. On the other hand, the wheat differential hosts carrying the resistance genes Sr McN, Sr10, Sr9a, Sr30, Sr9g, Sr8a, Sr6, Sr7b and Sr21 were ineffective to 100% of the isolates tested. Hence, the Sr resistance gene Sr24 can be used as sources of resistance in wheat breeding program.
| Keywords |
| Wheat stem rust; Race; Puccinia graminis f.sp.tritici; Sr genes; Disease prevalence; Disease severity; Disease incidence |
| Introduction |
| Ethiopia is the largest wheat producer in sub-Saharan Africa [1]. West and southwest Shewa zones are among the major wheat producing areas in Oromia region [2]. Wheat is the staple food for 4.5 bil |
| Although the productivity of wheat has increased in the last few years in Ethiopia, it is still very low as compared to other wheat producing countries. The national average productivity is estimated to 2.4 tons/ha [2], which is by far below the world’s average of 3.3 tons/ha [5]. The low productivity is attributed to a number of factors including: Biotic (Diseases, insect pests, and weeds), abiotic (moisture, soil ferti |
| However, Stem rust or black rust (caused by Puccinia graminis f. sp. tritici) is a serious wheat disease causing a decrease of wheat production in many areas of the world [9]. Yield loss due to stem rust in Ethiopia was estimated to reach up to 100% on susceptible wheat varieties at times of disease epidemics [10].According to Leppik [11] and Singh et al. [12] the highland of Ethiopia is considered as a hot spot for the development of stem rust races diversity. |
| In a study conducted in Germany, Admassu et al. [13] reported 22 stem rust races from 152 collections made in Ethiopia in 2006. Similarly, due to lack of infrastructure, race analyses of stem rust samples collected in Ethiopia 56 was done in St. Paul and Winnipeg. Surveys made from 1996 to 2005 in Bale indicated that stem rust was the most damaging to the crop with severity levels of 40% in ‘Genna’ and 90% in ‘Bona’ [14]. Similarly, Wheat stem rust disease was recorded with 44.1% prevalence, 19.2% incidence and 11.3% of severity in west and southwest Shewa zones in 2008 cropping season [15]. Moreover, due to sudden changes in stem rust race patterns, commercial varieties tend to become vulnerable. Hence, detailed information on the wheat stem rust status and physiological race variabi |
| Materials and Methods |
| Description of the study area |
| The wheat stem rust survey was carried out in West and Southwest Shewa zones of Oromia Regional State in Ethiopia. West Shewa zone is located at 8º57´N latitude and 38º07´ E longitude and within elevation ranges between 1380-3300 m.a.s.l. Annual mean maximum and minimum rain fall is 1900 mm and 600 mm, respectively. The mean minimum and maximum air temperature of the area is 11.7°C and 25.4°C, in that order. Southwest Shewa zone is located at 8º16-9o 56´ N latitude and 37º 05´-38º 46´ E longitude and altitude ranging from 1600-3576 m.a.s.l. It receives annual rainfall ranging from 900 -1900 mm. The mean minimum and maximum air temperature of the area is 10ºC and 35°C, respectively. Stem rust race analysis was done in Ambo Plant Protection Research Center (APPRC). It is located at 080 96’ 885’’ N latitude and 370 85’ 923’’ E longitude and at an altitude of 2147 m.as.l. The annual average temperature and rain fall is 27.540C and 1077.68 mm, respectively. |
| Wheat stem rust field survey |
| A total of twelve districts that included seven from West Shewa zone (Ambo, Dendi, Che |
 |
| The disease severity under field condition was recorded as percentage of leaf/stem area covered by rust disease followed modified Cobb’s scale as developed by Peterson et al.[16] According to this scale, at 100% disease severity, the actual leaf/stem area covered by rust pustules is 37%. Disease severity was assessed by selecting 10 plants from a single quadrant and five quadrants were used for the estimation of disease severity from a single wheat field. |
 |
| The prevalence of rust disease was measured by using the number of fields affected divided by total number of fields and expressed in percentage. It is calculated as: |
 |
| In addition, data on geographical information (latitude, longitude and elevation) of each field was recorded using GPS (e Trex Legend GPS system, Garmin). Crop growth stage was assessed based on the decima |
| Collection of stem rust samples |
| Stems and/or leaf parts of wheat plants infected with stem rust were cut in to small pieces of 5-10 cm using scissors and put in paper bags after the leaf sheath was separated from the stem in order to keep stem and/or leaf sheath dry. The samples collected in the paper bags were tagged with the name of the Zone, district, variety and date of collection. The samples within the paper bags were air dried and kept in refrigerator at 4°C for race analysis purpose in the greenhouse until the survey in all districts between zones completed. A total of 45 stem rust samples (27 and 18 from West and Southwest Shewa zones of Oromia regions, respectively) were collected. |
| Isolation and multip |
| The inoculum was multip |
| Green house inoculations were carried out using the methods and procedures developed by Stakman et al. [19]. Uredio spores of the stem rust were collected from the diseased wheat parts by using motorized spore collector in a capsule container and diluted by using |
| After seven to ten days of inoculation (when the flecks/symptoms was clearly visible) leaves containing single fleck that produce single pustule was selected from the base of the leaves and the remaining seed |
| After two weeks of inoculation (when the monopustule was well developed) each monopustule was sucked using electric power operated machine (vacuum pump) and collected in capsule container separately. A suspension, prepared by mixing urediospores of the monopustule in |
| After inoculation of 15 days, the spores of each monopustule/ isolate were collected in separate test tubes and stored at 4°C until they were inoculated on the standard differential |
| Inoculation of wheat stem rust isolates on the differential |
| Five seeds each of the 20 stem rust differential |
| Stem rust infection types were scored 14 days after inoculation using the 0-4 scale (Table 1) of Stakman et al. [19]. Infection types were grouped in to two, where, Low (resistance) = incompatibi |
| Designation of races |
| Race designation was done by grouping the 20 differential |
| Each isolates was assigned a five letter race code based on its reaction on the differential |
| Data analysis |
| Survey data (prevalence, incidence and severity) were analyzed by using the descriptive statistical analysis (means) over districts, varieties, altitude range and crop growth stages. Similarly, race analysis was analyzed using the descriptive statistics. |
| Result and Discussion |
| Survey of wheat stem rust in west and southwest shewa zones of oromia region |
| Survey of wheat stem rust was carried out in west and southwest Shewa zones in October, 2014. A total of 86 wheat fields were surveyed mainly for assessment of wheat stem rust intensity. During the surveys, the crop was at flowering to hard dough growth stages (Table 3). From 86 fields inspected, 21 (24.4%), 42 (48.8%), 7 (8.1%), 4 (4.7%) and 12 (14%) of wheat fields were at flowering, milk, soft dough, dough and hard dough stages, respectively. In the same order, stem rust was observed in 17 (81%), 38 (90.5%),7(100%), 4 (100%) and 9 (75%) of 21, 42, 7, 4 and 12 wheat fields inspected in the mentioned growth stages. Thirteen wheat varieties were grown by farmers such as Digelu, Kakaba, Danda’a, Kubsa, ET-13A2, Shorima, Kulutu, Ki |
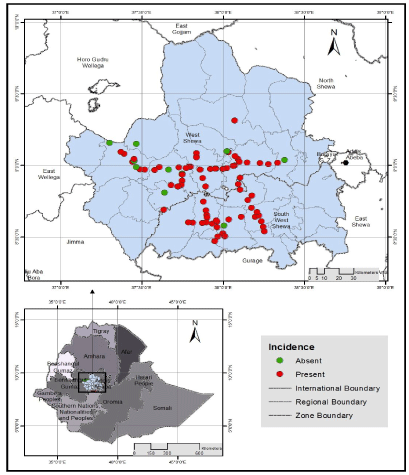
| Intensity of stem rust across locations: Of the 86 wheat fields assessed in the two zones, 87.2% were infected by the stem rust disease (Figure 1). The mean field prevalence of stem rust was 96.3% in southwest and 83.1% in west Shewa zones (Table 4). Whereas, the mean incidence was 34.7% and 31.2% in west and southwest Shewa zones, respectively. Similarly, mean severity was 14.5% in west and 7.1% in southwest Shewa zones. The assessed wheat fields showed susceptible (S), moderately susceptible (MS) and resistance (R) types of responses to stem rust infection. Hundred percent stem rust prevalence was recorded from 7 districts i.e., 4 districts from southwest and 3 from west Shewa zones. The least field prevalence was observed in west Shewa zone from Ejere (50%) and Che |
| The overall mean incidence of wheat stem rust in both zones was 33%. The highest stem rust incidences (100%) were recorded in Ambo (Senkale loca |
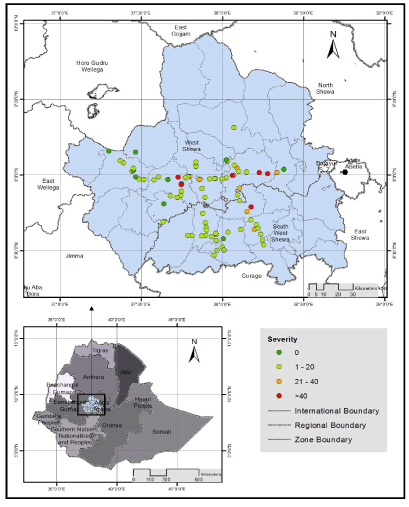
| The mean severity of stem rust ranged from 0.9% in Che |
| Intensity of stem rust in different altitude ranges: The survey was conducted in the altitude ranges between 1925-2915 m.a.s.l. Based on CSA [22] altitude agro-ecology classification, out of 86 wheat fields observed, 5 (5.8%), 69 (80.2%) and 12 (14%) fields were found at lowaltitude (1500-2000), mid-altitude (2001-2500) and high-altitude (2501-3560 m.a.sl.), respectively. Of the 5 wheat fields inspected in the altitude ranges between 1500-2000 m.a.s.l, stem rust was observed in 5 (100%) wheat fields which had 30.8% mean incidence and 9.6% mean severity. Of the 69 wheat fields surveyed in the elevation that ranges between 2001-2500 m.a.s.l, black rust was recorded in 62 (89.9%) fields, with mean incidence and severity of 36.3% and 13.5%, following the same order mentioned. Similarly, 66.7% of stem rust disease prevalence was recorded at high-altitude which had 3.4% mean incidence and 1.5% mean severity. The survey result indicated that, mean incidence and severity increased from low-altitude to mid-altitude and decreased at high altitude (Table 5). Maximum stem rust disease severity (80%) was recorded at mid-altitude followed by 40% at low-altitude. Similarly, maximum stem rust incidence (100%) was recorded at mid and low-altitude. The highest level of stem rust infection has been cited in |
| Intensity of stem rust in different wheat varieties grown in the surveyed areas: Of the 86 assessed wheat fields, only six (7%) fields were covered by five different local varieties and the remaining 80 (93%) were covered by eight different released varieties. The local varieties such as Bedu Gela and Chofero have shown resistance response to stem rust infection during survey in west Shewa zone. The absence of stem rust in local varieties may probably be due to their relative resistance and/or may be cultivated at a relatively higher altitude ( ≥ 2810 m.a.s.l ) (Table 6), where stem rust disease is not a threat to wheat crop [25] The most widely grown wheat variety was Digalu and it covered 55.8% of surveyed wheat fields in west and south west Shewa zones, Oromia region with 1 to 80% ranges of stem rust severity (Table 6). It showed susceptible to moderately susceptible reactions with 39.2% mean incidence and 14.5 mean severity. The second commonly grown variety Kakaba was also infected with stem rust at different intensity levels and its coverage was 16.3% surveyed wheat fields in both Zones. This variety showed moderately susceptible to resistance stem rust reaction with mean incidence and severity of 12.5% and 3.4%, respectively. Variety Danda’a was the third widely grown (8.1% wheat fields) in west Shewa zone only and it also showed similar field response as Kakaba for stem rust disease with 12.7% mean incidence and 2.1% mean severity. Hidasie variety was released in 2012 by KARC/EIAR and was not widely cultivated in the assessed areas except one field in Che |
| Of the 48 inspected Digalu variety fields, 100% stem rust incidence was recorded in 12 (25%) fields. Similarly, the highest disease severity of 80% was recorded on Digalu variety followed by Ki |
| In general, the survey result indicated that the intensity of stem rust varied across locations, elevation, varieties, growth stage. In addition, out of 75 (87.2%) stem rust disease infected wheat fields, only 2 (2.7%) wheat fields were sprayed with fungicide (Tilt 250EC). This low percentage use of fungicide by farmers was due to lack of awareness, unaffordable price of the fungicide and low technical support from agricultural experts according to farmers. |
| Physiological races and virulence diversity of stem rust on wheat in west and southwest zones |
| Race analysis is done based on the reaction of differential |
| In this study, of the total 45 stem rust samples, 43 from farmer’s fields and 2 from experimental plot of Ambo Plant Protection Research Center (APPRC) were collected. Of these, 2 samples from west Shewa zone did not yield viable spores at the time of inoculation on the susceptible check McNair701 in the green house. Forty-three viable isolates were identified and further multip |
| Virulence and physiological race composition of wheat stem rust: Of the 43 isolates tested, 5 races were identified from west and southwest Shewa zones. The result showed that, most of the isolates collected from different wheat fields belonged to the same race group, except Ambo and Wo |
| In general, the new, TKTTF race was distributed in the altitude range of 1925-2588 m.a.s.l in 12 districts of west and south west Shewa zones, Oromia region. This showed that, TKTTF is the most virulent race on wheat varieties and it is rapidly spreading to a wide altitude ranges. This might be due to favorable environmental conditions as well as cultivation of susceptible wheat varieties in those districts. In contrast, other 3 new races were found in the altitude range of 2054- 2154 m.a.s.l. only from two districts (Table 7). The race TTKSK (Ug99) was detected from elevations of 2059 and 2147 m.a.s.l. |
| Out of 5 races, the most frequently and predominantly occurred race was TKTTF with a frequency of 88.4% (Table 8). The second frequently race was TTKSK with a frequency of 4.7%. This might be widely growth of resistant variety |
| The observed/recorded virulence spectrum varied between 13-18 Sr genes (Table 8). The most wide virulence spectrum was recorded on the race of TTTTH that exhibited virulence on 18 Sr genes. The second broad virulence spectrum was recorded on the TKTTF and TTKSK races that showed virulence on 17 Sr genes. The most devastating stem rust race TTKSK (commonly known as Ug99) virulence on gene Sr31 was first detected in Uganda in 1999 [27] and had been spread to most of the wheat growing areas of Kenya in 2002 and Ethiopia in 2003 [28]. In 2005, Ethiopian reports confirmed its presence in six dispersed locations [29] and was spread to most of wheat growing areas in the country and becoming the main threat for wheat production [30]. Similarly, TTKSK has been reported by Teklay in southern Tigray zone with a virulent spectrum on the 17 resistance gene of differential |
| TTKSK (Ug99) was avirulent to Sr36, Sr24, and SrTmp (Table 8). In the same way, the new race TKTTF (Digalu race) was avirulent to Sr11, Sr31, and Sr24. Virulence on the resistance gene SrTmp is considered the main factor behind the complete susceptibi |
| Generally, the identified races had wider range of virulence in the study areas (Table 8). High virulence diversity of stem rust races were reported ear |
| The race spectrum in Ethiopia was clearly different from other parts of the world. For example surveys in Canada [21,34-36] USA, Russia and South Africa detected fewer races such as 15, 5, 6 and 7, respectively. Whereas, more races were identified from Ethiopia, i.e. 60, 41, 17, 44, 22 and 20 [24,30,31,37-39] at different times and locations in the country.However, the present study is dissimilar to the previous works that have been done in Ethiopia. It is evident that only 5 races have been identified from two zones and TKTTF was the most dominant across the locations and it covered 88.4% of the race frequency occurrence in those 12 districts. |
| Most of Ethiopian races varied from one another by single gene/ step changes Belayneh et al.[30] Abebe et al.[24] also reported that, 40% of the races that were identified from Southern Tigray in 2010 cropping season varied by single gene changes. Such single step changes in virulence were reported to be the main process of evolutionary change in P. graminis f. sp. tritici populations [40]. However, the present study showed that all identified races were not varied by single step changes (Table 8). There might be other factors for race variation in the studied area |
| Virulence frequency of P. graminis f. sp. tritici isolates to Sr resistance genes: The results showed that the majority of the stem rust resistance genes were found ineffective against most of the isolates tested in this study. 85% of the Sr genes were ineffective to 88.4% of the isolates. The wheat differential |
| In contrast, the stem rust resistance gene Sr24 was found effective to all 43 stem rust isolates collected from west and south west Shewa zones of Oromia region (Table 9). This was previously confirmed by the reports of Roelfs et al.[9] Abebe et al.[24] and CIMMYT [41] as Sr24 gene is amongst the effective genes in different countries. Even though, in Kenya 2006, a |
| Conclusion |
| The study confirmed the presence of high virulence spectrum among the five identified wheat stem rust races. This indicated that, West and southwest Shewa zones are hot spot areas for appearance of virulent genetic diversity of stem rust races. Therefore, regular assessment and physiological stem rust race identification will be mandatory for virulence and/or avirulence information in west and southwest Shewa zones. Sr24 gene was the only effective gene that showed resistant for all identified races. Hence, the Sr resistance gene Sr24 can be used as sources of resistance in wheat breeding program. |
| Acknowledgement |
| I am deeply grateful and indebted to EIAR for allowing me to pursue postgraduate study at Ambo University. In this regard, I would |
| References |
References
- id="Reference_Titile_
- nk" value="1">FAOSTAT (2014). FAO Statistical database.>
- id="Reference_Titile_
- nk" value="2">CSA (2014). Agricultural Sample Survey. Report on Area and production of Major crops.>
- id="Reference_Titile_
- nk" value="3">Braun HJ, At
- n G, Payne T (2010). Multi-location testing as a tool to identify plant response to global c
- mate change.C
- mate Change and Crop Production, edn. MP Reynolds 7: 115–38.
>
- id="Reference_Titile_
- nk" value="4">Pena RJ(2002). Wheat for bread and other foods. FAO Corporation document Repository.>
- id="Reference_Titile_
- nk" value="5">FAO (2007).Crop prospects and food situations: Global cereal production brief: 4.>
- id="Reference_Titile_
- nk" value="6">Zegeye T, Taye G, Tanner D, Verkuiji H, Agidie A,et al.(2001). Adoption of improved bread wheat varieties and inorganic ferti
- zer by small-scale farmers in YelmanaDensaand Farta districts of Northwestern Ethiopia. EARO and CIMMYT.
>
- id="Reference_Titile_
- nk" value="7">Park RF, Bariana HS, Wel
- ngs CS(2007).Stem rust of wheat in Austra
- a. PrefaceAustra
- an Journal of Agricultural Research 58: 469.>
- id="Reference_Titile_
- nk" value="8">Mengistu H, Getaneh W, Yeshi A, Rebka D, Ayele B (1991).Wheat pathology research inEthiopia. Wheat research 173-218.>
- id="Reference_Titile_
- nk" value="9">Roelfs AP (1978). Estimated losses caused by rust in small grain cereals in the united states 1918-76. Miscellaneous pub
- cation 1363.
>
- id="Reference_Titile_
- nk" value="10">Bechere E, Kebede H, Belay G(2000). Durum wheat in Ethiopia: An old crop in an ancient land. Institute of Biodiversity Conservation and Research (IBCR) :68.>
- id="Reference_Titile_
- nk" value="11">Leppik EE (1970). Gene centers of plants as sources of disease resistance. Ann Rev Phytopathol 8: 323-344.>
- id="Reference_Titile_
- nk" value="12">Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG,et al.(2006). Current status,
- kely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviewes 1: 054.
>
- id="Reference_Titile_
- nk" value="13">Admassu B,
- nd V, Friedt W, Ordon F (2009)ne
- brary.wiley.com="" doi="" j.1365-3059.2008.01976.x="" title="C
- ck here"> Virulence analysis of Pucciniagraminisf. sp.triticipopulations in Ethiopia with special consideration of Ug99.Plant Pathol 58:362-369
>
- id="Reference_Titile_
- nk" value="14">SARC (2004). Progress report for year 2004. Department of cereal pathology, Sinana, Ethiopia.>
- id="Reference_Titile_
- nk" value="15">APPRC (2010). Progress report for the year 2010. Department of cereal pathology, Plant Protection Research Center, Ambo, Ethiopia.>
- id="Reference_Titile_
- nk" value="16">Peterson R.F, Campbell AR, Hannah AE(1948)cation="" title="C
- ck here">. A diagrammatic scale for estimating rust intensity on leaves and stem of cereals. Canadian Journal Research 26: 490-500
>
- id="Reference_Titile_
- nk" value="17">Zadoks JC, Chang TT,Kanzak CF (1974)ne
- brary.wiley.com="" doi="" j.1365-3180.1974.tb01084.x="" title="C
- ck here">a decimal code for the growth stage of cereals. Weed Research 14: 415-421.
>
- id="Reference_Titile_
- nk" value="18">Roelfs AP, Singh, RP, Saari EE (1992). Rust Diseases of Wheat: Concept and Methods of Disease Management. CIMMYT: 81.>
- id="Reference_Titile_
- nk" value="19">Stakman EC, Steward DM, Loegering WQ(1962). Identification of physiologic races of Pucciniagraminis var. tritici. Agric Res Serv E-617: 1-53.>
- id="Reference_Titile_
- nk" value="20">Jin Y. Singh RP, Ward RW,Wanyera R, Kinyua M et al. (2007). Characterization of seed
- ng infection types and adult plant infection responses of monogenic Sr gene
- nes to race TTKS of Pucciniagraminisf.sp. tritici. Plant Dis91:1096–1099
>
- id="Reference_Titile_
- nk" value="21">Fetch TG, Dunsmore KM(2004)ne.com="" doi="" abs="" title="C
- ck here">. Physiological specia
- zation of P. graminis on wheat, barley, and oat in Canada in 2001. Canadian Journal of Plant Pathology 26: 148-55.
>
- id="Reference_Titile_
- nk" value="22">Central Statistical Authority (CSA). (2008). Agricultural Sample Survey 1998/99. Report on Area and Production of Major Crops Volume 1. Statistical Bulletin 200. CSA, Addis Ababa, Ethiopia 111.>
- id="Reference_Titile_
- nk" value="23">Ayele B, Eshetu B, Betelehem B, Bekele H, Melaku D et al.(2008). Review of two decades of research on diseases of small cereal crops. In: AbrhamTadesse (eds). Increasing crop production through improved plant protection volume I. Proceedings of 14th annual conference of plant protection society of Ethiopia (PPSE) 19-22 December. 2006 Addis Ababa, Ethiopia 375-416.>
- id="Reference_Titile_
- nk" value="24">Abebe T, Woldeab G, Dawit W (2010)cation="" title="C
- ck here"> Distribution and Physiologic Races of Wheat Stem Rust in Tigray, Ethiopia. J Plant PatholMicrob 3:142.
>
- id="Reference_Titile_
- nk" value="25">Dagnatchew Y(1967). Plant disease of economic importance in Ethiopia. Haileslassie I University, College of Agriculture, Environmental station bulletin . Addis Ababa, Ethiopia:30.>
- id="Reference_Titile_
- nk" value="26">Wanyera R, Macharia JK, Kilonzo SM, Kamundia JW(2009)cations="" plantdisease="" september="" pages="" title="C
- ck here">. Fo
- ar fungicides to Control wheat stem rust, race TTKS (Ug99), in Kenya. Plant Disease 93:929-932.
>
- id="Reference_Titile_
- nk" value="27">Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000)cations="" plantdisease="" february="" pages="" title="C
- ck here">. Detection of virulence to wheat stem rust gene Sr31 in Pucciniagraminisf. sp. triticiin Uganda. Phytopathology84: 203.2.
>
- id="Reference_Titile_
- nk" value="28">Wanyera R, Kinyua MG, Jin Y, Singh RP(2006). The spread of stem rust caused by Pucciniagraminisf. sp. tritici, with virulence on Sr31 in wheat in Eastern Africa. Plant Dis90: 113.>
- id="Reference_Titile_
- nk" value="29">Singh RP(1991). Pathogenicity variation of Pucciniarecondita f. sp. tritici and P.graminis f. sp.tritici in wheat growing areas of Mexico during 1988-1989. Plant Disease 75: 790-794.>
- id="Reference_Titile_
- nk" value="30">Belayneh A,
- nd V, Friedt W, Ordon F (2009)ne
- brary.wiley.com="" doi="" j.1365-3059.2008.01976.x="" title="C
- ck here">. Virulence analysis of Pucciniagraminisf. sp. Triticipopulations in Ethiopia with special consideration of Ug99. Plant Pathol 58: 362-369.
>
- id="Reference_Titile_
- nk" value="31">Mert Z, Karakaya A, Dusunce
- F, Akan K, Cetin L (2012). Determination of Pucciniagraminisf.sp. tritici races of wheat in Turkey. Turk J Agric For 36: 107-120.>
- id="Reference_Titile_
- nk" value="32">Belayneh A, Emebet F (2005). Physiological races and virulence diversity of P. graminisf. sp.triticion wheat in Ethiopia. Phytopathol. Mediteer 44: 313-318. >
- id="Reference_Titile_
- nk" value="33">Van Ginkel M, Getinet G, Tesfaye T (1989). Stripe, stem and leaf rust races in major wheat producing areas in Ethiopia. IAR Newslett. Agric Res 3: 6-8.>
- id="Reference_Titile_
- nk" value="34">Jin Y(2005). Races of Pucciniagraminis identified in the United States in 2003. Plant Disease 75: 1125-1127.>
- id="Reference_Titile_
- nk" value="35">Lekomtseva SN, Volkova VT, Zaitseva LG,Skolotneva ES(2007)cations="" article="" title="C
- ck here">. Races of Pucciniagraminis f. sp. tritici in the Russian Federation in 2007. Moscow, Russian Federation.Annual Wheat Newsletter 55: 178-179.
>
- id="Reference_Titile_
- nk" value="36">Pretorius ZA, Bender CM, Visser B, Terefe T(2010). First report of a Pucciniagraminisf. sp.triticirace virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. PlantDis94: 784-785.>
- id="Reference_Titile_
- nk" value="37">SPL (1988). Annual report for the period of 1985-1988. Ambo, Ethiopia. 5-16.>
- id="Reference_Titile_
- nk" value="38">Ayele B, Alemtaye A, Bedada G, Payne T(2001). Double sources of resistance to Pucciniastriformis and P. graminis f. sp. tritici. In: CIMMYT bread wheat
- nes.Proceeding of 9th Annual Conference 22-23 June, CSSE and EIAR. Addis Ababa, Ethiopia.Sebil 9: 11-19>
- id="Reference_Titile_
- nk" value="39">Serbessa N(2003). Wheat Stem Rust (P. graminis f. sp. tritici) Intensity and Pathogenic Variabi
- ty in Arsi and Bale zones of Ethiopia. M.Sc. Thesis. Alemaya University Ethiopia 92.>
- id="Reference_Titile_
- nk" value="40">Green GJ (1975). Virulence changes in Pucciniagraminis f. sp. tritici in Canada. Canadian Journal of Botany 53: 1377-1386.>
- id="Reference_Titile_
- nk" value="41">CIMMYT (2005). Sounding the alarm on global stem rust: an assessment of race Ug99 in Kenya and Ethiopia and the potential for impact in neighboring countries and beyond. Mexico city, Mexico.>
- id="Reference_Titile_
- nk" value="42">Jin Y,Szabo LJ, Pretorius ZA, Singh RP, Ward R, et al. (2008). Detection of virulence to resistance gene Sr24 within race TTKS of Pucciniagraminisf. sp. tritici.PlantDis 92: 923-926.>
- id="Reference_Titile_
- nk" value="43">Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, et al. (2008). Will stem rust destroy the world’s wheat crop? Adv. Agron98:271–309.>
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
| Table 6 | Table 7 | Table 8 | Table 9 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 19673
- [From(publication date):
November-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 14578
- PDF downloads : 5095
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
