Research Article Open Access
Distribution and Seasonal Variation of Heavy Metal in Surface Sediments from Arvand River, Persian Gulf
Abdolah Raeisi Sarasiab1, Zohreh Mirsalari2 and Mehdi Hosseini3*1Department of Marine Ecology, Farhangian Science University of Iran, Iran
2Department of Biodiversity, Science and Research Branch, Islamic Azad University, Ahvaz, Iran
3Department of Marine Biology, Faculty of Biological Science, Shahid Beheshti University, Tehran, Iran
- *Corresponding Author:
- Mehdi Hosseini
Department of Marine Biology
Faculty of Biological Science
Shahid Beheshti University, Tehran, Iran
Tel: 98 21 29901
E-mail: smhbio@yahoo.com
Received date: March 14, 2014; Accepted date: July 03, 2014; Published date: July 10, 2014
Citation: Sarasiab AR, Mirsalari Z, Hosseini M (2014) Distribution and Seasonal Variation of Heavy Metal in Surface Sediments from Arvand River, Persian Gulf. J Marine Sci Res Dev 4:150. doi:10.4172/2155-9910.1000150
Copyright: © 2014 Sarasiab AR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Distribution and seasonal variation of heavy metals copper (Cu), nickel (Ni), cadmium (Cd), cobalt (Co), lead (Pb) and mercury (Hg) were measured in the surface sediments samples taken from Arvand river in the northeast Persian Gulf. Concentration of heavy metals varied depending on different season and sampling sites. The concentration order of heavy metals in sediment were Ni > Cu > Co > Pb > Cd > Hg. There was significant difference (p<0.05) in metals levels between different seasons and stations. The highest concentration of Ni, Cu, Cd, Pb and Hg was detected in S3 in July and the highest concentration of Co was detected in S3 in April. The percentage of mercury associated with different fractions in the sediment from was in the order of residual > organic matter > easily and exchangeable > acid reduction. Igeo values calculated for Cu (4.5) and Ni (3.6) showed higher values in the July month in the upstream region of the river than in the other seasons. BAFs of Ni were extremely higher than other metals followed by Cu, Co, Pb, Cd and Hg, respectively. These results indicate that Ni and Cu are more easily available than other elements. The analysed heavy metals were found in sediment samples at mean concentration in the sediment quality guideline proposed by NOAA and ROPME, except for Ni concentration in some cases.
Keywords
Heavy metal; Surface sediment; Seasonal variation; Arvand River
Introduction
Heavy metals can be classified as potentially toxic elements such as cadmium, lead and mercury [1] and essential elements such as copper, zinc and iron [2]. Toxic metals naturally occur in aquatic environments in very low concentrations, but their concentration levels have increased due to anthropogenic pollutants [3] over time. Industrial activities as well as agriculture and mining create a potential source of metals pollution in aquatic environment [4]. As a result, living things inhabiting contaminated waters show rather high metal concentrations. It is well known that metals accumulate in sediments of aquatic environments and hence metals measured in the sediments can reflect past exposures [1,5,6].
Sediments are important sinks for various pollutants such as heavy metals and also play a useful role in the assessment of heavy metal contamination [1,5,6]. Sediments, particularly surficial sediments, may serve as a metal pool that can release metals to the overlaying water via natural anthropogenic processes, causing potential adverse health effects to the ecosystems because of their serious toxicity and persistence [7,8]. Bottom sediments are known to act as a sink for heavy metals introduced to the marine environment [9]. Therefore, benthic organisms, which occupy the bottom layer of the water column, tend to accumulate the highest levels of heavy metals in comparison to pelagic organisms [9-11].
A study of the distribution of trace metals in the sediments is important to the assessment of the probable influence of the mining of iron ore in this region on the estuarine environment. The measurement of trace element concentrations and distribution in the marine environment leads to better understanding of their behaviour in the aquatic environment and is important for detecting sources of pollution [6]. Studies of various physicochemical, biological and mineralogical aspects of the Persian Gulf have been carried out, but limited data are available on the metal concentrations and seasonal variation in the sediments of the Persian Gulf. The trace metal data provide new information on the surface sediment geochemistry in the region whilst the study of element interrelationships, including organic carbon, gives information on their possible origin [12]. The distribution of metals in surficial sediments in the Arvand river, northwest of Persian Gulf has been examined, therefore, in order to enhance the data inventory for the region, characterize the geochemistry of Cd, Co, Cu, Ni, Pb and Hg in surficial sediments and to help understand the influence of anthropogenic activities in this tropical estuarine system.
Materials and Methods
Four sampling sites were selected along the northwest coasts of the Persian Gulf (Figure 1). The first one (S1) was located near the Khoramshahr city, the second one (S2) was located near Minoo island, the third one (S3) was located near the Abadan city, and the last one (S4) was located near Arvand river’s mouth. The Arvand river, the border between Iraq and Iran, is the biggest river in the Persian Gulf. It passes three main cities including Al-Basre in Iraq, Abadan and Khoramshahr in Iran. For people of these cities, the Arvand river is considered as a main resource of seafood and drinking water. For this reason, striking quantities of marine organisms in the markets of these cities are caught from Arvand river.
Surface sediments were collected in Spring (April), Summer (July) and Autumn (October) of 2010 and Winter (January) of 2011, using a Van Veen Grab from 4 stations in the Persian Gulf (Figure 1) across the upper, mid, and lower regions, covering a distance of 50 km and a range of salinities from freshwater in the upstream area to seawater at the mouth. Subsamples were taken from the uppermost layer of the sediment taking care to minimize contamination. The samples were frozen after collection and later thawed, dried at 50-60°C in an oven and disaggregated in an agate mortar, before chemical treatment for total metal analysis. For each sample a known quantity (1 g) of sediment was digested with a solution of concentrated HClO4 (2 ml) and HF (10 ml) to near dryness. Subsequently, a second addition of HClO4 (1 ml) and HF (10 ml) was made and the mixture was evaporated to near dryness. Finally, HClO4 (1 ml) alone was added and the sample was evaporated until white fumes appeared. The residue was dissolved in concentrated HCl and diluted to 25 ml [13].
Concentrations of Cd, Cu, Ni, Pb and Hg were measured with a GBC (Dandenong, Vic. 3175, and Australia) Flam Atomic Absorption Spectrometer (Savant AA sigma) with hyper pulse deuterium background corrector. Mercury concentration was determined by a cold vapor atomic absorption spectrometer (Unicam, model 919).
The accuracy of the analytical procedures was assessed using the certified reference material BCR-1 and yielded results within the reference value range [14]. The recovery means for Cd, Cu, Co, Ni, Pb and Hg were 91.4%, 90.5%, 93.5%, 94%, 92.1% and 108.4% respectively.
All data were tested for normal distribution with Shapirowilk normality test. Significant differences between heavy metals concentration in the samples of various stations and between seasons were determined using One-Way analysis of variance (ANOVA) fallowed by Duncan post hoc test.
Results and Discussion
The mean concentrations of studied metals in sediment are given in Table 1. The heavy metals concentration in sediments at all sampling sites occurs in descending order of Ni > Cu > Co > Pb > Cd > Hg, except for station 1 where Cu concentration was higher than Ni.
| Metal | Station | Season | |||
|---|---|---|---|---|---|
| April | July | October | January | ||
| Cu | S 1 | 20.3 ± 0.01c | 22.5 ± 0.02c | 18.1 ± 0.05c | 14.4 ± 0.05a |
| S 2 | 12.l ± 0.02c | 12.4 ± 0.01b | 11.3 ± 0.01c | 10.2 ± 0.06c | |
| S 3 | 15.5 ± 0.03b | 16.2 ± 0.04c | 14.2 ± 0.05c | 13.4 ± 0.05b | |
| S 4 | 10.4 ± 0.04b | 15.2 ± 0.40c | 17.2 ± 0.02c | 13.8 ± 0.02b | |
| Ni | S 1 | 34.6 ± 0.01c | 38.2 ± 0.01c | 34.1 ± 0.01b | 20.8 ± 0.01b |
| S 2 | 40.4 ± 0.05c | 51.4 ± 0.04c | 42.2 ± 0.05c | 35.2 ± 0.06b | |
| S 3 | 51.5 ± 0.01c | 64.5 ± 0.06c | 45.1 ± 0.04c | 42.5 ± 0.05c | |
| S 4 | 23.1 ± 0.b | 28.2 ± 0.04c | 35.3 ± 0.06b | 21.5 ± 0.01b | |
| Co | S 1 | 17.2 ± 0.05b | 15.1 ± 0.06c | 16.3 ± 0.01c | 17.5 ± 0.01c |
| S 2 | 18.4 ± 0.03b | 17.1 ± 0.05c | 16.5 ± 0.01c | 16.2 ± 0.01b | |
| S 3 | 25.1 ± 0.02c | 23.5 ± 0.01c | 22.2 ± 0.01c | 21.2 ± 0.06c | |
| S 4 | 16.2 ± 0.01b | 14.4 ± 0.04c | 15.1 ± 0.01b | 14.5 ± 0.04b | |
| Pb | S 1 | 7.1 ± 0.02b | 8.2 ± 0.04c | 6.2 ± 0.04c | 3.31± 0.06c |
| S 2 | 3.4 ± 0.06c | 5.1 ± 0.03c | 2.4 ± 0.01c | 1.22 ± 0.03c | |
| S 3 | 7.1 ± 0.03b | 6.1 ± 0.01c | 3.1 ± 0.05c | 0.71 ± 0.04a | |
| S 4 | 2.2 ± 0.05c | 4.2 ± 0.03c | 1.1 ± 0.03c | 0.52 ± 0.09a | |
| Cd | S 1 | 0.36 ± 0.01a | 0.28 ± 0.04a | 0.19 ± 0.02a | 0.21 ± 0.05c |
| S 2 | 0.21 ± 0.06c | 0.18 ± 0.03a | 0.14 ± 0.01b | 0.15 ± 0.04b | |
| S 3 | 0.30 ± 0.04c | 0.19 ± 0.04a | 0.16 ± 0.01b | 0.15 ± 0.03b | |
| S 4 | 0.15 ± 0.03b | 0.12 ± 0.01b | 0.15 ± 0.06a | 0.11 ± 0.04c | |
| Hg | S 1 | 0.03 ± 0.03c | 0.07 ± 0.05a | 0.04 ± 0.01a | 0.04 ± 0.03a |
| S 2 | 0.20 ± 0.02b | 0.30 ± 0.01c | 0.10 ± 0.02c | 0.08 ± 0.01b | |
| S 3 | 0.30 ± 0.06b | 0.60 ± 0.03c | 0.20 ± 0.01c | 0.10 ± 0.06b | |
| S 4 | 0.01 ± 0.07a | 0.05 ± 0.02b | 0.02 ± 0.04a | 0.01 ± 0.03a | |
Table 1: Mean concentration of heavy metals (μgg-1) in sediments during different seasonsfrom Arvand river, northwest of Persian Gulf
Ni
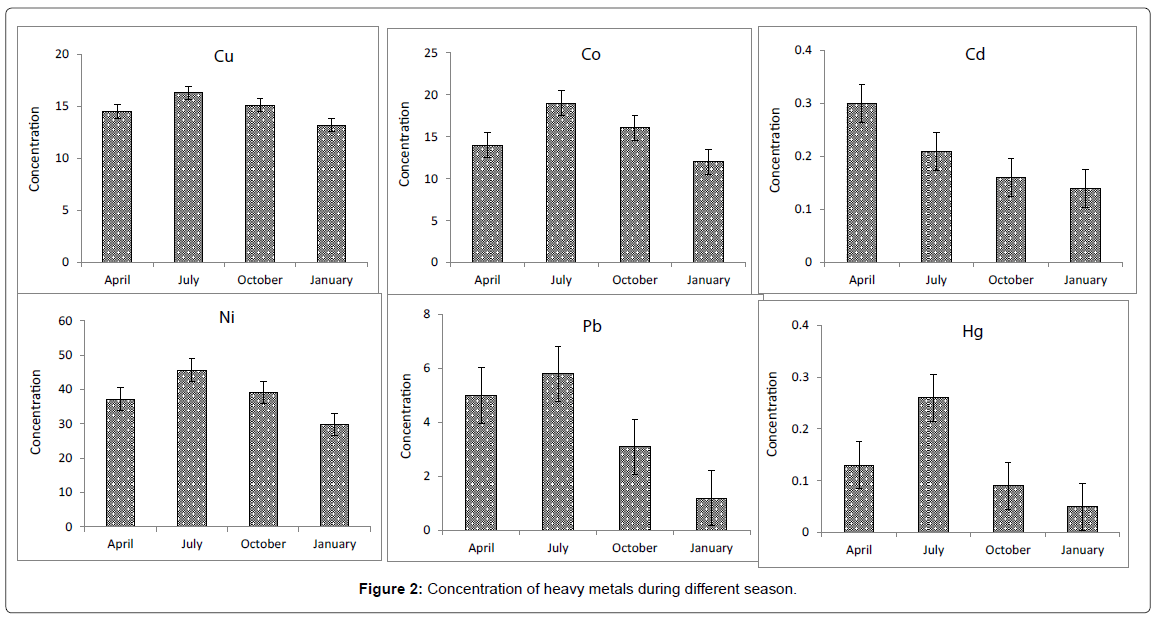
In the sediments at all sampling station, concentration of Ni ranged from of 21 to 64.5 μg g-1. The highest concentration of Ni (64.5 μg g-1) was detected in station 3 in July. There was significant difference (p<0.05) in Ni levels between different seasons. It is likely that this high concentration of Ni was related to the oil tankers traffic in this site [15]. This area is surrounded by Abadan petrochemical complex and petroleum refinery.
Cu
In the sediments at all sampling station, concentration of Cu ranged from 10.3 to 22.5 μg g-1. The highest concentration of Cu (22.5 μg g-1) was detected in site 1 in July. The high concentration of lead at site 1 was situated at the pH of the sediment was the lowest. Samecka-Cymerman and Kempers [16] have showed that the highest concentrations of copper were found in lakes which had the lowest sediment pH. Tokalioglu et al. [17] have indicated that copper concentrations in the lake samples, decreased with increasing pH, because the metal binding abilities are decreased with decreasing pH due to proton binding. A decrease in pH will increase the competition between metals and hydrogen ions for binding sites and may dissolve metal complexes, releasing free metal ions into the sediment [18]. There was significant difference (p<0.05) in Cu levels between different seasons. It may be mentioned here that there is no significant relation between salinity and the copper concentration for sediment along the Arvand River.
Co
The concentration of Co ranged from 14.3 to 26.81 μg g-1. The highest concentration of Co (26.81 μg g-1) was detected in station 3 in April. There was no significant difference (p<0.05) in Co levels between different seasons. No strongly elevated concentrations of Co were observed, although Co is apparently enriched above natural values. It should be noted that the choice of reference value for normalization alters the degree of enrichment.
Pb
The concentration of Pb ranged from 1.03 to 8.11 μg g-1. The highest concentration of Ni (8.11 μg g-1) was detected in station 1 in July. There was significant difference (p<0.05) in lead levels between different seasons. Nolting et al. [19] observed that the low and constant Pb concentrations indicating the minor importance of anthropogenic input from the Laptev Sea in contrast to other areas of world as anthropogenic inputs are considered to be the major source of elevated Pb concentrations in marine sediments [19]. The high concentration of lead at site 1 was situated at the boat stations where high boating activities takes place and in located where the pH of the sediment was the lowest. Byrd and Perona [20] have showed that the highest concentration of lead was found in the Turlok Lake when there were high boating activities.
Cd
In the sediments at all sampling station, concentration of Cd ranged from 10.3 μg g-1 to 22.5 μg g-1 with an average of14.8 μg g-1. The highest concentration of Cd (22.5 μg g-1) was detected in station 3 in July. There was significant difference (p<0.05) in Cu levels between different seasons. The high concentrations of cadmium in sediment at sampling site 3, seems to corresponds to low pH sediment in the former site while the latter site showed agricultural runoff entering river.
Hg
In the sediments at all sampling station, concentration of Hg ranged from 0.01 to 0.60 μg g-1. The highest concentration of Hg (0.60 μg g-1) was detected in station 3 in July. There was significant difference (p<0.05) in Hg levels between different seasons. As similar to Cd, there is a general trend observed that for mercury decreasing its concentration in July. Mercury concentrations in the Arvand river sediments showed generally lowest values than other metals. Mercury can enter the aquatic environment from a number of sources including hazardous substance spills from various refineries, petrochemical and oil industries [15].
Correlation analysis also showed relationship between individual elements including Cu and Pb (r=0.65), Pb and Cd (r=0.71) and between Cd and Cu (r=0.76). Pearson correlation showed that there was no significant correlation among these metals (p>0.05).
The comparison between the sampling stations showed that the amount of metals varied from site to site, and the variation could be related to variability in the sources of metals input. Maximum concentration of Hg, Ni, Co and Cd were observed in sit 3 and Maximum concentration of Cu and Pb were observed in sit 4. The site 3 is the nearest station to Abadan petrochemical complex and petroleum refinery [15]. Site 3 was relatively rich in Ni concentration. It is likely that this high concentration of Ni was related to the oil tankers traffic in this site. About 800 offshore platforms and 25 major oil terminals as well as oil supertankers traffic exert tremendous stress by oil pollution in the Persian Gulf [15,21]. Therefore, site 3 receives different types of pollution such as heavy metal from the surrounding areas. Maximum concentration of Cu was observed in site 1. The concentrations of Cu were higher than the other metals in site 1, suggesting that there is an influence of the industrial activities in surroundings of the sampled stations and which confirmed the increased anthropogenic activities with time [21].
Minimum concentration of metals was observed in site 4. This station was located near the mouth of Persian Gulf. In the area river water with water of Persian Gulf to be mixed and concentration of metals was low. As the interactions between salt water and fluvial water in the mouth of rivers could lead to sediment deposition, a considerable quantity of the sediment is deposited in the mouth of the Arvand river [21,22]. Also, there is no industrial activity near this station, which is a relatively remote area compared to other stations.
This study can provide information on possible chemical forms of heavy metals in sediments. The effects of heavy metals in the environment depend to a large extent on whether they occur in forms that can be taken up by plants or animals. According to Elith and Garwood [18], lead may be strongly adsorbed onto sediment particles and therefore largely unavailable, while cadmium ions can be directly absorbed to sediment and it is known to be most mobile among the other metals [23]. A wide range of values for heavy metal concentrations was observed for the sediments. On the average, the percentage of cadmium associated with different fractions in the sediment from all sites was in the order of residual (49.4%) > organic matter (31.6%) > easily and exchangeable (11.2%) > acid reduction (7.8%). The percentage of copper from all sites was in the order of residual (70.3%) > organic matter (27.4) > easily and exchangeable (1.7%) > acid reduction (0.6%). The percentage of nickel was in the order of residual (73.5%) > organic matter (22.5%) > easily and exchangeable (3.6%) > acid reduction (0.4%). The percentage of mercury was in the order of residual (80.6%) > organic matter (17.5%) > acid reduction (1.3%) > easily and exchangeable (0.6%).The percentage of lead was in the order of residual (63.5%) > organic matter (30.5%) > easily and exchangeable (4.4%) > acid reduction (16.5%). The percentage of cobalt was in the order of residual (60.5%) > organic matter (29.5%) > easily and exchangeable (6.8%) > acid reduction (3.2%). Assuming that bioavailability is related to solubility, then metal bioavailability decreases in the order of exchangeable forms > acid reduction forms > organic forms > residual forms [6,24]. The residual forms are not expected to be released under normal conditions in nature [6] and could be considered as an inert phase [24].
Metal availability is also influenced by other characteristics of the sediment system such as pH and organic matter content, which control the solubility and therefore the availability of metals [25]. Among the non-residual fractions, the organic matter fraction was much higher than other fractions in all sites. The percentage of cadmium in the nonresidual fractions was greater than the residual fraction. About 73% of the cadmium in sediment was associated with the exchangeable, acid reduction and oxidation fractions. Forstner [26] reported that cadmium was characteristically enriched in the more mobile fractions and more mobile than most of other heavy metals [27]. The present results indicate that cadmium, cobalt and lead have greater potential for mobilization from the sediments than copper, nickel and mercury because of their higher concentration at the acid reduction fraction.
Organic matter has a high specific storage capacity for heavy metals [18]. Acidic pH condition is known to influence the sorption of lead by organic matter fraction in sediments [6]. Analysis of pH and percentage of organic matter present in sediments concluded that there were strong correlations between each of these factors and the concentrations of copper and lead. Results showed that the concentration of heavy metals at oxidation fraction increased with increasing pH levels and percentage of organic matter present in the sediments.
A direct correlation was carried out between organic matter percentages, pH and concentration of heavy metals at the oxidation fraction. There was no significant correlation between concentration of cadmium with organic matter percentage and pH (p>0.05). The result of Pearson correlation (r) for organic matter for copper was 0.75 (p<0.001), which means that there was a strong linear correlation with organic matter. There was a linear correlation between lead and the percentage of organic matter in the sediment (r=0.55 and p<0.01), copper (r=0.64 and p<0.01). Thus, organic matter shows a significant to bind copper and lead the sediment in Arvand river. There was a linear correlation between copper and pH (r=0.69 and p<0.01) and lead and pH (r=0.80 and p<0.01).
There was significant difference (P<0.05) between the levels of heavy metals during different seasons. Relatively high concentrations were observed in sediments collected during July. It may be mentioned here that there is significant relation between salinity and temperature and the heavy metals concentration for sediments along the coast of Persian Gulf [15,28]. Temperature could increase metal concentration in summer than winter [12,19]. Also, this variation could result variation in bioavailability of metal in environment [29]. Such this condition was happened during present study and metals levels in different sites showed higher in summer season compared to other seasons. Similarly in the present study, more metals levels uptake was showed during summer season. According to different studies the heavy metal levels in sediments showed higher in summer season [15,29]. It was revealed that sediments all show similar seasonal patterns in metal concentrations it would seem likely that environmental factors (discharges to the estuary, pH, salinity, suspended matter, etc.) are having a greater overall influence on seasonality of metal concentrations.
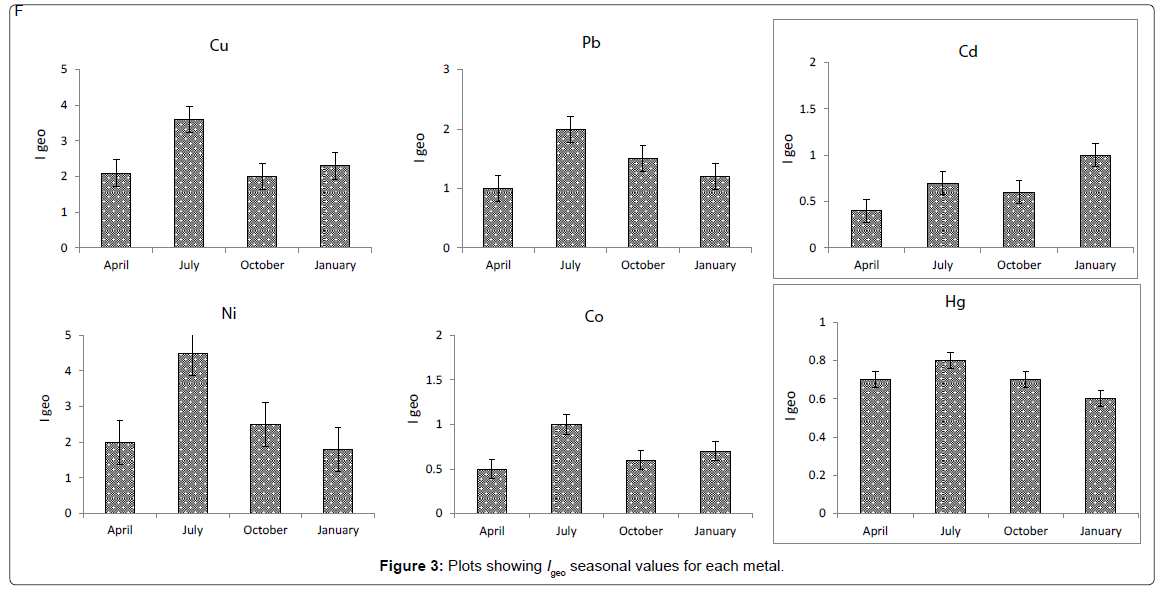
Index of geoaccumulation
Possible sediment enrichment of metals was evaluated in terms of the Igeo of Muller [30]. The formula used for the calculation of Igeo is log2 (Cn/1.5Bn), where Cn is the measured content of element ‘‘n’’, and Bn the element’s content in ‘‘average shale’’ [31]. The geoaccumulation index (Igeo) was originally defined by Muller [30] for a quantitative measure of the metal pollution in aquatic sediments [32] and is shown for the Arvand river sediments in (Figure 2). Elevated Igeo values identified for Cu and Ni in the same areas indicate that surface sediments are contaminated to some extent, most probably as a result of anthropogenic activities. Igeo values calculated for Cu (4.5) and Ni (3.6) showed higher values in the July month in the upstream region of the river than in the other seasons. This trace metal enrichment is attributed to iron ore processing in the higher reaches.
Bioaccumulation factors (BAFs)
BAFs significantly differed between heavy metals and sediments. BAFs of Ni were extremely higher than other metals followed by Cu, Co, Pb, Cd and Hg, respectively. These results indicate that Ni and Cu are more easily available than other elements. The bioavailability of metals in sediments could be controlled by species of metals and physicochemical factors such as pH, salinity, temperature, hardness, alkalinity, oxidation and reduction potential, particulate matter, organic carbon content, cation exchange capacity and differences in the grain size [12,33-36]. The present study also showed that the highest BAFs of essential and non-essential metals occurred in the station 3 followed by station 1, 2 and 4, respectively (Figure 3).
Conclusion
This study provides new information on the distribution and seasonal variation of metals in surface sediments along the Arvand river from northwest of Persian Gulf. The results showed that the concentration of metals varied among station and season sampling. The heavy metal concentration in the sediments are described in the descending order of Ni> Cu > Co > Pb > Cd > Hg at all sampling sites, except for station 1 where Cu concentration was higher than Ni. Results of this study also showed sediments from S3 showed greater concentration of the mercury than those from the other areas. The high concentration of metals in sediments at the S3 sampling could result from industrial effluents. BAFs showed that the risk of Ni is higher than the risk of other metals in this study. Therefore, the Arvand river as a major source of sediments and a source of metals can affect the concentration of metals in sediment of the area. Therefore, in order to enhance the data inventory for the region, characterize the geochemistry of meals in surficial sediments and to help understand the influence of anthropogenic activities in this tropical estuarine system.
Acknowledgements
The authors would like to thank all the field technicians helping in the field. This work was funded by Khoramshahr University of Marine Science and Technology and Environmental Protection of Tehran, Iran.
References
- Clements W, Newman M (2002) Community ecotoxicology. New York, Wiley.
- Tuzen M (2009) Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food ChemToxicol47: 1785-1790.
- Kargin F, Donmez A,Cogun HY (2001) Distribution of heavy metals in different tissues of the shrimp Penaeussemiculatus and Metapenaeusmonocerus from the Iskenderun Gulf, Turkey: seasonal variations. Bull Environ ContamToxicol 66: 102-109.
- Unlu E, Akba O, Sevim S, Gumgum B (1996) Heavy metal levels in mullet, Liza abu (Heckel, 1843) (Mugilidae) from the Tigris River, Turkey. Fresenius Environmental Bulletin 5: 107-112.
- Chen MH (2002) Baseline metal concentrations in sediments and fishes, and the determination of bioindicators in the subtropical Chi-ku Lagoon, S. W. Taiwan. Mar Pollut Bull 44: 703-714.
- Ikem A, Egiebor NO, Nyavor K (2003) Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water, Air, and Soil Pollution 149: 51-75.
- Davies PJ (1974) Arsenic in sediments on the continental shelf of southeast Australia. Searches 5: 394-397.
- McCready S, Birch GF, Long ER (2006) Metallic and organic contaminants in sediments of Sydney Harbour, Australia and vicinity-a chemical dataset for evaluating sediment quality guidelines. Environ Int 32: 455-465.
- Yi Y, Wang Z, Zhang K, Yu G, Duan X (2008) Sediment pollution and its effect on fish through food chain in the Yangtze River. International Journal of Sediment Research 23: 338-347.
- de Mora S, Fowler S, Wyse E, Azemard S (2004) Distribution of heavy metals in marine bivalves, fish and coastal sediments in Persian Gulf and Gulf of Oman. Mar Pollut Bull 49: 410-424.
- Dalman O, Demirak A, Balci A (2006) Determination of heavy metals (Cd, Pb) and trace elements (Cu, Zn) in sediments and fish of the Southeastern Aegean Sea (Turkey) by atomic absorption spectrometry. Food Chemistry 95: 157-162.
- Alagarsamy R (2006) Distribution and seasonal variation of trace metals in surface sediments of the Mandovi estuary, west coast of India. Estuarine, Coastal Shelf Science 67: 333-339.
- Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry 51: 844-851.
- Flanagan FJ (1973) 1972 Values for international geochemical reference samples. GeochimicaCosmochimicaActa 37: 1189-1200.
- AbdolahpurMonikh F, Safahieh A, Savari A, Doraghi A (2012) Heavy metal concentration in sediment, benthic, benthopelagic, and pelagic fish species from Musa Estuary (Persian Gulf). Environ Monit Assess 185: 215-222.
- Samecka-Cymerman A, Kempers AJ (2001) Concentrations of heavy metals and plant nutrients in water, sediments and aquatic macrophytes of anthropogenic lakes former open cut brown coal/mines differing in stage of acidification. Sci Total Environ 281: 87-98.
- Tokalioglu S, Karta S, Elçi L (2000) Speciation and determination of the heavy metals in lake waters by atomic absorption spectrometry after sorption on Amberlite XAD- 16 Resin. Analytical Sciences 16: 1169-1174.
- Elith M, Garwood S (2001) Investigation into the levels of heavy metals within Manly Dam Catchment. In: Freshwater ecology report 2001. Sydney: Department of Environmental Sciences, University of Technology.
- Nolting RF, van Dalen M, Helder W (1996) Distribution of trace and major elements in sediment and pore waters of the Lena Delta and Laptev Sea. Marine Chemistry 53: 285-299.
- Byrd JE, Perona MJ (1980) The temporal variations of the lead concentration in a freshwater lake. Water, Air, and Soil Pollution 13: 207-220.
- Sheppard C, Al-Husiani M, Al-Jamali F, Al-Yamani F, Baldwin R, et al. (2010) The Gulf: A young sea in decline. Mar Pollut Bull 60: 13-38.
- ROPM (1999) Overview on Land-based Sources and Activities Affecting the Marine Environment in the ROPME Sea Area. UNEP/GPA Coordination Office & ROPME 127.
- Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants (3rd ed.) Boca Raton, FL: CRC Press.
- Xiangdong L, Zhenguo S, Onyx WHW, Yok-sheung L (2000) Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chemical Speciation and Bioavailability 12: 17-25.
- Vogel-MikusK, Drobne D, Regvar M (2005) Zn, Cd and Pb accumulation and arbuscularmycorrhizal colonization of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133: 233-242.
- Forstner U (1985) Chemical forms and reactivities of metals in sediment. In R. Leschber, R. D. Davids, L. Hermite (Eds), Chemical methods for assessing bio-available metal in sludges and soils (pp. 1–30). London: Elsevier.
- Kong IH, Liu SH (1995) Determination of heavy metals distribution in the anoxic sediment slurries by chemical sequential fraction. Ecotoxicol Environ Saf 32: 34-38.
- Karbassi AR, Amirnezhad R (2004) Geochemistry Ni, Cu, Zn, Pb, Cd, Co, Mn,V, Fe, Al and Ca in sediments of North Western part of the Persian Gulf. Intl J Env Studies 54: 205-212.
- Bordin G, Mc-Court J, Rodriquez A (1992) Trace metal in the marine bivalve Macomabalthica in the westerschelde estuary the Nerthlands. Part 1:analysis of total copper, cadmium, zinc and iron concentrations; local and seasonal variations. Science of the Total Environ 127: 255-280.
- Muller G (1979) Schwermetalle in den sedimenten des Rheins e Veranderungenseit 1971. Umschau 79: 778-783.
- Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geological Society of America Bulletin 72: 175-192.
- Ridgway J, Shimmield G (2002) Estuaries as repositories of historical contamination and their impact on shelf seas. Estuarine, Coastal and Shelf Science 55: 903-928.
- Caussy D, Gochfeld M, Gurzau E, Neagu C, RuedelH (2003) Lessons from case studies of metals: investigating exposure, bioavailability, and risk. Ecotoxicol Environ Saf 56: 45-51.
- Sekhar KC, Chary NS, Kamala CT, Suman Raj DS, Sreenivasa Rao A (2003) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int29: 1001-1008.
- Zhang W, Yu L, Hutchinson SM, Xu S, Chen Z, et al. (2001) Chinas Yangtze Estuary: I. Geomorphic influence on heavy metal accumulation in intertidal sediments. Geomorphology 41: 195-205.
- Bellucci IG, Moumni El, Collavini B, Frignani FM, Albertazzi S (2003) Heavy metals in Morocco Lagoon and river sediments. Journal de Phys 107: 139-142.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 16400
- [From(publication date):
December-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10913
- PDF downloads : 5487