Discovery and Testing of a New Biologically Produced Acne Cream: ��?LACNEND�
Received: 30-Jun-2017 / Accepted Date: 07-Aug-2017 / Published Date: 15-Aug-2017
Abstract
Background: Acne is one of the predominant causes for a lack of self-confidence in young adults, especially because physical appearance is very closely linked peer relationships. Fortunately, there are creams that are designed to decrease acne; however, many of these current creams use harsh chemicals. These ingredients require extra cosmetics to be used such as soothing lotions to counteract the side effects of the chemicals.
Objective: To resolve this issue, the current study focused on producing an organic, biologically active cream with the main ingredient as lactic acid. To our knowledge there are no other studies that combine a biologically produced lactate with organic coconut oil to make an acne cream.
Methods: In the present study, the strain that was chosen was L. rhamnosus (Lactobacillus rhamnosus ATCC 9595). Through fermentation, purification, making and testing of the cream, it is shown that the cream made from biologically produced lactate (called “LACNEND”) is able to inhibit the growth of bacteria. There were 30 “LACNEND”, 48 control and 18 coconut oil samples.
Results: A one-way ANOVA indicated that the growth in the “LACNEND” condition was significantly lower than the growth in coconut and control conditions.
Conclusion: It was concluded that the “LACNEND” cream was able to inhibit the growth of bacteria better than the coconut oil.
Implications and future research: It is important to note that this cream is one of the first acne creams to have an ingredient that is biologically produced through bacteria. Also, it is the first acne cream to utilize only lactic acid and coconut oil in a cream, which is effective against acne. Although the cream has proven to inhibit the growth of bacteria, further research can be done to legitimize “LACNEND”.
Keywords: Acne cream; Biological product; Lactate; Fermentation; Purification
42643Introduction
Discovery and testing of a new biologically produced acne cream: “LACNEND”
Acne affects 40-50 million people in the United States. 79-90% of the U.S.’s population of 16 to 18 year olds has acne. The most common type of acne is Acne vulgaris, which occurs mostly in teens and young adults [1-5]. There are other types such as Acne conglobata, a rare type mostly seen in young men, Acne fulminans, a severe form affecting teen boys, which also causes muscle and bone pain, or Infantile acne, occurs in babies of 3 to 16 months with higher hormone levels [6].
The current study is focused on Acne vulgaris as it is the predominant form. Acne vulgaris occurs when sebum gets trapped in hair follicles, also known as pores, and grows bacteria. Sebum is made up of various lipids such as glycerides, fatty acids, cholesterol, etc. It is produced by sebaceous glands, which are found over most of the body, except for the palms of hands and soles of feet. These glands are much larger and more numerous on the forehead, chin, and mid-back, which explains the reason as to why most acne occurs in these regions.
Acne occurs in two main types: non-inflammatory and inflammatory. Non-inflammatory acne is when microcomedones, the precursor to acne, heal or become comedones, non-inflamed skin blemishes. Comedones include whiteheads and black heads. Whiteheads form when trapped sebum and bacteria stay under the skin; they are usually tiny white spots or even invisible to the naked eye.
Blackheads form when a pore opens to the surface and the sebum containing melanin oxidizes and turns into a black colour. Noninflammatory acne is the hardest to treat because they lie under the follicles and are usually overlooked. Topical retinoids are good at preventing comedones and clearing the ones that exist. Retinoids are derivatives of Vitamin A, which unclog pores, reduce fine lines, and smooth the skin over a period of weeks [7].
Common retinoids include tretoinin, tazarotene, and adaplene. The downsides of retinoids include severe irritation, which is usually solved through moisturizers [8-10]. Azelaic acid is used in patients with atopic diseases since retinoids cause irritation. Azelaic acid prevents comedones and is antibacterial; however, it causes hypopigmentation, loss of skin colour.
Inflammatory acne occurs when the comedones’ follicle walls rupture which can be the result of picking or touching skin. There are also two types of inflammatory acne: papules and pustules. Papules occur when the follicle wall breaks, the white blood cells rush in, and the area becomes inflamed. Pustules are what people call “pimples” and “zits” and take place over a couple days when the white blood cells reach the surface of the skin.
If papules or pustules collapse, they severely infect the skin and can cause cysts or nodules. Cysts are large pus filled lesions and nodules are large, inflamed bumps from the break along the bottom of a follicle wall. For inflammatory acne, benzoyl peroxide at 2.5-10% is the most common form of treatment. Similar to retinoids, benzoyl peroxide causes irritation, which is solved by less dosage and in a cream [11].
The bacteria found in acne, Propionibacterium acnes, sometimes resists antibiotics and doctors do suggest combining topical clindamycin and erythromycin (antibiotics) with benzoyl peroxide, oral antibiotics, or isotretinoin (prescription drug used to treat severe acne).
If acne gets extremely severe or begins to resist topical treatment, then most doctors prescribe oral antibiotics such as tetracycline at 1 gram a day. Other oral antibiotics include doxycycline or minocycline at 75-200 milligrams a day. Patients that do use oral antibiotics have a higher risk of getting sunburnt. It can be observed that many of these treatments have side effects that require extra cosmetics and skin care.
One of the treatments with minimal side effects may involve utilization of lactic acid, which is the primary focus of this paper. The current study aims to show that the lactic acid is a safer, gentler, and more natural method for treating acne and, to suggest that it potentially improves the emotional well-being of many teenagers.
Lactic acid and cream
Lactic acid is the most common carboxylic acid in nature and is a three carbon organic acid. The peak of lactic acid production hit in 1982, where the worldwide production amounted to 24-28 × 103 tons [12-16]. It was used in food processing, pharmaceutics, and technical applications such as biodegradeable plastics. There are two isomeric forms of lactic acid: D-lactic acid and L-lactic acid. L-lactic acid is the “biologically important isomer”.
Lactic acid is used in many cosmetic and pharmaceutical applications such as topical ointments, lotions, skin peels and anti-acne solutions. Lactic acid was chosen for this study because of its current uses in cosmetics and its positive results. Because lactic acid is an alpha hydroxyl acid, it has moisturizing properties that sets it apart from benzoyl peroxide, a common acne treatment. Lactic acid skin peels are known to make the person’s skin cleaner, smoother, and firmer (because of its ability to remove dead skin and make the new skin thicker and stronger). The unique quality of lactic acid is its ability to work on clearing up acne, while not drying up the user’s skin.
Currently, two methods are used in manufacturing lactic acid: chemical synthesis or fermentation. Chemical synthesis is a cheaper method which is why the microbiological production of lactic acid has been abandoned in the United States [17]. The commercial process in producing lactic acid through chemical synthesis is based on lactonitrile or cellulose [18-20]. Lactonitrile is a solvent in the industrial production of lactic acid [21].
Many companies that use lactic acid, especially in acne creams use chemical synthesis. This is the major difference between the cream that is produced in this study and the creams on market. This is important to the study, because of its effect on how the cream can be sold. A recent study discussed how the international market prefers natural form of polymer(s) for medical purposes compared to that produced by chemical or enzymatic process [22]. This new acne cream is fermented using the desirable characteristics of industrial microorganisms.
In the present study, the strain that was chosen was L. rhamnosus (Lactobacillus rhamnosus ATCC 9595) because of its availability on the market and it is known to ferment L-lactic acid. Lactic acid bacteria produce a variety of antimicrobial compounds and one of these strains is L. rhamnosus , the focus of this study. L. rhamnosus is a gram-positive strain of bacteria and is widely studied because of its use in probiotics and its lack of pathogenicity.
It is typically found in the gastrointestinal tract of humans and has a considerable amount of assistance to the immune system with combating intestinal and urinary tract pathogens. The health benefits of L. rhamnosus are numerous, in fact, there was a study where it exerted antibacterial activity against Salmonella typhimurium , a common disease that occurs in the intestines of humans and animals.
The cream that is produced in this study has two ingredients: lactic acid and coconut. The benefits of lactic acid make it the primary focus of this study; however, coconut is needed to provide an organic consistency to the cream. In other words, the coconut will make the liquid lactic acid into a cosmetic cream.
Coconut oil is known to have many benefits. One of its most interesting benefits is that lauric acid, found in coconut oil, fuses with the cell membrane of the acne-causing bacteria where it then releases its fatty acid thus killing the bacteria. However, the lauric acid in coconut oil may not have the same effects on every person when applied to skin.
There have been many studies dealing with lactic acid but few about lactic acid and acne. One study uses a 5% aqueous solution of lactate as an application for the entire face over the course of a year for 22 patients. They studied comedones, inflammatory lesions, and cysts separately and for once a month. It was found that at the end of one year, 40.9% of patients achieved 90-100% reduction in inflammatory lesions and 22.7% of patients achieved it in non-inflammatory lesions. It was concluded that “topical applications of lactic acid would thus reinforce the natural mechanism for controlling the bacterial proliferation on the skin,”.
The present study
Many of the current creams use harsh chemicals. These ingredients require extra cosmetics to be used such as soothing lotions to counteract the side effects of the chemicals. To resolve this issue, the current study focuses on producing an organic, biologically active cream with the main ingredient as lactic acid.
The present study aims to answer the following research questions
• Does lactic acid mixed with coconut oil produce an effective cream (referred here on as “LACNEND”) which inhibits the growth of E. coli ?
• Does coconut oil itself inhibit the growth of E. coli ?
It was hypothesized that “LACNEND”, which is a combination of biologically produced lactic acid and coconut oil, prevents the propagation of E. coli more than coconut oil on its own.
Methods
Microorganisms
A group of rod-shaped, gram-positive, non-spore-forming bacteria, called Lactobaccilaecae, produce lactic acid as a by-product of glucose metabolism. Lactic acid bacteria are facultative anaerobes that are nutritionally fastidious. Their growth is dependent on such factors as pH, temperature, and the accumulation of metabolic end products.
In the present study, L. rhamnosus stock was prepared from KwikStik pouch of L. rhamnosus ATCC 9595 . The primary culture plate was prepared by activating the bacterial pellet in the KwikStik pouch and streaking on MRS agar plates to facilitate colony isolation. The primary culture plate was incubated at 37°C overnight and wrapped in parafilm, and stored in the refrigerator. Every month, a new plate was streaked to preserve the bacteria.
Fermentation of lactic acid
The fermentation media was made by suspending 47.2 g MRS Broth powder (38944 MRS Broth modified, Vegitone) in 1000 ml distilled water containing 1 ml polysorbate 80 and its pH was adjusted to pH 4.5 using glacial acetic acid. The media was sterilized by autoclaving at 121°C for 15-30 minutes. The culture was started by taking a colony from the L. rhamnosus plate and mixed into a 250 ml baffled flask (Corning 4113-0250) containing 50 ml MRS Broth and incubated overnight at 37°C on an orbital shaker at 150 rpm.
The next day, 600 ml media was inoculated with the 50 ml culture in a 2 Litre baffled flask (Corning 431256) and incubated on the shaker at the same conditions for 7-10 days. The culture was sampled for lactate accumulation on days 4 through 10, and a commercial analyzer (Cedex Bio HT, Roche) was used for the measurement of glucose and lactate concentrations both in fermentation media and during purification steps. Around 500 μl of a sample was pipetted into micro-tubes before loading onto sample trays. If the concentration glucose and lactate was greater than 7.5 and 1.4 g/l, respectively, then the sample was diluted 1:4–1:16.
When all the glucose has been converted to lactate (8-10 days), the cells were separated by centrifugation at 2,500 rpm for 10 minutes in two 500 ml centrifuge bottles. The supernatant was filtered by vacuuming through a 0.2 μm PES membrane filter and stored at 4-8°C in a refrigerator.
Purification
Harvested supernatant’s lactate concentration was measured using Cedex Bio HT analyzer. The solution was concentrated by 8-folds by evaporation at 100°C. Liquid-liquid extraction was used to purify lactate. The extraction of lactic acid involved two steps:
(1) An extraction from an aqueous solution of lactate into a butanol phase as the free acid.
(2) Back-extraction to an aqueous solution as sodium lactate.
The two steps were executed as follows: An equal amount of butanol was added to the concentrated lactate solution and its pH was adjusted to pH 2 using hydrochloric acid.
The butanol phase was separated from the aqueous phase using a separating funnel. An equal amount of distilled water was added to the butanol phase and its pH was adjusted to pH 6 using sodium hydroxide. The sodium lactate in aqueous phase was separated from the butanol phase using a separating funnel. Extraction and backextraction was repeated twice to recover more sodium lactate. All sodium lactate was combined to accumulate the lactic acid. After extracting the lactic acid, it was further concentrated by boiling it at 100°C. The final amount of lactate was measured using Cedex Bio HT analyzer.
Preparation of cream
After researching and conducting a pilot study, 10% of a lactic acid cream mixed with coconut oil was found desirable. Based on the amount of lactate, virgin coconut oil was added to bring the lactate concentration to 10%. After mixing the cream, it was sterilized by autoclaving at 121°C for 15 minutes.
Testing of cream on E. coli
A colony from the E. coli stock (K12, Carolina Biological Supply Company) was mixed it into a 250 ml baffled flask containing 50 ml Tryptic Soy Broth and incubated overnight at 37°C. 1 ml of the overnight culture was mixed it into 9 ml of a new Tryptic Soy Broth. 200 μl of the mixture was spread on a plate using sterilized glass beads. The cream was dabbed with a sterile cotton swab onto two of the quadrants on the plate. The other two quadrants were left empty as the control. The plates were incubated until the bacterial lawn grows and the results were observed through the zone of inhibition.
Results
The present study consisted of three main experiments, all with their own sets of data. The first experiment was the fermentation of lactic acid from glucose using L. rhamnosus bacteria. The second experiment was the purification of lactic acid from fermentation broth. The third experiment was the making and testing of cream using E. coli as model organism.
Fermentations
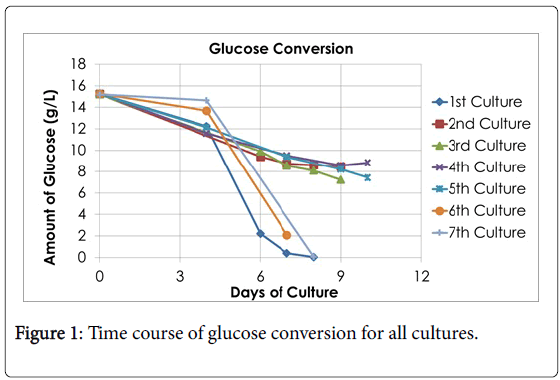
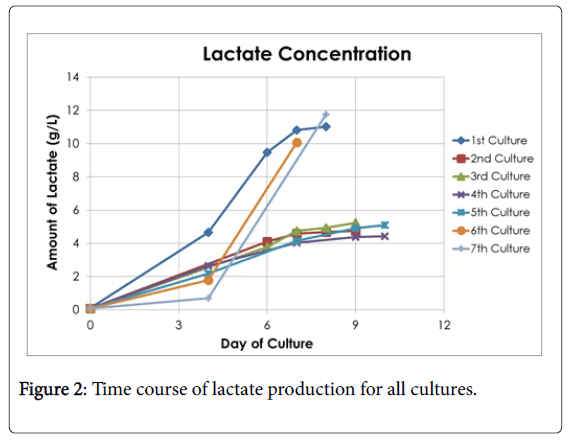
Seven cultures were fermented throughout this study and each one was documented based on its conversion of glucose to lactate. The fermentation continued over the course of 7-10 days, and the lactate and glucose concentration were measured on days 4, 6, 7, 8, 9, or 10. The comparisons of glucose conversion and lactate accumulation for each culture can be seen in Figures 1 and 2, respectively. The calculated results are shown in Table 1. The fluctuation for the yield of lactic acid can be explained that from cultures 1-5 the same bacteria was used from one KwikStik being maintained on agar plates over a course of several months. In other words, one plate was streaked from one KwikStik then grown and stored in refrigerator for a month, then streaked again. This was repeated many months, instead of starting from a new swab. Cultures 6 & 7 were a new KwikStik swab since it was realized that the yield of lactic acid was declining.
| Calculated Results | Glucose | Lactic Acid | Yield of Lactic Acid | ||
|---|---|---|---|---|---|
| Starting Amount (g/l) | Ending Amount (g/l) | Starting Amount (g/l) | Ending Amount (g/l) | ||
| 1st Culture | 15.29 | 0.05 | 0.09 | 11.03 | 71.78% |
| 2nd Culture | 15.26 | 8.47 | 0.08 | 4.75 | 68.78% |
| 3rd Culture | 15.2 | 7.24 | 0.07 | 5.25 | 65.08% |
| 4th Culture | 15.32 | 8.86 | 0.08 | 4.43 | 67.34% |
| 5th Culture | 15.25 | 7.42 | 0.07 | 5.09 | 64.11% |
| 6th Culture | 15.29 | 2.09 | 0.08 | 10.08 | 75.76% |
| 7th Culture | 15.22 | 0.06 | 0.07 | 11.77 | 77.18% |
Table 1: All fermentation cultures with calculated results.
Summarizing the fermentations, the final average amount of lactic acid concentration of all 7 cultures was 7.49 g/l with a standard deviation of 3.30 g/l. The Kolmogorov-Smirnov test of normality showed that the distribution was marginally normal (p=0.03) and there was no outlier. Also, the average amount of glucose converted was 10.39 g/l with a standard deviation of 3.98 g/l.
According to the Kolmogorov-Smirnov test of normality, the distribution of the sample points was normal (p>0.05) and there were no outliers. These analyses show that the fermentations were executed over a period of time and that the fluctuation for the yield of lactic acid was within the expected range.
Purification
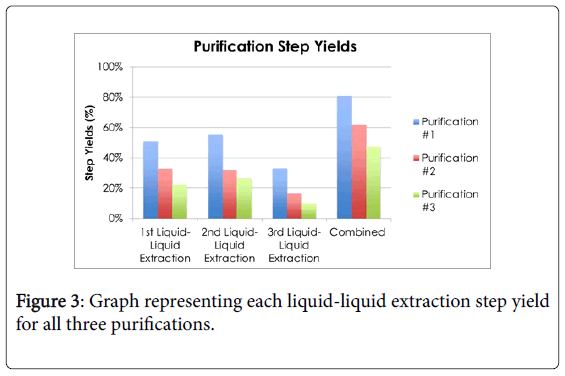
The second experiment was purification of the fermented lactic acid. Three purifications were conducted in this study. Each purification consisted of three liquid-liquid extractions using butanol. The calculated purification yields are shown in Figure 3.
The first purification’s liquid-liquid extraction step yields were 51%, 55.8%, and 34.2%, respectively. A portion of the 1st culture (2.0 g of lactate) was purified and the final lactate recovered was 1.63 g, which is 81.5% overall yield. In an attempt to first precipitate the lactate as salt, the second purification started with the addition of magnesium chloride. The procedure called for the removal of the precipitate; however, the precipitate did not contain all the lactate and the disposed supernatant contained most of the lactate. Therefore, the liquid extraction started off with 0.19 g of lactate and was able to recover 0.169 g of lactate, which was an overall yield of 61.8%. The liquidliquid extraction step yields were 32.9%, 31.7%, and 16.4%, respectively.
The 3rd and final purification was used for the main testing of the cream on 24 plates. This purification’s liquid-liquid extractions had step yields of 22.3%, 26.3%, and 10.0%, respectively. The purification started off with 5.02 g of lactate, and 3.35 was recovered which is an overall yield of 47.6%. The lower yields may be due to not enough time to extract lactate from aqueous phase to butanol phase.
Summarizing the purifications, the average of the overall yields for three purifications was 63.5%, with a standard deviation of 16.8%. Considering that a commercial purification would be closer to 100% yield, a One-Sample T-test analysis showed that 63.5% is not significantly different than 100% (t(2)=-3.76, p>0.05).
Making & testing of cream
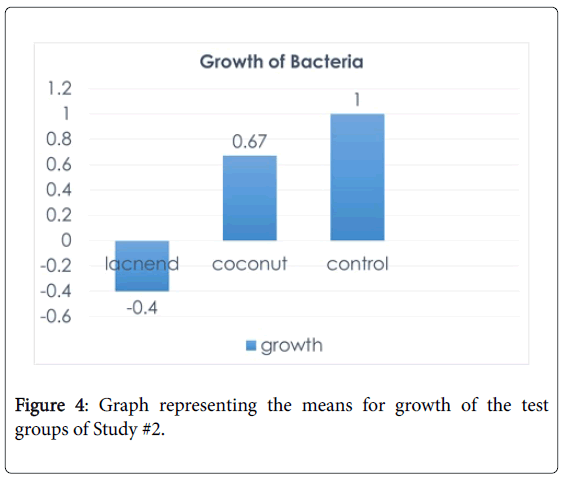
After each purification, the lactic acid was mixed with coconut oil to make the “LACNEND” cream then tested on agar plates with E. coli . There were also plates with just coconut oil in order to show the effectiveness of the lactic acid in the cream. Before any testing of the lactate that was produced from the fermentations, a commercially produced L -lactic acid (TCI) was mixed with coconut oil at amounts of 10% and 15%. It was then used in a pilot study to see which concentration would be effective. Tables 2–4 depict the results from pilot studies and first study. The first pilot study showed that the cream inhibited the growth of the bacteria; however, there was too little cream, thus, a second pilot study was conducted with more cream on the plate to show a larger zone of inhibition. It was decided from this pilot study that 10% concentration of lactic acid would be enough since both were equally effective. Then the first purification’s lactic acid was used to make a 10% cream and tested on E. coli . The cream, when compared to the control in the plate, proved to inhibit the bacteria; however, the lactic acid was too dilute, meaning that it wasn’t concentrated down enough from the boiling. Therefore, the second study had the lactic acid concentrated down until there was less than 0.5 g of water. The second purification was never used as a cream because there was too little lactate. Table 5 and Figure 4 depict the results of the last study that had 24 plates and used the third purification (with 3.35 g lactate and total of 33 g of “LACNEND” was produced). For the “LACNEND” plates, 9 quadrants had growth and 21 quadrants had no growth. The quadrants with growth had bacteria because there was not enough application of cream.
| Parameters | Control | 10% | 15% | Coconut |
|---|---|---|---|---|
| Positive | 12 | 2 | 1 | 4 |
| Negative | 0 | 2 | 3 | 0 |
Table 2: Pilot Study #1 Testing of cream on E. coli .
| Parameters | Control | 10% | 15% | Coconut |
|---|---|---|---|---|
| Positive | 12 | 0 | 0 | 4 |
| Negative | 0 | 4 | 4 | 0 |
Table 3: Pilot Study #2 Testing of cream on E. coli .
| Parameters | Control | Lacnend 10% |
|---|---|---|
| Positive | 4 | 2 |
| Negative | 0 | 2 |
Table 4: Study #1 Testing of cream on E. coli .
| Parameters | Control | Lacnend 10% | Coconut |
|---|---|---|---|
| Positive | 48 | 9 | 15 |
| Negative | 0 | 21 | 3 |
Table 5: Study #2 Testing of cream on E. coli .
A statistical analysis was done of this study in order to show the effectiveness of the cream. There were 30 “LACNEND”, 48 control and 18 coconut samples. Bacterial growth was coded as -1 (no growth) to 1 (growth). Therefore, scores closer to 1 show that there is stronger growth. A one-way ANOVA (levels: control, coconut, “LACNEND”) indicated that the growth in “LACNEND” condition (mean:-0.4) was significantly lower than the growth in coconut (mean: 0.67) and control (mean: 1) conditions, F (2,95)=48.61, p=0.00. The “LACNEND” condition was different than the other two conditions (p=0.00), but the control and coconut conditions did not differ significantly (p>0.1). Thus, it was concluded that bacteria growth was the smallest in “LACNEND” condition than the other two conditions.
Discussion
The purpose of this project was to effectively produce lactic acid, purify it, make it a cream, and test it. This new cream is called “LACNEND” because of its intended use to treat acne. Acne is not only a skin disease but it is also one of the predominant causes for a lack of self-confidence in young adults, especially because physical appearance is very closely linked peer relationships. “LACNEND” is a cream that provides hydration to the skin because of the lactic acid and the coconut oil, and it also has antimicrobial properties because of the lactic acid. This cream, to our knowledge, is the first to combine biologically produced lactate and organic coconut oil to make an effective acne cream. Three experiments were carried out in order for “LACNEND” to be created.
The first study aimed to ferment the lactic acid using L. rhamnosus bacteria. The bacteria undergoes homolactic fermentation using glucose to produce lactic acid. This study conducted seven fermentations in order to produce ample lactic acid. Each fermentation was executed over the course of 7-10 days and the results were recorded accordingly. The fermentation results showed a fluctuation in the amount of lactic acid produced; however, this difference did not prove to be significant after a statistical analysis. Therefore, the fermentations all produced lactic acid as expected by the age of the bacterial stock. The yield of lactic acid was lower that what was reported by others.
The second study aimed to purify the lactic acid. Not every fermentation was purified because only a certain amount of lactic acid was needed. The three purifications allowed a learning process towards the most effective way to purify the lactate. The first purification was from half of the first fermentation and it underwent a liquid-liquid extraction. The yield of lactic acid seemed insufficient at 81.5%, thus, a different purification method was used which was the formation of a precipitate using magnesium chloride. Therefore, the second purification used magnesium chloride to precipitate the remaining lactate from the first fermentation; however, very little amount of precipitate formed and the supernatant was disposed of. After solubilizing the precipitate with water, the liquid-liquid extraction was conducted. The yield was less in the second purification and the amount of lactate was a very small amount, thus, the supernatant which was disposed of contained most of the lactate. The third purification from the sixth culture gave the most amount of lactic acid, but the overall yield was lower due to a higher amount of starting lactate and not enough time for separation between the two phases. Purification yields were slightly lower that what was reported by others.
The making and testing of the cream required a pilot study in order to find the concentration of lactic acid needed for the cream. 10% proved sufficient and was used throughout the main studies. There were two set of experiments; one set used the lactate obtained from the first purification and the other set from the third purification. The lactate obtained from the second purification was not used because of its small amount of 0.17 g recovered. The first set of experiments showed that the lactic acid needed to be more concentrated before mixing with coconut. In other words, there needed to be very little water in order for the cream to inhibit the growth of the E. coli effectively. Therefore, the second study concentrated the lactic acid down and the results showed that the cream worked. Multiple trials were conducted to show the effectiveness of the cream.
In sum, through the fermentation, purification, making and testing of the cream, it was shown that the cream made from biologically produced lactate was able to inhibit the growth of bacteria and the “LACNEND” cream was able to inhibit the growth of bacteria better than the coconut oil. It is important to note that this cream is one of the first acne creams to have an ingredient which is biologically produced through bacteria. Also, it is the first acne cream to utilize only lactic acid and coconut oil in a cream, which is effective against acne.
Conclusion
This study successfully produced a biological lactic acid, purified it, made it an acne cream called “LACNEND” which provides hydration to the skin and has antimicrobial properties. It consists three main experiments. The first study showed that the fermentations all produced lactic acid as expected by the age of the bacterial stock. The second study purified the lactic acid. The third study tested this cream and showed that it effectively inhibits the growth of the E. coli bacteria. This study makes an important contribution by producing and testing the first acne cream that is biologically produced through bacteria, and to utilize only lactic acid and coconut oil in a cream.
Although the cream has proven to inhibit the growth of bacteria, further research can be done to legitimize “LACNEND”. Firstly, bacteria other than E. coli should be tested such as P. acnes , which is known to generate acne vulgaris. Propionibacterium acnes is widely known as existing in the oil glands of hair follicles and causing acne; however, it is more hazardous to use in a school laboratory and takes longer to grow in culture. Stricter safety regulations must be considered if the cream is to be tested on P. acnes too. Some methods that cosmetic companies use to test their product can be applied in future research too. The most common is stability testing which has physical/chemical, microbiological, and packaging tests. The physical/ chemical tests include temperature variations through cycles, centrifuge testing to see oil-in-water emulsion, light exposure tests for discolorations, and constant monitoring of pH, colour, odour, and viscosity. The microbiological guidelines are to ensure that the product does not get contaminated during packaging and shipment, thus, screening tests using sampling and evaluations can be used. Packaging tests ensure the safety of the glass, make sure that there is no leakage, and no weight loss through evaporation. Other future research also includes perfecting the purification process in order to yield close to 100% of the lactate. One thing that can be changed from observation is that there needs to be more time allotted for purification. Waiting for separation between the two phases in the separating funnel needs to be longer. Also, for the fermentations to produce a lot of lactate, a new bacterial stock must be used every time. Finally, next step for this project would be to achieve a patent for “LACNEND” to maintain rights for it to be marketed and commercially produced. It is noteworthy that in these studies, the lactate was fermented in shake flasks and purified using a separating funnel Due to the biological production of lactate, the manufacturing of it can be scaled up to large commercial scale by simply increasing the production vessel from a shake flask to bioreactors (>10 kl) and this will allow to maintain a low-cost for the manufacturing of lactate. Therefore, the future scale of operations and the results of this study exhibit that “LACNEND” is a cream that can be introduced into commercial use to treat acne.
Acknowledgements
Special thanks to my supervisor, Ms. Bettie Bechtel for assisting me in the lab, and teaching me proper procedures to complete this research. Also special thanks to my father Semsi Ensari for providing the laboratory materials and for his mentorship.
References
- Benthin S, Villadsen J (1995) Production of optically pure D-lactate by Lactobacillus bulgaricus and purification by crystallization and liquid/liquid extraction. Applied Microbiology and Biotechnology 42: 826-829.
- Chawong K, Rattanaphanee P (2011) N-butanol as an extractant for lactic acid recovery. International Journal of Chemical, Molecular, Nuclear, Materials, and Metallurgical Engineering 733-736.
- Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, et al. (2002) Acne Vulgaris: A disease of western civilization. Arch Dermatol 138: 1584-1590.
- De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, et al. (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbial Lett 259: 89-96.
- Erickson LE, Fayet E, Kakumanu BK, Davis LC (2004) Lactic acid fermentation. In carcass disposal: A comprehensive review. Kansas: National Agricultural Biosecurity Center 1-8.
- Lee K (2005) Comparison of fermentative capacities of lactobacilli in single and mixed culture in industrial media. Process Biochemistry 40: 1559-1564.
- Litchfield JH (1996) Microbiological production of lactic acid. Adv Appl Microbiol 42: 45-95.
- Niju N, Pradip KR, Aradhana S (2004) L (+) lactic acid fermentation and its product polymerization. Electronic Journal of Biotechnology 7.
- Ren J (2010) Biodegradable poly (lactic acid): Synthesis, modification, processing, and applications. Tsingshua University Press, Beijing, China 4-14.
- Rojan JP, Nampoothiri MK, Ashok P (2007) Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Applied Microbiological Biotechnology 74: 524-534.
- Siebold M, Frieling PV, Joppien R, Rindfleisch D, Schugerl K, et al. (1993). Comparison of the production of lactic acid by three different lactobacilli and its recovery by extraction and electrodialysis. Process Biochemistry 30: 81-95.
- Garg T, Ramam M, Pasricha JS, Verma KK (2002) Long term topical application of lactic acid/lactate lotion as a preventive treatment for acne vulgaris. Indian J Dermatol Venereol Leprol 68: 137-139.
- (2004) The European Cosmetic Toiletry and Perfumery Association. Guidelines on stability testing of cosmetic products. Brussels: Colipa.
- Wang Y, Deng W, Wang B, Zhang Q, Wan X, et al. (2013) Chemical synthesis of lactic acid from cellulose catalyzed by lead (II) ions in water. Nature Communications 1-7.
- Williams HC, Dellavalle RP, Garner S (2012) Acne vulgaris. Lancet 379: 361-372.
- Yang D, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D (2009) The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials 30: 6035-6040.
- (2017) "What Type of Acne Do You Have? Types of Acne Explained." BioClarity. Web.
Citation: Pelin E (2017) Discovery and Testing of a New Biologically Produced Acne Cream: “LACNEND”. J Biol Med Science 1: 102.
Copyright: © 2017 Pelin E. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 3708
- [From(publication date): 0-2017 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 2892
- PDF downloads: 816