Research Article Open Access
Optimisation of Sample Preparation for Direct SPME-GC-MS Analysis of Murine and Human Faecal Volatile Organic Compounds for Metabolomic Studies
Reade S1, Mayor A1*, Aggio R1, Khalid T1, Pritchard DM1, Ewer AK2, and Probert CS11Department of Gastroenterology, Institute of Translational Medicine, University of Liverpool, UK
2School of Clinical and Experimental Medicine, University of Birmingham, Birmingham UK
- *Corresponding Author:
- Arno Mayor
Department of Gastroenterology
Institute of Translational Medicine
University of Liverpool, Crown Street
Liverpool, L69 3GE, UK
Tel: +44 (0)151 794 6863
Fax: +44 (0)151 794 6825
E-mail: arnom@liverpool.ac.uk
Received date: February 21, 2014; Accepted date: March 21, 2014; Published date: March 24, 2014
Citation: Reade S, Mayor A, Aggio R, Khalid T, Pritchard DM, et al. (2014) Optimisation of Sample Preparation for Direct SPME-GC-MS Analysis of Murine and Human Faecal Volatile Organic Compounds for Metabolomic Studies. J Anal Bioanal Tech 5:184. doi: 10.4172/2155-9872.1000184
Copyright: © 2014 Reade S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The analysis of volatile organic compounds (VOCs) emitted from biological fluids such as blood, urine, breath and faeces, has been receiving more attention from researchers and clinicians due to their contribution to the metabolome and potential use as diagnostic tools in clinical settings. The faecal metabolome represents the final product of a complex interaction involving the gut microbiota and cell metabolism and an accurate measurement of the faecal volatile organic metabolome enables a better understanding of disease-related metabolic pathways and, eventually, identifying potential biomarkers. However, there is a lack of published evidence evaluating the sample preparation steps for faecal metabolome analysis and no well-defined protocol has been established. Consequently, different research groups employ diverse methodologies, which, ultimately, prevent comparison of results between laboratories.
We evaluated different aspects of sample preparation when processing murine and human faecal samples through a pipeline involving solid phase micro-extraction (SPME) coupled to gas chromatography-mass spectrometry (GC-MS). We identified the sample volume, the SPME fibre coating, the extraction conditions and the vial volume that produce the most accurate and reproducible results. Finally, we propose an optimized method for the direct SPME-GC-MS analysis of VOCs in murine and human faecal samples.
To the best of our knowledge, this is the first work evaluating different aspects of sample preparation for direct SPME-GC-MS analysis of VOCs and the first method proposed for the analysis of murine and human faecal samples. In addition, our proposed method can be coupled to the Automated Mass Spectral Deconvolution and Identification System (AMDIS) and the R software package, Metab, in order to produce results in a reliable and high-throughput manner.
Keywords
Volatile organic compounds (VOCs); Gas chromatography-mass spectrometry (GC-MS); Solid phase microextraction (SPME); Faeces
Introduction
Metabolomics is considered one of the newest omics technologies and has grown rapidly in the last 10 years. It involves screening a large number of low molecular mass metabolites (<1.5Kd) present in biological samples [1,2]. Metabolomic techniques have been largely and successfully applied in the identification of biomarkers and the dissection of metabolic pathways associated with specific diseases [3]. More recently, a number of studies have emphasized the role of volatile organic compounds (VOCs) in identifying disease-specific metabolome patterns [4-8].
VOCs are a large and diverse group of carbon-based molecules whose accurate identification has the potential to revolutionize patient care. Most vapours emitted from biological samples (e.g. breath, sweat, blood, urine and faeces) contain VOCs which may have a potential link to specific diseases [9]. For example, 3-methylhexane, decane, caryophyllene naphthalene have been detected at significantly lower levels in the breath of breast cancer patients, while lauric acid and palmitic acid have been detected at high levels in biopsied tissue from melanoma patients [10,11]. In addition, VOCs analysis of urine, breath or faeces is non-invasive and can be performed at a low cost, which makes it suitable for use as a screening tool in clinical diagnosis and in monitoring the efficacy of therapies [12].
The faecal metabolome is a result of the complex interaction between the intestinal microbiome and the cell metabolism [13]. Therefore, the analysis of VOCs present in faecal samples is of particular relevance when dealing with gastrointestinal (GI) diseases. Healthcare professionals commonly observe unusual smells from the faeces of patients suffering from GI diseases such as celiac disease, Crohn’s disease, chronic pancreatitis or intestinal infections [14]. Therefore, there is an increasing use of faecal metabolomics to investigate both the pathophysiology of GI diseases and the role of the intestinal microbiota in the metabolism in states of health and disease.
The analysis of VOCs relies on an accurate and reliable extraction of metabolites from the biological sample [15]. A solid phase microextraction (SPME) fibre is a solvent-free extraction technique ideally suited to metabolite extraction, as it minimises contact with possible infectious agents from blood, stool and urine samples [16]. SPME can be coupled to high-performance liquid chromatography (HPLC), inductively coupled plasma (ICP) and gas chromatography-mass spectrometry (GC-MS), however, SPME-GC-MS is considered one of the most popular methods for the analysis of VOCs emitted from faecal samples [16,17]. The different possible SPME configurations and the distinct sample preparation steps directly affect the results produced by SPME-GC-MS analyses [18]. Parameters such as the SPME fibre coating, the extraction conditions (temperature and time) and the sample volume used ultimately determine the number of VOCs identified their abundances and the repeatability across replicates [18]. Therefore, an evaluation of each of these parameters is essential in order to obtain reliable and reproducible results.
Although SPME-GC-MS has been used extensively for the analysis of VOCs from faecal samples [4,19-21], there is a lack of published evidence evaluating sample preparation steps and potential SPME extraction configurations prior to GC-MS analysis. Using human faecal samples, Dixon and co-workers [22] investigated the effect of different extraction times on VOCs when using 4 different SPME fibres. In addition, they compared the number of VOCs identified when using 8 different SPME fibres and an extraction time of 20 min. Couch et al. [23] studied 2 SPME extraction times (20 min and 18 hrs) using 3 different fibres. On the other hand, a thorough literature review resulted in no published protocols or methods regarding sample preparation for the direct SPME-GC-MS analysis of murine faecal samples. Therefore, different research groups have to evaluate the same method-related parameters prior to analysis of experimental samples. In addition, there is a lack of standardization between laboratories performing metabolomics. To the best of our knowledge, there is no single gold standard method for the direct SPME-GC-MS analysis of VOCs extracted from human and/or murine faecal samples, which results in different findings when analysing the same subject [20-22] and prevents comparing results between labs. Thus, there is a current need for well-established method for the analysis of VOCs in murine and human faecal samples.
Here, we have evaluated the effect of different parameters for sample preparation and different SPME configurations on the analysis of VOCs from murine and human faecal samples. The number of VOCs identified and their abundances have been assessed when using different sample masses, distinct vial volumes, salting out of samples, and the effect of leaving samples at 1°C whilst loaded onto auto-sampler tray for 14 hours (overnight), applying different SPME extraction times and temperatures and using distinct SPME fibre coatings. Finally, we propose an optimized method for a reliable and high-throughput analysis of VOCs present in faecal samples.
Materials and Methods
Mouse faecal samples
Four inbred wild-type C57BL/6 mice were purchased from Charles River Laboratories (Margate, UK) at 9 weeks old and acclimatised under standard specific pathogen free (SPF) animal house conditions at the University of Liverpool for a minimum of 1 week prior to faecal sample collection. Animals were housed in a single cage under conventional conditions with food and water ad libitum and kept on a 12:12 hrs light-dark cycle. Samples of 3, 5, 10 and 20 pellets of mice faeces were collected during 3 consecutive weeks, placed in 10 ml glass vials and stored at -20°C. An additional four male inbred wild-type C57BL/6 mice were purchased at 5 weeks old and housed individually in the same conditions. Samples of 10 pellets were collected from each of these four additional mice in a single week and used to assess the effect of vial volume, extraction time and temperature and SPME fibre coating, on the number and abundances of VOCs identified, as described below
Human faecal samples
Eleven faecal samples of variable masses were collected fresh from 5 premature babies hospitalized across the UK. The 5 donors were chosen at random from a larger cohort study. In accordance to a clear protocol, samples were collected by nurses and stored at -20°C. The sampling took place after written parental consent, with research ethics committee approval (11/WM/0078).
GC/MS conditions
A Perkin Elmer Clarus 500 GC/MS quadruple bench top system (Beacons field, UK) was used in combination with a Combi PAL autosampler (CTC Analytics, Switzerland) for the analysis of all samples. The GC column used was a Zebron ZB-624 with inner diameter 0.25 mm, length 60 m, film thickness 1.4 μm (Phenomenex, Maccles field, UK). The carrier gas used was helium of 99.996% purity (BOC, Sheffield, UK). The SPME fibres used were CAR-PDMS 85 μm and DVB-CAR-PDMS 50/30 μm (1 cm) (Sigma-Aldrich, Dorset, UK). Both fibres were pre-conditioned before use, in accordance with the manufacturer manual. Vials with magnetic caps of 2 ml (Crawford Scientific, Lanarkshire, UK) and 10 ml (Sigma-Aldrich, Dorset, UK) volume were used. The fibre desorption conditions were 5 minutes at 220°C. The initial temperature of the GC oven was set at 40°C and held for 1 minute before increasing to 220°C at a rate of 5°C/min and held for 4 min with a total run time of 41 min. A solvent delay was set for the first 6 min and the MS was operated in electron impact ionization EI+ mode, scanning from ion mass fragments 10 to 300 m/z with an interscan delay of 0.1 sec and a resolution of 1000 at FWHM (Full Width at Half Maximum). The helium gas flow rate was set at 1 ml/min. The sensitivity of the instrument was determined with 2-pentanone only and will vary for other compounds. The limit of detection, as being 3 times the signal/noise ratio, of the method for 2-pentanone with DVBCAR- PDMS is 16 ppm and with CAR-PDMS is 40 ppm.
Sample mass optimisation
The number of VOCs identified and their abundances were evaluated according to different sample masses. Murine samples of 3, 5, 10 and 20 pellets, in triplicate, were pre-incubated at 60°C for 30 min before VOC extraction using a CAR-PDMS SPME fibre at 60°C for 20 min prior to desorption into the GC oven. Only VOCs identified in every sample were then used for comparing the VOC abundances according to the sample mass used. The same procedure was applied to human faecal samples, where one single sample was initially divided into triplicates of 100, 450 and 700 mg. Then 4 additional samples were divided into triplicates of 50 and 100 mg. A pilot study (data not published) using human faecal samples between 130 and 1320 mg showed a plateau in the number of VOCs identified initiating at 700 mg. Therefore, sample masses greater than 700 mg were not investigated here.
Vial volume optimisation
The number of VOCs identified and their abundances were evaluated according to different vial volumes used for SPME-GC-MS analysis. For each vial volume of 2 and 10 ml, murine samples of 10 pellets (n=4) and aliquots of 100 mg from one human samples (n=4) were analysed. Each sample was pre-incubated at 60°C for 30 min before VOC extraction using a CAR-PDMS SPME fibre at 60°C for 20 min prior to desorption into the GC oven.
Salting–out optimisation
The number of VOCs identified was evaluated according to the addition of 0, 0.5 or 1 ml of saturated sodium chloride solution. Three human faecal samples were divided into 9 aliquots of 100 mg, stored in 10 ml vials and analysed with either 0 (n=3), 0.5 (n=3) or 1 ml (n=3) of sodium chloride solution added. Each sample was pre-incubated at 60°C for 30 min before VOC extraction using a CAR-PDMS SPME fibre for 20 min at 60°C prior to desorption into the GC oven.
Leaving samples at 1°C overnight (14 hrs)
The abundances of identified VOCs were evaluated according to waiting time periods of 0 and 14 hrs at 1°C prior to SPME-GC-MS analysis. One human sample was divided into six aliquots and stored in 10 ml glass vials at -20°C. Three aliquots were analysed immediately after being taken from -20°C freezer (0 hrs), while three aliquots were analysed after being left at 1°C on the auto-sampler tray overnight (14 hrs). Each sample was pre-incubated at 60°C for 30 min before VOC extraction onto a CAR-PDMS SPME fibre at 60°C for 20 min prior to desorption into the GC oven.
Extraction time optimisation
The number of VOCs identified was evaluated according to different SPME extraction times. Murine samples of 10 pellets were pre-incubated at 60°C for 30 min before VOC extraction using a CARPDMS SPME fibre at 60°C for 10 (n=3), 20 (n=3) or 30 min (n=3) prior to desorption into the GC oven.
Extraction temperature optimisation
The number of VOCs identified was evaluated according to different SPME extraction temperatures. Murine samples of 10 pellets were pre-incubated at 60°C for 30 min before VOC extraction using a CAR-PDMS SPME fibre at 50 (n=3), 60 (n=3) or 70°C (n=3) for 20 min prior to desorption into the GC oven.
SPME fibre optimisation
The number of VOCs identified was evaluated according to two SPME fibres. Murine samples of 10 pellets were pre-incubated at 60°C for 30 min before VOCs extraction using CAR-PDMS (n=5) or DVBCAR- PDMS (n=5) SPME fibres at 60°C for 20 min prior to desorption into the GC oven. The same procedure was applied to a single human faecal sample divided into 10 aliquots of 100 mg.
Repeatability and multiple analyses
In order to assess the repeatability of the final method proposed in this paper, the VOC profiles of ten human samples were further used to calculate the variation within samples. Each individual sample was divided in triplicate, stored in 10 ml vials and pre-incubated at 60°C for 30 min before VOCs extraction using a CAR-PDMS SPME fibre for 20 min at 60°C prior to desorption into the GC oven. The abundances of the VOCs identified within each sample (n=3 per sample) were used to calculate their coefficient of variation. Finally, 3 of these human samples were reanalysed 3 additional times in order to determine the effect of multiple analyses of a single sample.
Data processing
All GC-MS data were processed using the Automated Mass Spectral Deconvolution System (AMDIS-version 2.71, 2012) in conjunction with the NIST mass spectral library (version 2.0, 2011) and the R package Metab [24]. VOCs were identified using an in-house library built with AMDIS in combination to the NIST library. All statistics were performed using R version 3.0.2 [25,26]. A t-test or a one-way analysis of variance (ANOVA) followed by Tukey’s HSD test were applied to test differences between data classes. A principle component analysis (PCA) was used to show similarities within data classes. Final p-values were adjusted for multiple comparisons using Bonferroni correction. P-values<0.05 were considered as significant.
Results
Sample mass optimization
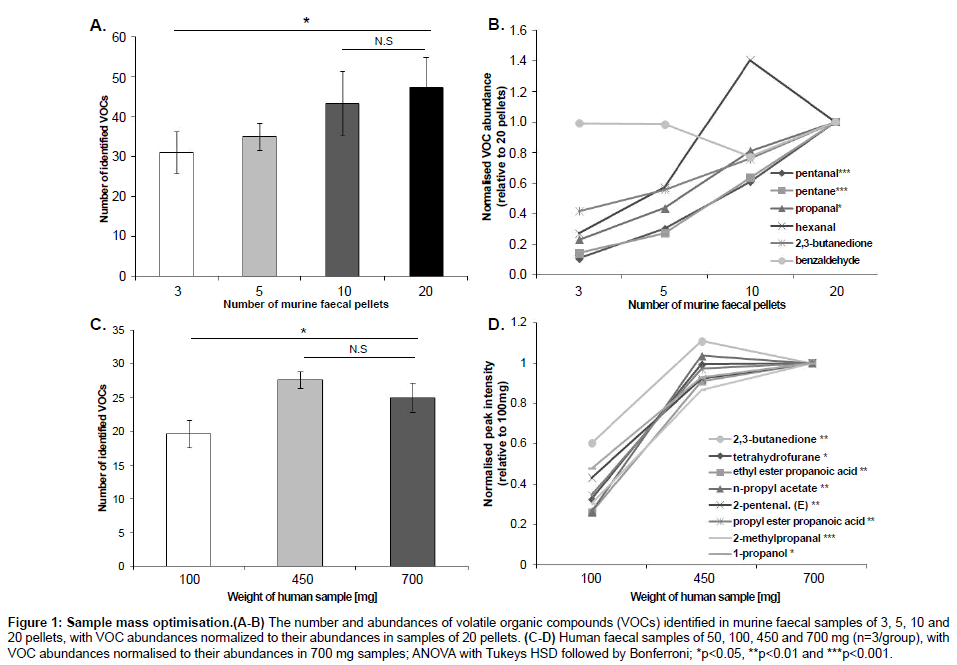
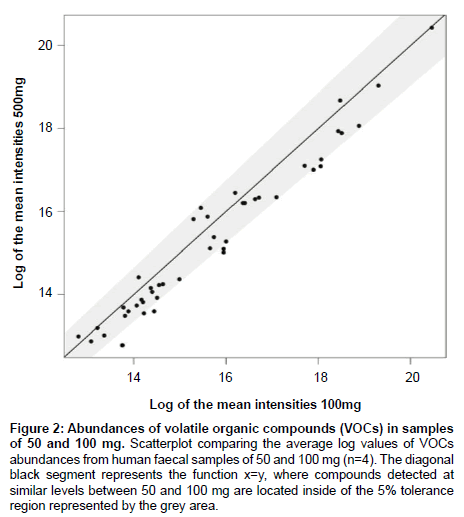
In order to optimize the sample mass for direct SPME-GC-MS analysis, we compared the number and abundances of VOCs identified in murine faecal samples (mean ± SEM) of 3 (40.0 ± 14.1 mg), 5 (76.7 ± 25 mg), 10 (133.3 ± 7 mg) and 20 (233.0 ± 25 mg) pellets; and in human faecal samples of 100 mg (100.3 ± 0.6 mg), 450 mg (455.1 ± 1.1 mg) and 700 mg (700.6 ± 2.8 mg) (Figure 1) and then 50 mg (52.4 ± 0.4 mg) and 100 mg (102.5 ± 0.2 mg) (Figure 2). Murine samples of 3 pellets showed a significantly lower number of VOCs than 20 pellets (p=0.05), with no significant difference in the number of VOCs between 10 and 20 pellets (p=0.87). Six compounds (pentanal, pentane, propanal, hexanal, 2,3-butandione and benzaldehyde) were consistently present in every murine sample with the compounds pentane and pentanal detected at significantly lower abundances in 3 and 5 pellets compared to 10 and 20 pellets. The compound propanal was present at significantly different levels between 3 and 20 pellets. In human samples, 450 and 700 mg showed a significantly higher number of VOCs than samples of 100 mg. There was no difference in the number of VOCs between 450 and 700 mg. Eight compounds were identified in every human sample (2, 3-butanedione, tetrahydrofurane, ethyl ester propanoic acid, n-propyl acetate, 2-pentenal (E), propyl ester propanoic acid, 2-methylpropanal, 1-propanol) and their intensities were significantly higher in samples of 450 and 700 mg than samples of 100 mg. The additional comparison between 50 and 100 mg showed no difference in both the number of VOCs identified (data not shown) and their abundances. All the statistical analyses for sample mass optimisation were performed by ANOVA followed by Tukey’s HSD test and Bonferroni.
Figure 1: Sample mass optimisation.(A-B) The number and abundances of volatile organic compounds (VOCs) identified in murine faecal samples of 3, 5, 10 and 20 pellets, with VOC abundances normalized to their abundances in samples of 20 pellets. (C-D) Human faecal samples of 50, 100, 450 and 700 mg (n=3/group), with VOC abundances normalised to their abundances in 700 mg samples; ANOVA with Tukeys HSD followed by Bonferroni; *p<0.05, **p<0.01 and ***p<0.001.
Figure 2: Abundances of volatile organic compounds (VOCs) in samples of 50 and 100 mg. Scatterplot comparing the average log values of VOCs abundances from human faecal samples of 50 and 100 mg (n=4). The diagonal black segment represents the function x=y, where compounds detected at similar levels between 50 and 100 mg are located inside of the 5% tolerance region represented by the grey area.
Vial volume and salting–out optimisation
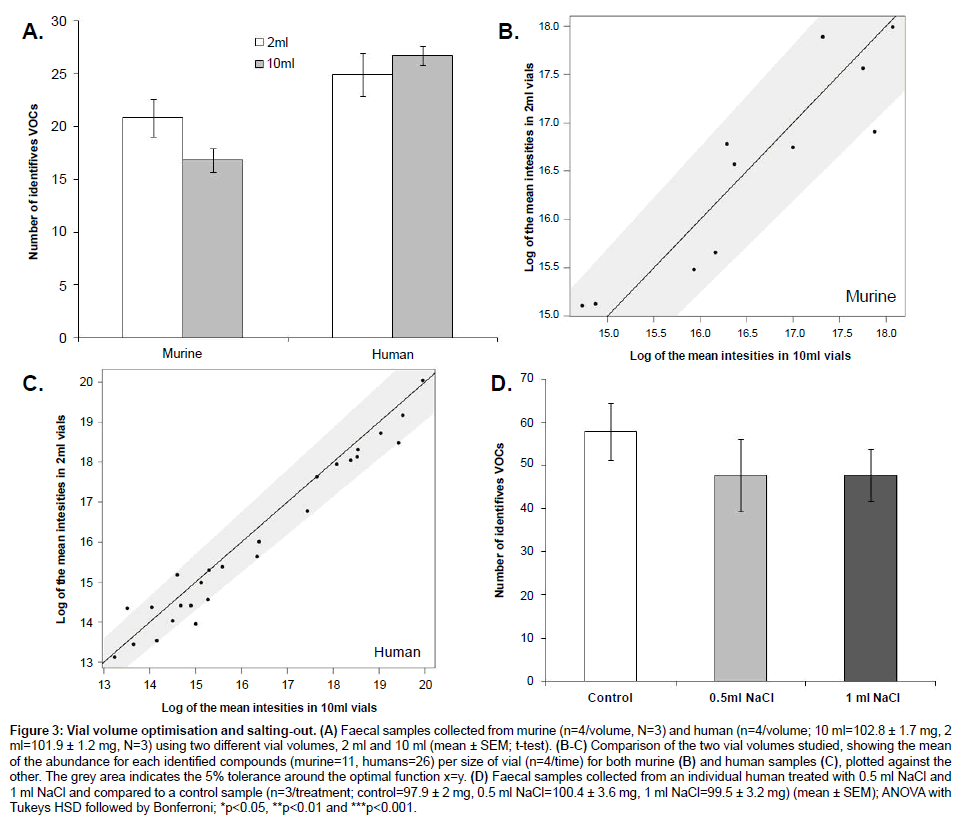
Vials of 2 ml showed a higher number of VOCs than vials of 10 ml when analysing murine samples (Figure 3A). However, there were no significant differences in VOCs abundances between vials (T-test; p-value>0.05). On the other hand, human samples showed no significant differences in both the number and the abundances of VOCs detected (T-test; p-value>0.05) (Figure 3B and 3C). The addition of sodium chloride showed no significant differences in the number and abundances of VOCs (ANOVA followed by Tukey’s HSD test and Bonferroni; p-value>0.05) (Figure 3D).
Figure 3: Vial volume optimisation and salting-out. (A) Faecal samples collected from murine (n=4/volume, N=3) and human (n=4/volume; 10 ml=102.8 ± 1.7 mg, 2 ml=101.9 ± 1.2 mg, N=3) using two different vial volumes, 2 ml and 10 ml (mean ± SEM; t-test). (B-C) Comparison of the two vial volumes studied, showing the mean of the abundance for each identified compounds (murine=11, humans=26) per size of vial (n=4/time) for both murine (B) and human samples (C), plotted against the other. The grey area indicates the 5% tolerance around the optimal function x=y. (D) Faecal samples collected from an individual human treated with 0.5 ml NaCl and 1 ml NaCl and compared to a control sample (n=3/treatment; control=97.9 ± 2 mg, 0.5 ml NaCl=100.4 ± 3.6 mg, 1 ml NaCl=99.5 ± 3.2 mg) (mean ± SEM); ANOVA with Tukeys HSD followed by Bonferroni; *p<0.05, **p<0.01 and ***p<0.001.
Leaving samples at 1°C overnight (14 hrs)
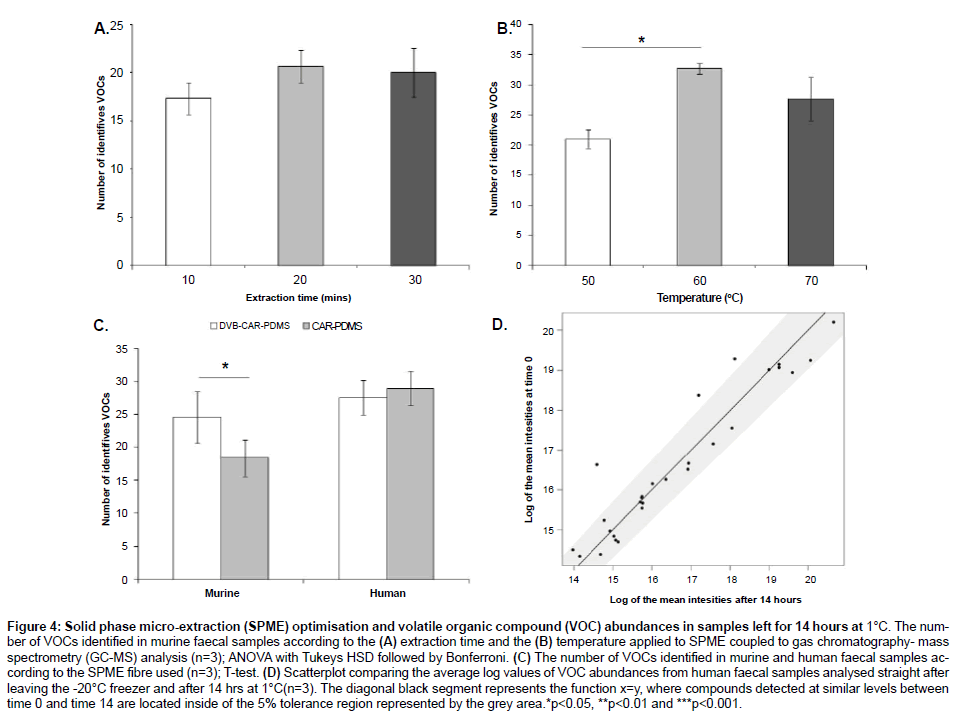
Although there were no significant differences in the number of VOCs identified (T-test; p-value > 0.05) there were three compounds detected at slightly lower abundances at 14hrs (Figure 4D).
Figure 4: Solid phase micro-extraction (SPME) optimisation and volatile organic compound (VOC) abundances in samples left for 14 hours at 1°C. The number of VOCs identified in murine faecal samples according to the (A) extraction time and the (B) temperature applied to SPME coupled to gas chromatography- mass spectrometry (GC-MS) analysis (n=3); ANOVA with Tukeys HSD followed by Bonferroni. (C) The number of VOCs identified in murine and human faecal samples according to the SPME fibre used (n=3); T-test. (D) Scatterplot comparing the average log values of VOC abundances from human faecal samples analysed straight after leaving the -20°C freezer and after 14 hrs at 1°C(n=3). The diagonal black segment represents the function x=y, where compounds detected at similar levels between time 0 and time 14 are located inside of the 5% tolerance region represented by the grey area.*p<0.05, **p<0.01 and ***p<0.001.
Extraction time and temperature optimization
Although there was no difference in the number of VOCs reported by the exposure times tested, 20 minutes produced the highest number of VOCs (p=0.5) (Figure 4A). The exposure temperatures of 60 and 70°C showed a significantly higher number of VOCs than 50°C (p=0.03). Although there was no significant difference between 60 and 70°C (p=0.33), the exposure temperature of 60°C produced the highest number of VOCs (Figure 4B) (ANOVA followed by Tukey’s HSD test and Bonferroni).
SPME fibre optimization
DVB-CAR-PDMS showed a significantly higher number of VOCs when analysing murine samples (T-test; p=0.04). Seven compounds were exclusively detected by DVB-CAR-PDMS, while 2 compounds were exclusively detected by CAR-PDMS. Human samples showed no significant difference between SPME coatings, although five compounds were exclusively reported by CAR-PDMS and another five compounds were exclusively reported by DVB-CAR-PDMS (Figure 4C and Table 1).
| Murine | Human | ||
|---|---|---|---|
| CAR-PDMS | DVB-CAR-PDMS | CAR-PDMS | DVB-CAR-PDMS |
| 2,2-dimethylpropanoic acid | 2-methyl-1-propanal | propanoic acid | 2-methyl-2-butenal |
| 2-methylpentanal | 2-pentanone | 2-methylpropanoic acid | butanoic acid, propyl ester |
| 2-methylfuran | 2,2-dimethylpropanoic acid | cyclopentane | |
| 2-nonenal | N,N-dimethylacetamide | 2-pentenal, (E) | |
| 3-methylbutanal | 1-propanol | 2-ethyl-1-hexanol | |
| 1-penten-3-ol | |||
| 2-heptanal | |||
Table 1: SPME fibre optimisation. Murine and human faecal samples were analysed using a CAR-PDMS (n=5) or DVB-CAR-PDMS (n=5) SPME fibre. There were 2 and 5 specific compounds found exclusively by CAR-PDMS in murine and human samples respectively, and 7 and 5 found exclusively by DVB-CAR-PDMS in murine and human samples, respectively.
Optimized method
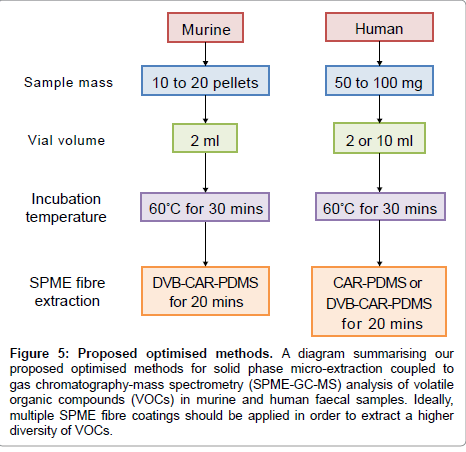
Based on the results presented above, we propose a SPME-GC-MS method for analysing murine and human faecal samples (Figure 5). We suggest murine samples are analysed using 10 to 20 pellets, stored in 2 ml glass vials, incubated for 30 min at 60°C and extracted by DVBCAR- PDMS for 20 min. Alternatively, human samples of 450 mg should be stored in 2 or 10 ml glass vials, incubated for 30 min at 60°C and extracted by CAR-PDMS or DVB-CAR-PDMS for 20 min.
Figure 5: Proposed optimised methods. A diagram summarising our proposed optimised methods for solid phase micro-extraction coupled to gas chromatography-mass spectrometry (SPME-GC-MS) analysis of volatile organic compounds (VOCs) in murine and human faecal samples. Ideally, multiple SPME fibre coatings should be applied in order to extract a higher diversity of VOCs.
Repeatability and multiple analyses
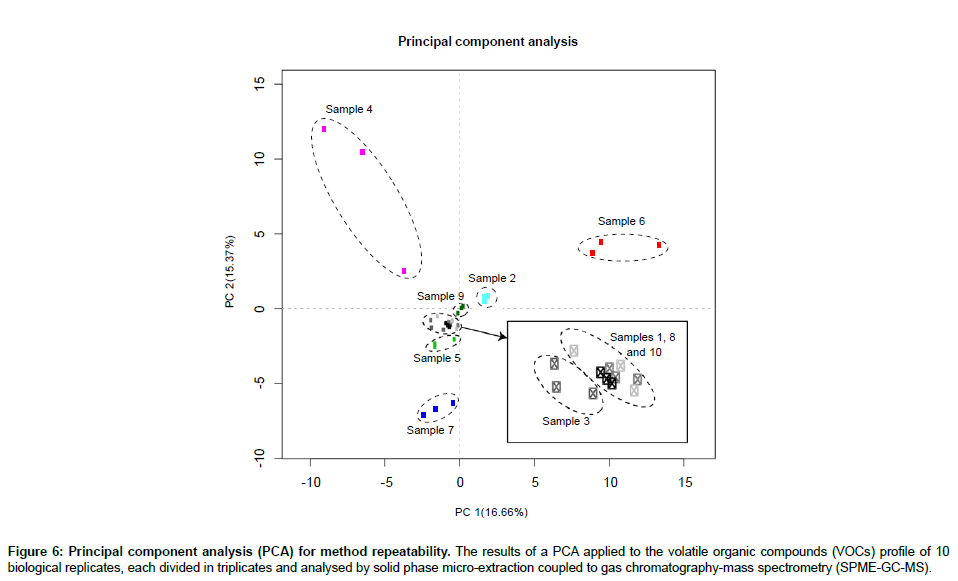
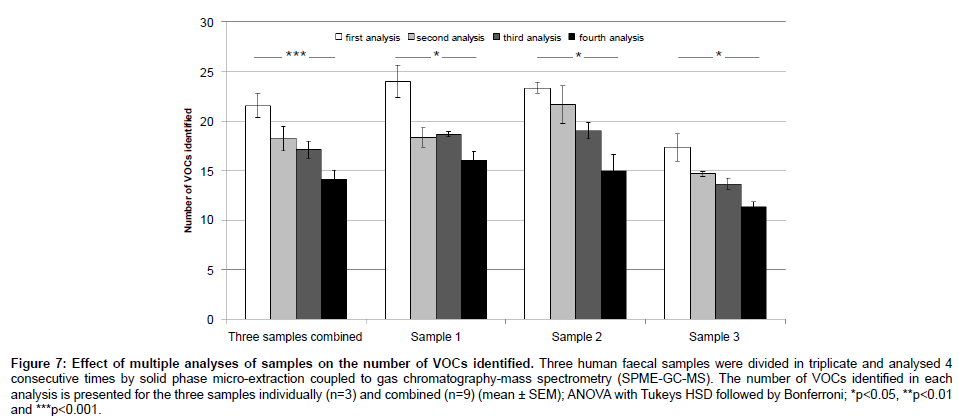
In order to assess the repeatability of our proposed method, we performed a principal component analysis (PCA) on the VOC profiles of 10 human faecal samples, each divided in triplicate (Figure 6). In addition, the mean, standard deviation and coefficient of variation (CV) were calculated across triplicates for each sample (Table 2). The PCA yielded results showing that each sample was clustered together. In average, 31.3 ± 10.5 VOCs were identified per human sample (mean ± SD). The standard deviation for each sample was 2.9 ± 1.3 compounds and, in average, 90% of the VOC abundances showed a coefficient of variation smaller than 30%, which is considered adequate for diagnostic sensitivity [27]. Furthermore, the effect of analysing a single sample multiple times was assessed. The number of VOCs identified and their abundances were compared across 4 GC-MS runs (Table 3). There was a significant decrease in the number of VOCs identified (Figure 7) and 40% of these VOCs were detected at significantly lower or higher abundances after 4 GC-MS runs (ANOVA; p-value<0.05).
| Samples | Number of VOCs ± S.D. (VOCs in every technical replicate) |
VOCs with CV < 30% (%) |
|---|---|---|
| Sample 1 | 24± 3 (19) | 100 |
| Sample 2 | 23± 1 (18) | 100 |
| Sample 3 | 20± 2 (12) | 92 |
| Sample 4 | 48± 5 (36) | 89 |
| Sample 5 | 37± 4 (26) | 81 |
| Sample 6 | 50± 4 (39) | 95 |
| Sample 7 | 32± 2 (21) | 90 |
| Sample 8 | 26± 4 (18) | 67 |
| Sample 9 | 26± 2 (19) | 95 |
| Sample 10 | 27± 2 (18) | 94 |
Table 2: Repeatability of method. Ten biological replicates of human faecal samples were divided in triplicates and analysed by solid phase micro-extraction coupled to gas chromatography-mass spectrometry (SPME-GC-MS). The average number of volatile organic compounds (VOCs) and the standard deviation associated to each biological replicate is presented together with the number of VOCs in every technical replicate and the percentage of compound abundances showing a coefficient of variation (CV) smaller than 30%.
| SPME-GC-MS analyses | VOCs present in triplicate | Percentage of significantly different abundances(%) |
|---|---|---|
| Sample 1 | 16 | 56 |
| Sample 2 | 14 | 64 |
| Sample 3 | 10 | 40 |
Table 3: Effect of multiple analyses of samples on the abundances. Three human faecal samples were divided in triplicate and analysed 4 consecutive times by solid phase micro-extraction coupled to gas chromatography-mass spectrometry (SPME-GC-MS). The number of volatile organic compounds (VOCs) present in at least 2 consecutive analyses is presented together with the percentage of compounds for which the intensities differed significantly after 4 analyses.
Figure 6: Principal component analysis (PCA) for method repeatability. The results of a PCA applied to the volatile organic compounds (VOCs) profile of 10 biological replicates, each divided in triplicates and analysed by solid phase micro-extraction coupled to gas chromatography-mass spectrometry (SPME-GC-MS).
Figure 7: Effect of multiple analyses of samples on the number of VOCs identified. Three human faecal samples were divided in triplicate and analysed 4 consecutive times by solid phase micro-extraction coupled to gas chromatography-mass spectrometry (SPME-GC-MS). The number of VOCs identified in each analysis is presented for the three samples individually (n=3) and combined (n=9) (mean ± SEM); ANOVA with Tukeys HSD followed by Bonferroni; *p<0.05, **p<0.01 and ***p<0.001.
Discussion
In this section, we discuss the results obtained when evaluating different aspects of sample preparation and SPME configurations for direct SPME-GC-MS analysis. Murine and human faecal samples have shown similar behaviour regarding the mass selection; however, they differed considering SPME coatings affinity and the vials volume optimisation.
Sample mass optimization
The sample mass optimization showed the same pattern for murine and human samples (Figure 1). An increase in sample mass led to an increase in both the number of VOCs detected and their abundances, therefore reaching a limit of VOCs identified; a result of fibre overload. However, as the amount of mass per sample may be limited according to the study being conducted, we searched for the minimum sample mass that would produce the highest number of VOCs with the highest abundances. Murine samples of >20 faecal pellets were not included in this present study as no significant difference was found between samples of 10 and 20 pellets and it was found experimentally difficult to collect >20 pellets from individual mice. Therefore, we would predict that any number of murine faecal pellets higher than 20 would also produce a non-significant result. Figure 1C showed an increase in the number of VOCs identified between samples of 100 and 450 mg and between samples of 100 and 700 mg. The lack of difference between samples of 450 and 700 mg indicates that the SPME fibre reached its limit of absorbance at 450 mg. Therefore, samples of 10 pellets and samples of 450 mg showed the best results for murine and human samples respectively. Although human samples of 450 mg showed higher number of VOCs identified and higher abundances, samples of this mass are not always available. For example, studies with premature babies generally involve samples of 100 mg or less [28-30]. Therefore, we compared the results between samples of 50 and 100 mg in order to find the lowest possible sample mass able to produce a reasonable number of VOCs and reproducible abundances. Results showed no significant differences, which suggests that samples from 50 to 100 mg are appropriate in these cases (Figure 2).
Vial volume and salting–out optimisation
Although no significant differences were observed between the vials tested when analysing both murine and human samples (Figure 3), 2 ml vials showed a much higher number of VOCs identified than 10 ml vials when analysing murine samples. However, when analysing human samples, 10 ml vials showed a slightly higher number of VOCs identified than 2 ml vials (2 ml=24, 10 ml=26 VOCs). Therefore, 2 ml vials are recommended for the analysis of murine samples and 2 ml or 10 ml vials when analysing human samples. This difference of behaviour might be explained by sample concentration. Certain compounds are more concentrated and at higher abundances in human samples compared to murine samples. Therefore, if the volume of headspace decreases then the levels of compounds in human samples will remain mostly unchanged whereas in murine samples the concentration increases leading to better coverage of the VOCs profiles in the samples. In addition, no significant differences were observed when salt was added to the samples. This could reflect the solid or semi-solid nature of the sample, which is only partially dissolved in the sodium chloride solution.
Leaving samples at 1°C overnight (14 hrs)
There were no significant changes in VOC abundances when samples were left for 14h at 1°C prior to SPME-GC-MS analysis (Figure 4D), which indicates that samples can be analysed overnight without significantly changing the VOC profiles or abundances. This result is particularly important for those experiments using GC-MS combined to auto-samplers that have a cooling tray with a minimum temperature of 1°C, where samples are generally sitting for up to 14 hours prior to injection.
Extraction time and temperature optimization
Typically, the SPME extraction process is considered complete when the analyte concentration has reached distribution equilibrium between the sample matrix and the fibre coating. Until the distribution equilibrium is not reached, the extraction process is considered in preequilibrium state. Our results showed that an increase in extraction time and temperature resulted in higher numbers of VOCs identified (Figure 4A and 4B). However, extraction times higher than 20 minutes and extraction temperatures higher than 60°C showed no improvement. These results indicate that in the pre-equilibrium state, a small change in extraction time and temperature result in a large change in the amount of analyte being absorbed. However, once the distribution gets close to the equilibrium, there are either no changes or only small changes in the amount of analyte absorbed. Therefore, the extraction time and temperature are not critical once equilibrium is nearly reached. These results are in agreement with previous work from our group and others (Figure 4A and 4B) [4,22,31].
SPME fibre optimization
Although there were no differences between the SPME fibres tested when analysing human samples, five compounds were exclusively detected by each specific fibre. On the other hand, the DVB/CAR/PDMS fibre was able to identify a higher number of compounds than the CAR/ PDMS fibre when analysing murine samples (Figure 4C), with seven compounds exclusively identified by DVB/CAR/PDMS. Therefore, when possible, we suggest the use of different SPME fibres in order to extract a greater diversity of compounds, which is in agreement with previous work from Dixon and collaborators [22]. However, if a single SPME fibre must be used, we suggest DVB/CAR/PDMS for murine studies whereas both types of SPME fibre can be equally applied for human samples.
Optimized method
Our optimized method (Figure 5) shows two main differences when applied to murine or human samples. When a single fibre is available, murine samples should be analysed by DVB/CAR/PDMS fibre and using 2 ml vials, while human samples can be analysed by DVB/CAR/ PDMS or CAR/PDMS and using 2 or 10 ml vials. Our proposed method involves almost no sample preparation steps and results in reproducible and reliable VOC identification and quantification. Furthermore, our method can be easily combined to AMDIS software and Metab package in order to frame a powerful pipeline able to analyse VOCs in faecal samples in a reliable and high-throughput manner.
Repeatability and multiple analyses
The PCA results (Figure 6) showed technical replicates clustering together, which indicates low variability across samples and high reproducibility. The PCA results are consistent with the low standard deviation across technical replicates (Table 2) and with the fact that the great majority of VOC abundances (90%) showed a CV lower than 30%. Finally, multiple SPME-GC-MS analyses of a single sample showed significant influence on the results, with a significant decrease in the number of identified VOCs (Figure 7) and difference in the intensity of at least 40% of the VOCs (Table 3) after 4 SPME-GC-MS re-runs of each sample. These differences confirmed the hypothesis that the sample’s headspace is affected by its analysis. Heating the faecal samples releases the majority of VOCs into the headspace air which are extracted and removed during the first SPME-GC-MS analysis. During the second, third and fourth analysis, the diversity and levels of specific VOCs are decreased, as confirmed in Figure 7. Therefore, each sample should be analysed once. The inter-individual variation between samples should also be taken into consideration, explaining the lower number of identified VOCs in sample 3 compared to sample 1 and 2 (Figure 7).
This proposed optimised method for the analysis of human and murine faecal samples using SPME-GC-MS is specific for the GC-MS conditions described in the ‘Materials and Methods’ section. Therefore, any change introduced by altering these configurations may result in different outcomes compared to those described in this study.
Conclusion
We have evaluated several aspects involving the direct VOC analysis of murine and faecal samples via SPME-GC-MS. As a result, we proposed a new optimised method and demonstrated its high repeatability across technical replicates. To the best of our knowledge, this is the first study evaluating different aspects of SPME-GC-MS analysis of murine and human faecal samples and the first study suggesting an optimised method. Furthermore, our proposed method can be combined to the data analysis tools AMDIS and Metab in order to produce reliable results in a high-throughput way.
Acknowledgements
We thank Action Medical Research for partly funding this work. Nicolas Ellaby is thanked gratefully for his help with the management of the human sample database. Rachel Jackson, Elizabeth Simcox and all the staff at the Birmingham Women’s Hospital, Royal Wolverhampton Hospital, Sheffield Teaching Hospitals, Birmingham Heartlands Hospital and University Hospitals of Leicester for collecting human samples on-site.
References
- Liu D, Hoynes-O'Connor A, Zhang F (2013) Bridging the gap between systems biology and synthetic biology. Front Microbiol 4: 211.
- Kell DB, Goodacre R2 (2014) Metabolomics and systems pharmacology: why and how to model the human metabolic network for drug discovery. Drug Discov Today 19: 171-182.
- Fedrizzi B, Carlin S, Franceschi P, Vrhovsek U, Wehrens R, et al. (2012) D-optimal design of an untargeted HS-SPME-GC-TOF metabolite profiling method. Analyst 137: 3725-3731.
- Garner CE, Smith S, Costello BD, White P, Spencer R, et al. (2007) Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J 21: 1675-1688.
- Garner CE, Ewer AK, Elasouad K, Power F, Greenwood R, et al. (2009) Analysis of faecal volatile organic compounds in preterm infants who develop necrotisingenterocolitis: a pilot study. J Pediatr Gastroenterol Nutr 49: 559-565.
- Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, et al. (2013) Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 100: 144-150.
- Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, et al. (2013) Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 11: 868-875.
- De Lacy Costello B, Ewen R, Ewer AK, Garner CE, Probert CS, et al. (2008) An analysis of volatiles in the headspace of the faeces of neonates. J Breath Res 2: 037023.
- Cicolella A (2008) [Volatile Organic Compounds (VOC): definition, classification and properties]. Rev Mal Respir 25: 155-163.
- Mangler M, Freitag C, Lanowska M, Staeck O, Schneider A, et al. (2012) Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol Pol 83: 730-736.
- Abaffy T, Möller MG, Riemer DD, Milikowski C, Defazio RA (2013) Comparative analysis of volatile metabolomics signals from melanoma and benign skin: a pilot study. Metabolomics 9: 998-1008.
- de Lacy Costello B, Ratcliffe NM (2013) Volatile organic compounds (VOCs) found in urine and stool. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine 405-462.
- Xu X, Xu P, Ma C, Tang J, Zhang X (2013) Gut microbiota, host health, and polysaccharides. Biotechnol Adv 31: 318-337.
- Medline Plus (2014) Stools - foul smelling.
- Mikami T, Aoki M, Kimura T (2012) The application of mass spectrometry to proteomics and metabolomics in biomarker discovery and drug development. Curr Mol Pharmacol 5: 301-316.
- Mills GA, Walker V (2000) Headspace solid-phase microextraction procedures for gas chromatographic analysis of biological fluids and materials. J Chromatogr A 902: 267-287.
- Walton C, Fowler DP, Turner C, Jia W, Whitehead RN, et al. (2013) Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm Bowel Dis 19: 2069-2078.
- Grob RL, Barry EF (2004) Modern Practice of Gas Chromatography. 4thEdition. Sons JW, 578.
- Di Cagno R, Rizzello CG, Gagliardi F, Ricciuti P, Ndagijimana M, et al. (2009) Different Fecal Microbiotas and Volatile Organic Compounds in Treated and Untreated Children with Celiac Disease. Applied and environmental microbiology 75: 3963-3971.
- Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, et al. (2009) Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J Gastrointestin Liver Dis 18: 337-343.
- Garner CE, Smith S, Bardhan PK, Ratcliffe NM, Probert CSJ (2009) A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis. Trans R Soc Trop Med Hyg 103: 1171-1173.
- Dixon E, Clubb C, Pittman S, Ammann L, Rasheed Z, et al. (2011) Solid-Phase Microextraction and the Human Fecal VOC Metabolome. PloS one 6: e18471.
- Couch RD, Navarro K, Sikaroodi M, Gillevet P, Forsyth CB, et al. (2013) The approach to sample acquisition and its impact on the derived human fecal microbiome and VOC metabolome. PLoS One 8: e81163.
- Aggio R, Villas-Bôas SG, Ruggiero K (2011) Metab: an R package for high-throughput analysis of metabolomics data generated by GC-MS. Bioinformatics 27: 2316-2318.
- R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria.
- Ihaka R, Gentleman R (1996) R: A language for data analysis and graphics. Journal of Computational and Graphical Statistics 5: 299-314.
- Drabovich AP, Pavlou MP, Batruch I, Diamandis EP (2013) Proteomic and Mass Spectrometry Technologies for Biomarker Discovery. Proteomic and Metabolomic Approaches to Biomarker Discovery 17-37.
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, et al. (2009) 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944-954.
- Schwiertz A, Gruhl B, Lobnitz M, Michel P, Radke M, et al. (2003) Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res 54: 393-399.
- Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, et al. (2013) Bacterial Diversity in Meconium of Preterm Neonates and Evolution of Their Fecal Microbiota during the First Month of Life. PloS one 8: e66986.
- Aldrich S (2014) FAQ - Solid Phase Microextraction.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16957
- [From(publication date):
May-2014 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 12200
- PDF downloads : 4757