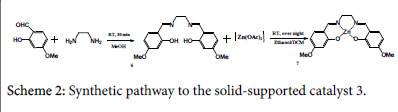Research Article Open Access
Direct Reaction of Carbon dioxide to Polycarbonate
Farah Bani Affan*Department of Chemical engineering, Koya University, Iraq
- *Corresponding Author:
- Affan FB
Department of Chemical Engineering
Koya University ,Iraq
Tel: +964 748 012 7520
E-mail: farah.ayad@koyauniversity.org
Received April 18, 2016; Accepted May 20, 2016; Published May 27, 2016
Citation:Affan FB (2016) Direct Reaction of Carbon dioxide to Polycarbonate. J Ecosys Ecograph 6:185. doi:10.4172/2157-7625.1000185
Copyright: © 2016 Affan FB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
At present, the main source of energy generation around the world is fossil fuel combustion (coal, oil and natural gas); it is also predicted to remain the dominant for the next few decades. A major drawback of combusting fossil fuels is the huge amounts of carbon dioxide (CO2) emissions into the atmosphere, especially with the evolving of the industrial revolution. Due to the fact that CO2 high levels in the atmosphere is linked to trapping sun light, hence global warming; much interest have been invested in the development of carbon capture and storage (CCS) approach. An efficient, valuable and profitable method of storing CO2 is to utilise it as a raw material in industries.
This paper is conducted based on experimental work regarding the conversion of CO2 from a challenging waste into a polymer; a high impact and temperature resistance, transparent, easily deformed without breaking, light material. The main features that are investigated in this paper are the synthesis of various types of Zn-based salen complexes (three catalysts, mostly novels), and their utilisation in copolymerisation reactions of CO2 with four different types of epoxides (Styrene oxide, n-hexane oxide, cyclohexene oxide, and propylene oxide. Zn-based salen catalysts have been chosen as no previous work has been carried out in the department regarding such complexes, as well as it is the main outcome from the technical review (preciously submitted as a part of this paper) as that the zinc catalyst can be recommended as the most beneficial among the other metal-centres based salen complexes in terms of CO2/epoxide copolymerisation. More than 30 copolymerisation runs have been accomplished under the effect of different epoxides, catalysts, solvents and different reaction conditions.
Overall, the results show that no specific relation can be drawn regarding whether a single catalyst demonstrates the optimum polymer yield through the different epoxide/CO2 copolymerisation; as for a certain epoxide, each catalyst exhibits different solubility drifts under the effect of the same solvent. For instance, complex 3 resulted in the highest yields for both styrene oxide and propylene oxide polymerisation, whereas complex 1 is more favorable for the n-hexane one. A wide range of low and high polymer yields has been observed from 12.2% (utilising styrene epoxide and complex 3) to 96.9% (utilising cyclohexene epoxide and complex 6). No/traceable amounts of cyclic carbonate have been detected in the final product after micro filtration; the case that supports Zn-based catalysts selectivity trend towards the production of polycarbonates over cyclic carbonates.
Keywords
Carbon dioxide; Poly-carbonate; Salen; Zn-based catalyst; Environment pollution; Light polymers; NMR
Introduction
Project overview
Nowadays, fossil fuel combustion (coal, oil and natural gas) is regarded as the main source of energy generation; furthermore, experts forecast that this approach might remain the dominate line for the next few decades, even though substitute bases are being established. On the other hand, the main downside behind the practice of fossil fuels combustion is the production of large amounts of carbon dioxide emissions. Statistics refer to the industrial revolution as the main reason behind the shape increases in carbon dioxide (CO2) levels in the atmosphere. As a consequence of elevated CO2 amounts in the atmosphere, climate is changing and sun-heat is being trapped in the environment surrounding us. With the intention of protecting the environment, thus life on earth, carbon capture and storage (CCS) approach has been introduced, where CO2 is separated from the flue gas, being purified, pressurized, and transported to be stored either underground and undersea for long term (which might be subjected to leakage possibility, long-term liability issues, for instance), or as a profitable industrial compound [1].
Carbon dioxide has been able to draw industrials attention not only because of its wide availability, but also for being nontoxic and distinctive renewable resource, as well as for being highly flexible in its nature; all these properties have promoted CO2 to be utilised in various industrial applications, although it is in not highly thermodynamically stabile [2]. Carbon dioxide can be utilised to form polycarbonates by reacting with different compounds, like epoxide, alcohol and others. North [3] indicates that CO2 polymerisation has been widely commercialized in the UK, USA and Germany in the latest years.
The reason after utilising CO2 in polycarbonates production is that polycarbonates are strong physically, light in weight, expected to last for a long time without breaking, transparent, heat resistant, biodegradable and good electrical insulation; besides they can be easily processed and coloured [2]. Therefore, polycarbonates are widely used in electronics, optical media, the automotive industry, the medical industries, and many others. Polymers can hold up to 47% weight CO2, thus a reduction of 0.173 tonne in CO2 emissions is achievable when producing 1 tonne of polycarbonate [4].
Carbon dioxide/epoxide copolymerization was discovered few decades ago. The initial catalytic system used was based on heterogeneous zinc catalyst, advanced experimental work have also utilised different metal catalysts like nickel, and furthermore various modifications have also been carried out for each metal-based catalyst [5]. Catalyst-based reactions offer the aids of reduced reagent consumption and by-product and waste formation, along with speed and selectivity [6].
Aims and objectives
The technical review paper also aimed to shed some light on the effect of different epoxides for the polymerisation part of the project. For instance, CO2 polymerisation with various epoxides, alcohol and different alkenes has been deliberated. It is also worth saying that researchers have utilised different catalysts and reaction conditions for the various epoxides types and even for the same epoxide type, resulting in a huge multi direction matrix.
In a group of four, efforts have been put to the issue of the CO2 cycle in the atmosphere, and the possible methods to diverge towards decreasing CO2 levels in the atmosphere via capture and utilization as a valuable row product in polymers syntheses. Exploration relating to the design of the capture unit has been carried out by one of the group members, where another has produced and investigated the capture adsorbing agents (different types of ionic liquids). Additionally, lab work has been held in the topic of utilising CO2 to produce polymers. Two methods have been supplied: direct and indirect reactions of CO2 and Epoxide with the aid of metal complexes (Zn-based, aluminiumbased and tin-based catalysts) to produce cyclic and polycarbonate. Process simulation, specifically for the design of the capture unit, HNMR spectroscopy along with spin-works software programme for the catalyst analyses have been used as part of the work. Proton-NMR has also been used to test the polymers yield after the polymerisation reactions due to limit access to advance testing methods.
This paper is mainly based on experimental work that has been carried out in the laboratory. Three different Zn-based salen catalysts have been made, some of them have been previously synthesised with different metal centres, whereas the rest are novel. Laboratory experiments have also been accompanied examining different epoxide for the direct CO2/epoxide copolymerisation, where different sets of runs have been carried out for four different epoxides at different reaction conditions and time slots. Styrene oxide, n-hexane oxide, cyclohexene oxide and propylene oxide have been chosen as the substrates; the first 3 were selected for their high boiling points, which help polymerisation in the reactor, whereas the final was chosen as the polypropylene carbonate (PPC) product is commercially interesting. For the epoxide it was essential to have a high boiling point due to the fact that the copolymerisation reactions were carried out in Radleys carousel reactor rather than an autoclave. An autoclave is designed to such reactions and to withstand very high pressures, however the available Radleys carousel reactor was pressurized up to only 2 bars, considering safety limits. Investigating the economical aspect would have added more weight to the paper, in addition to the biodegradability and other properties of the polymers that couldn’t be explored due to the limited testing methods, such as mass and gas chromatography. The catalysts reactions and copolymerisation run sets are to be explained in the methodology section next in this paper, and the results to be discussed in the result and discussion section later on. Reactions schematic diagrams along with the operation conditions are provided where necessary. Appendices are further attached containing all the proton-NMR that has been made along with the polymerisation products NMR tests.
Experimental Methods
As copolymerisation reactions, catalysts synthesis has been a major part of this paper and the experimental work. Three different types (between previously established and novels) have been prepared. “Catalysis” was first used by Berzelius in 1836 to categorize a new item that has the ability to promote the occurrence of a chemical reaction by a “catalytic contact”. The catalyst was viewed as an entity that is added to the reaction to speed it (catalytic force) without being consumed or produced in the process. However, with the beginning of the twentieth century, catalysis started to play a major influence on the chemical industry; where currently more than 95% of chemicals are being produced with the aid of one catalytic step as a minimum. In addition, in the 1990s, the US market has reported more than 130 examples of new catalysts or catalyst improvements for operational processes, signifying the crucial role of the catalytic technology to many industrial processes [7].
By tradition, catalysts were classified into homogeneous and heterogeneous; heterogenized catalysts were consequently introduced. This division is associated to the fact that some catalysts operate respectively in the same phase in which the reaction occurs (homogeneous catalysts), whereas others operate in a different phase (heterogeneous or heterogenized catalysts). In principle, most of the processes utilising homogeneous catalysts take place in a liquid phase while for the heterogeneous catalysts, the catalyst is usually solid, and the reaction may occur either in the liquid or gaseous phase. The main difference between the two is the fact that every single homogeneous catalyst can act as a single active site, which makes it naturally more active and selective in comparison with traditional heterogeneous catalysts [7].
In order to determine the catalyst unique structure, as well as the polymerisation product composition, NMR or nuclear magnetic resonance spectroscopy has been used. It sets apart the carbonhydrogen context of an organic compound (in this case, the ligand and metal complexes). The working mechanism of the NMR is similar to that of the infrared and mass spectrometry, it drives the atoms to spin all in the same direction and records peaks where specific atoms are present in the path of flow, via these peaks experts are able to determine the complete structure of a compound. H-NMR or proton magnetic resonance in particular has been used; it is the first and the most popular type of the nuclear magnetic resonance spectroscopies. Different types of solvents are usually used to dissolve the samples prior to testing in the NMR, for this experimental work Deuterated chloroform (CDCl3) and Dimethyl sulfoxide (DMSO) have been used [8].
Metal complexes synthesis paths are to be explained by details with diagrams and H-NMR results, followed by the copolymerisation reactions, again with schematic diagrams and tests results.
Metal complexes preparation
4-[(2-aminoethyl)imino]-2-Penten-2-ol ligand
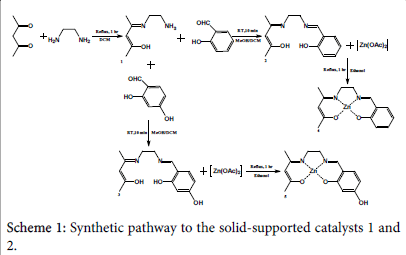
A solution of access 1,2-diaminoethane (2.57 g, 40 mmol) in 8 cm3 dichloromethane has been added into a solution of 3.66 g (37 mmol) 2,4-pentanedione in 8 cm3 dichloromethane (Scheme 1). The mixture has then been heated at reflux with continuous stirring for 1 hour. Evaporation under vacuum and 60°C has been done in order to remove the access amine. The final product has been tested using NMR, the following result has been obtained: δH [(CDCl3); 400 MHz] 10.8 (1H ,s, -OH), 4.9 (1H, s, -CH), 3.25 (2H, m, CH2), 2.8 (2H, m, - CH2), 1.9 (3H, s, -CH3), 1.8 (3H, d, CH3), 1.4 (2H, s, -NH2). The ligand resulted amount (5.254 g, 37 mmol) has been used once with salicylaldehyde and once with 2,4-dihydroxybenzaldehyde in order to produce 2 different types (4 and 5) of Zn-based complexes.
4-[(2-aminoethyl)imino]-2-Penten-2-ol ligand
A mixture of 2.73 g (17 mmol) C7H14N2O ligand in 16 cm3 dichloromethane has been reacted with a solution of 2.0 g (16 mmol) salicylaldehyde in 8 cm3 methanol and further 8 cm3 dichloromethane (Scheme 1). The reaction has been carried out at room temperature for 10 minutes with stirring. Vacuum evaporation has then been subjected to remove the solvents. An amount of 4.2 g (17 mmol) C14H18N2O2 ligand has been produced, and the H-NMR test revealed the following: δH [(CDCl3); 400 MHz] 13.0 (1H, s, -OH), 10.9 (1H, s, -OH), 8.33 (1H, s, -CH), 7.25 (3H, m, -CH), 6.9 (3H, m, -CH2), 5.0 (1H, d, -CH), 3.75 (2H, m, -CH2), 3.6 (2H, m, -CH2), 2.0 (3H, d, -CH3), 1.85 (3H, s, - CH3).
2-[[[2-[(3-hydroxy-1-methyl-2-buten-1-ylidene) amino] ethyl] imino] methyl]-Phenol ligand
The second half of C7H14N2O ligand (2.52 g, 18 mmol) has been mixed with 18 cm3 dichloromethane and into it was added a solution of 2.0 g (14 mmol) of 2,4-dihydroxybenzaldehyde in 8 cm3 methane and 8 cm3 dichloromethane (Scheme 1). The mixture has been stirred at room temperature for 10 minutes, and then the solvents have been removed via vacuum evaporation. An amount of 1.11 g (4 mmol) product has been yielded. H-NMR test has been carried out over a sample, however no clear result has been provided due to the fact that the sample did not dissolve in the testing solvent.
[Zn(salenac)] (metal complex one)
Similar number of moles of C14H18N2O2 ligand (4.20 g, 20 mmol) and zinc acetate tetrahydrate (3.73 g, 2 mmol) in 30 cm3 ethanol have been mixed together and stirred under reflux for 1 hour (Scheme 1). The resulted light-orange powder (2.3 g, 7 mmol) has been collected and stored for the second experimental step (CO2/epoxide copolymerisation). H-NMR test has revealed the following: δH [(CDCl3); 400 MHz] 8.3 (1H, s, -CH), 7.2 (1H, t, -CH=N), 7.15 (1H, t, -CH), 6.95 (1H, m, -CH), 6.65 (1H, t, -CH), 3.9 (2H, s, N-CH2), 3.35 (2H, m, N-CH2), 3.25 (3H, s, -CH3), 2.1 (1H, s, -CH), 2.0 (3H, s, - CH3).
[Zn(salenac-OH)] (metal complex two)
The C14H18N2O3 ligand (1.11 g, 4 mmol) has been mixed with similar amount of zinc acetate tetrahydrate (0.92 g, 4 mmol) in 30 cm3 ethanol at reflux for 1 hour (Scheme 1). The yielded complex (lightpink powder) (1.41 g, 4 mmol) has been collected and the residual has been evaporated from solvents and stored for future utilisation. HNMR test has indicated the following: δH [(DMSO); 400 MHz] 9.4 (1H, s, -OH), 8.25 (1H, s, -CH=N), 7.15 (1H, t, -CH), 6.9 (1H, d, -CH), 6.0 (1H, s, -CH), 5.95 (1H, d, -CH), 4.4 (2H, t, N-CH2), 3.6 (2H, s, NCH2), 3.5 (6H, m, 2x-CH3), 2.1 (1H, s, -CH).
2,2'-[1,2-ethanediylbis(nitrilomethylidyne)]bis[5-methoxy- Phenol ligand
A solution of 2-hydroxy-4-methoxybenzaldehyde (0.6 g, 4 mmol) in 5 cm3 methanol was added to a solution of 1,2-diaminoethane (0.13 g, 2 mmol) in 5 cm3 methanol. The mixture was stirred at room temperature for 30 min (Scheme 2). An amount of 0.186 g (0.6 mmol) ligand has been obtained. In order to dissolve the solid precipitate, 5 cm3 of CH2CL2 has been added. H-NMR test has shown the following: δH [(CDCl3); 400 MHz] 3.83 (6H, s, 2x-OCH3), 3.9 (4H, s, - CH2CH2-), 6.43 (2H, m, 2x-CH), 6.45 (2H, m, 2x-CH), 7.1 (1H, s, - CH), 7.15 (1H, s, -CH), 8.25 (2H, s, 2x-N=CH-), 13.8 (2H, s, 2x-OH).
[Zn(salenac-OMe)] (metal complex 3)
A solution of zinc acetate (0.12 g, 0.57 mmol) in 5 cm3 methanol was added into the C18H20N2O4 ligand and CH2CL2 solution and the resulted mixture was stirred overnight at room temperature (Scheme 2). The mixture was cooled and the produced solid was collected to yield yellow crystals (0.13 g, 0.3 mmol), the residual crop has been stored for future supplies. H-NMR test has presented the following: δH [(DMSO); 400 MHz] 3.65 (4H, s, 2x-CH2), 3.73 (6H, s, 2x-CH3), 6.1 (2H, m, 2x-CH), 6.15 (2H, s, 2x-CH), 7.02 (1H, s, -CH), 7.04 (1H, s, - CH), 8.3 (2H, s, 2x N=CH-).
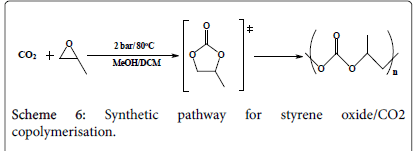
CO2/epoxide polymerization
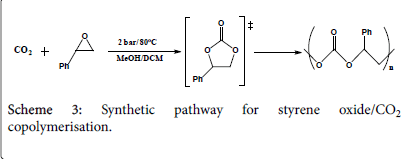
The first run: Three similar mixtures (Scheme 3) of 0.12 g (1 mmol) styrene oxide, 0.115 g (1 mmol) mesitylene oxide, 0.11 g (0.03 mmol) tetrabutylammonium iodide and 2.5 cm3 dichloromethane each has been introduced into a Radley tube where to each a different metal complex has been added: 9.29 g metal complex one, 9.77 g metal complex two, and 11.75 g metal complex three. All the five tubes have been pressurized under the same conditions in the Radley carousel reactor with 1.75 bar CO2 under 85°C for 20 hours. Due to fact the tubes were not well sealed, dichloromethane has been evaporated half way through the reaction and therefore the run has been regarded as an error one.
The second run: Similar to the first run, three similar mixtures of 0.12 g (1 mmol) styrene oxide, 0.115 g (1 mmol) mesitylene oxide, and 0.11 g (0.03 mmol) tetrabutylammonium iodide each has been introduced into a Radley tube where to each a different metal complex has been added: 9.29 g metal complex one, 9.77 g metal complex two, and 11.75 g metal complex three. All the three tubes have been pressurized under the same conditions in the Radley carousel reactor but well-sealed with 1 bar CO2 under 87°C for 24 hours.
The third run: Due to the fact that the Radley carousel reactor provided cannot reach high pressures and temperatures; different procedure has been verified. Atmospheric pressure CO2 has been introduced into two Radley tubes; each contains a solution of 0.24 g (2 mmol) styrene oxide in 2 cm3 DMSO, but with different catalysts; one with 62 mg (0.2 mmol) metal complex one and the other with 65 mg (0.2 mmol) metal complex two. Both tubes have been pressurized under 60oC for three hours.
The fourth run: Similar to the third run, 0.24 g (2 mmol) styrene oxide in 2 cm3 DMSO has once been mixed with 62 mg (0.2 mmol) metal complex one and the other with 65 mg (0.2 mmol) metal complex two into different Radley tubes. Both tubes have been pressurized with 1 atmosphere CO2 under 60°C over weekend, hopefully to reach a high yield conversion into polycarbonate.
The fifth run: A mixture of 0.24 g (2 mmol) styrene oxide in 2 cm3 methanol has been equipped in three Radley tubes, a catalyst has been added into each; 0.062 g of complex one, 0.065 g of complex two, and 0.07 g of complex three respectively. All the three tubes have been pressurized under 2 bar CO2 and stirred under 80°C for 3 hours. The products have been micro filtered (using silica gel and dichloromethane) and evaporated under vacuum and collected in small containers for testing. The experimental procedure is more likely to produce cyclic carbonates rather than poly carbonates where less work has been conducted towards styrene oxide polymerisation; however the reaction time has been extended from 2 hours to 3 hours hoping for the production of polymers. The results are to be discussed later in this paper.
The sixth run: Similar to the previous run, a mixture of 0.24 g (2 mmol) styrene oxide in 2 cm3 dichloromethane instead of methanol has been equipped in three Radley tubes, a catalyst has been added into each; 0.062 g of complex one, 0.065 g of complex two, and 0.07 g of complex three respectively. All the three tubes have been pressurized under 2 bar CO2 and stirred under 80°C for 5 hours instead of 3. The products have been micro filtered and evaporated under vacuum and collected in small containers for testing. The experimental procedure is more likely to produce cyclic carbonates rather than poly carbonates where less work has been conducted towards styrene oxide polymerisation; however the reaction time has been extended from 2 hours to 3 hours hoping for the production of polymers. The results are to be discussed later in this paper.
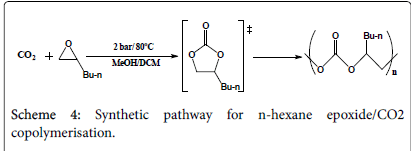
CO2/n-hexane epoxide polymerisation
A mixture of 0.2 g (2 mmol) n-hexane oxide in 2 cm3 methanol has been equipped in three Radleys tubes, a catalyst has been added into each; 0.062 g of complex one, 0.065 g of complex two, and 0.07 g of complex three respectively. All the three tubes have been pressurized under 2 bar pressure CO2 and stirred under 80°C for 3 hours (Scheme 4). The products have been micro filtered and evaporated under vacuum and collected in small containers for testing. The experimental procedure is more likely to produce cyclic carbonates rather than poly carbonates where less work has been conducted towards styrene oxide polymerisation; however the reaction time has been extended from 1.5 hours to 3 hours hoping for the production of polymers. The results are to be discussed later in this paper.
The run has been repeated with the same amounts and the same conditions, however dichloromethane has been used instead of methanol and the reaction has been carried out for 5 hours instead of 3.
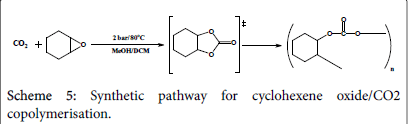
CO2/cyclo-hexane epoxide polymerization
A mixture of 0.196 g (2 mmol) n-hexane oxide in 2 cm3 methanol has been equipped in three Radley tubes, a catalyst has been added into each; 0.062 g of complex one, 0.065 g of complex two, and 0.07 g of complex three respectively. All the three tubes have been pressurized under 2 bar CO2 and stirred under 80°C for 3 hours (Scheme 5). The products have been micro filtered and evaporated under vacuum and collected in small containers for testing. The experimental procedure is more likely to produce cyclic carbonates rather than poly carbonates where less work has been conducted towards styrene oxide polymerisation; however the reaction time has been extended from 2.5 hours to 3 hours hoping for the production of polymers. The results are to be discussed later in this paper.
Again, the run has been repeated with the same amounts and the same conditions, however dichloromethane has been used instead of methanol and the reaction has been carried out for 5 hours instead of 3.
CO2/propylene epoxide polymerization
A mixture of 0.2 g (2 mmol) n-hexane oxide in 2 cm3 methanol has been equipped in three Radley tubes, a catalyst has been added into each; 0.062 g of complex one, 0.065 g of complex two, and 0.07 g of complex three respectively. All the three tubes have been pressurized under 1 bar CO2 and stirred under 80°C for 3 hours (Scheme 6). The products have been micro filtered and evaporated under vacuum and collected in small containers for testing. The experimental procedure is more likely to produce cyclic carbonates rather than poly carbonates where less work has been conducted towards styrene oxide polymerisation; however the reaction time has been extended from 2 hours to 3 hours hoping for the production of polymers. The results are to be discussed later in this paper.
The final run to carry out was to repeat with the same amounts and the same conditions, however dichloromethane has been used instead of methanol and the reaction has been carried out for 5 hours instead of 3. In general, the produced polymers will contain between 27-43% CO2 by weight.
Results
Zn-based metal complexes synthesis results
The proton-NMR test results have been already indicated in the experimental section corresponding to the reactions. The produced catalysts structures, properties and yields are listed in Table 1.
| Catalyst | Chemical structure | Colour | Yield (%) |
|---|---|---|---|
| Complex 1 | 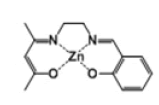 |
Dark-yellow powder | 40 |
| Complex 2 | 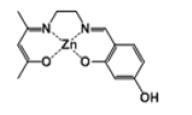 |
Light-pink powder | 25 |
| Complex 3 |  |
Light-yellow powder | 16.4 |
Table 1:Synthesizedcatalysts properties.
The complexes have shown different solubility trends; ligands for complexes 1 and 2 along with complex 2 show high solubility trends in CDCL3 and DMSO, however complex 1 in DMSO provided poor peaks to be calibrated, thus difficult to be integrated. Complex 3, on the other hand, dissolved well in DMSO.
Polymerisation reactions results
No traceable amounts of styrene carbonate have been detected, as well as small amounts of the starting material have been seen. Calculations regarding the polycarbonate amounts have been carried out and the results are presented in the following Table 2:
| Entry | Temp (├?┬░C) | Catalyst | Solvent | Press. (bar) | Reaction time (hr) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 60 | 1 | MeOH | 2 | 3 | 77.0 |
| 2 | 60 | 2 | MeOH | 2 | 3 | 22.0 |
| 3 | 60 | 3 | MeOH | 2 | 3 | 85.4 |
Table 2: Styrene oxide/CO2 copolymerisation in methanol conditions and yields percentages.
| Entry | Temp (├?┬░C) | Catalyst | Solvent | Press. (bar) | Reaction time (hr) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 70 | 1 | DCM | 2 | 5 | 0.0 |
| 2 | 70 | 2 | DCM | 2 | 5 | 22.2 |
| 3 | 70 | 3 | DCM | 2 | 5 | 12.2 |
Table 3: Styrene oxide/CO2 copolymerisation in DCM conditions and yields percentages.
N-hexane epoxide/CO2 copolymerisation has been also carried out in two sets, first with methanol (Table 4), and again with DCM (however as Tables 3 and 4 show that the reaction with methanol is more effective than with DCM, thus only the methanol samples have been tested with proton-NMR. No traceable amounts of n-hexane carbonate have been noticed, in addition to small amounts of the starting materials. Calculations regarding the polycarbonate amounts have been carried out and the results are presented in the following table:
| Entry | Temp (├?┬░C) | Catalyst | Solvent | Press. (bar) | Reaction time (hr) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 80 | 1 | MeOH | 2 | 3 | +95.0 |
| 2 | 80 | 2 | MeOH | 2 | 3 | 67.7 |
| 3 | 80 | 3 | MeOH | 2 | 3 | 17.4 |
Table 4: N-hexane oxide/CO2 copolymerisation in methanol conditions and yields percentages.
Similarly, cyclohexene epoxide/CO2 copolymerisation has been carried out in two sets, first with methanol (Table 5), and again with DCM (for the same reason as Tables 3 and 4 indicate, the reaction with methanol seems to be more effective than with DCM, therefore only the methanol samples have been tested with proton-NMR. No cyclohexene carbonate has been noticed, and small amounts of the starting materials have been seen. Calculations regarding the polycarbonate amounts have been carried out and the results are presented in the following table:
| Entry | Temp (├?┬░C) | Catalyst | Solvent | Press. (bar) | Reaction time (hr) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 80 | 1 | MeOH | 2 | 3 | 88.2 |
| 2 | 80 | 2 | MeOH | 2 | 3 | 92.3 |
| 3 | 80 | 3 | MeOH | 2 | 3 | 90.9 |
Table 5: Cyclohexene oxide/CO2 copolymerisation in methanol conditions and yields percentages.
Last of all, propylene epoxide/CO2 copolymerisation has been carried out, again in two sets, first with methanol (Table 6), and DCM (for the same reason as Tables 3 and 4 indicate, the reaction with methanol seems to be more effective than with DCM, therefore only the methanol samples have been tested with proton-NMR. Large methanol peaks have been clearly noticed in this run due to poor vacuum evaporation, and for the reason that the reactions have not been carried out in an autoclave but in Radleys reactor, and due to the low boiling point of propylene oxide, most of the starting material is believed to be evaporated during the reaction period, which makes it difficult to make a clear cut of how much has actually reacted and how much have been evaporated. However, no/traceable amounts of propylene carbonate have been noted, and small amounts of the starting materials have been also remarked. Calculations regarding the polycarbonate amounts have been carried out and the results are presented in the following Table 6:
| Entry | Temp (├?┬░C) | Catalyst | Solvent | Press. (bar) | Reaction time (hr) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 80 | 1 | MeOH | 2 | 3 | 37.3 |
| 2 | 80 | 2 | MeOH | 2 | 3 | 32.8 |
| 3 | 80 | 3 | MeOH | 2 | 3 | 73.5 |
Table 6: Propylene oxide/CO2 copolymerisation in methanol conditions and yields percentages.
Discussion
Catalysts synthesis
Nowadays, homogeneous catalysts synthesis is rapidly developing, where many sophisticated ligand systems are being conveyed, the case that adds functionality and selectivity to the central metal of the catalytic process. The instance catalyst, however, is very rarely recovered from the reaction mixture as indicated earlier; this bounds the industrial sustainability of homogeneous catalysts on the basis of decontamination in addition to the financial side. All the three salentype catalysts that have been synthesized for this project are homogenous ones. The salen-type complexes were selected and preferred to be set because of their simple structures, resulting in an efficient, straightforward and economical method for the synthesis of base ligands and their following alteration metal complexes. Phan et al. [6] reported the same ligands as 1-3 complexes, however they have finalized with nickel and aluminium metals, while the later uses zinc. Costes [9] reported different ligands similar to the ligands of complexes 1-3.
Complex 1 and 2 are quite similar where both have been prepared using the same row materials (1,2-diaminoethane and 2,4- pentanedione), however the first proceeded with salicylaldehyde while the later proceeded with 2-hydroxybenzaldehyde. Due to that difference in the middle step two different ligands, hence two different salen-metal complexes have been produced. It worth to mention the earlier is novel, whereas the second is previously reported by Phan et al. [6] but with different metal centers (Ni and Pb rather than Zn). Proton-NMR test results show trace amounts of impurities in both, yet a clear picture can be drawn. It can be noticed that the aromatic protons for the first complex are shifted farther than the second one; they lie between 6.4 – 8.45 for the complex 1, while they are between 5.9 and 8.3 for complex 2, and that is because the change in the chemical shift of the aromatic peaks indicates a change in electron density; the presence of electronegative elements reduces the electron density around the adjacent peaks. As the magnet field suck away all the electrons; the peak shifts downfield (and the shift number goes up), whereas the number goes down indicating that the electron density has increased and the peak has been shifted up field. The higher the electron density the lower is the number. That is the reason behind the low CH3 groups’ shift; as they are on the end of the chain outlying from any electronegative elements.
Both have been found to dissolve easily in DMSO than DCM, furthermore both dissolve in acetone at high temperatures. Complex 3, on the other hand, is the only symmetric one among the six complexes. Complex 3 with different metal center has been reported by Phan et al. [6].
The three different complexes exhibited different performances as the copolymerisation test has showed, however it is worth mentioning that an error of about 5% more or less might have occur due to many reasons. First of all, it is common for copolymerisation reactions to be held in an autoclave where properly sealed environment is initiated where loss in the materials amounts is seldom; especially for the volatile materials with low boiling points like propylene oxide (with a boiling point of 34°C). It can be clearly noticed that the yield percentages for poly (propylene carbonates are the lowest among the other polymers (to be discussed in the coming section).
Secondly, the maximum pressure Radleys curasel reactors can hold in the laboratory conditions provided to carry out the experimental work was 2 bar (within safety limits), while reaction in an autoclave can hold much higher pressures, there for the whole work has to be done under low pressure comparing with the previous work done by literatures and researchers. On the other hand, there is also a chance of the samples being contaminated in any stage over the whole process from synthesizing the catalysts themselves, up to the copolymerisation reactions, ending with the polymers sampling.
Copolymerisation reactions
The type of catalyst used in a polymerisation reaction can highly affect the reaction path; hence the product type (selectivity) and product yield. The selectivity to cyclic carbonates or polycarbonates depends heavily on the type of the catalyst used in the formation reaction. On the other hand, additives, the reaction conditions of temperature and pressure, in addition to the epoxide concentration also have a significant role on the process selectivity [2].
Concerning catalysts, the copolymerisation process is predominantly to be carried out catalysed by salen-based metal complexes. According to Supasitmongkol et al. [10], co-catalysts have a considerable influence on the reaction path as well; this to be discussed in yields percentages later in this chapter.
Diverse methods of comparison can be drawn regarding the catalysts performance and the polymer yield percentages. Firstly, comparison is to be held among the catalysts of the same copolymerisation reaction, followed by an overall evaluation concerning the effect of each catalyst on the different processes. Then, comparison with literature is to be provided. Table 7 summaries the yields structures and percentages.
To start with the first set of results, styrene oxide/CO2 polymerisation with methanol seems to have high polymer yields between 72-85.4%, however for the synthesis with complex 2 only 22% have been observed, it could be either the catalyst does not perform well in such conditions in which the reaction has been carried out in, or some contamination has occur resulting in only small amount of polymers to be detected by proton-NMR test. The symmetric complex 3 resulted with the highest yield; complexes 1 and 3 have positioned themselves in the second and third place recording yields of 77 and 74% respectively. On the other hand, when the reaction has been repeated with DCM dissimilar results have been noted; considerably low yields has been gained (below 25%) utilising all the catalysts each a time. Complexes 1 and 3 resulted with zero percent yields; furthermore surprisingly complex 3 recorded the lowest among the rest four forming only 12% yield polymers. For the reason of these very low yields, the rest of the DCM samples have been discarded, predicting low catalyst solubility in this particular solvent. A credit can be given to these reactions; although high amount of papers and research show that for such reaction composition and conditions the trend is usually towards the formation of cyclic carbonate, none of the reactions utilising each of the three complexes has resulted in cyclic carbonate. This can be regarded to the fact that most of the complexes are novel and none has been utilised in such reactions, in addition it can be stated that the Zn-metal salen-based complexes tendencies are selective towards poly carbonate rather than the cyclic ones.
In the concept of n-hexane epoxide/CO2 copolymerisation reactions demonstrate a wide range of polymer yields; complexes 1 and 2 come first with polymer yields of up to 95% and 67.7% respectively. Whereas the other four complexes demonstrated low yields in comparison with the first two; 17.4, 15.3, 33.6 and 12.2% have been detected for complex 3. Comparing to the polymerisation with styrene oxide, no specific relation can be drawn. Again, no cyclic carbonate has been detected in the product, the incident that supports the fact of Zn-based catalysts selectivity trends. The wide range of low and high polymer yields can be contributed to samples being contaminated or other errors regarding the catalysts solubility; where it is clear that for a certain epoxide, each catalyst exhibits different solubility drifts under the effect of the same solvent, and that can be strongly related to the fact that half the complexes are supported with a ring structure which makes them more stable, hence less flexible and less reactive.
In general, yields have been observed from the three complexes resulting in 88.2, 92.3, 90.9, 88.4 and 92.6% polymers for complexes 1, 2 and 3 respectively. A minimum of 88.2% can still be counted as a high polymer yield, where again cyclic carbonate is the product for other salen metal centres complexes.
Lastly, propylene epoxide and due to its low boiling point and the fact that Radley caurasel reactor has been used instead of an autoclave, generally low polymer yields have been noted. The major reason for such low yields can be contributed to the fact that the reactions have been carried out in a poor sealing condition where most of the propylene oxide has evaporated leaving poor amount to be converted into polymers. Nevertheless, complex 3 has performed more effective formation reaction than the others with polymer yield of 73.5%, whereas the others recorded below 50% yields; 37.3% by complex 1, and 32.8% by complex 2.
| Entry | Substrate | Product | Time (hr) | Yield (%) |
|---|---|---|---|---|
| 1 | 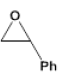 |
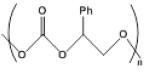 |
3 | 22.0 - 85.4 |
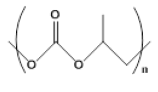
| 2 | 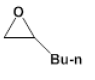 |
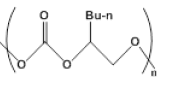 |
3 | 12.2 - +95.0 |
| 3 | 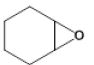 |
 |
3 | 88.2 - 96.9 |
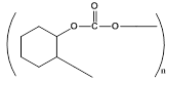
| 4 | 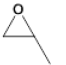 |
 |
3 | 29.41- 48.1 |
Table 7: Substrate/CO2 copolymerisation yields structures and percentages.
In summary, no single catalyst seems to dominate all over the four epoxides. Although catalyst 1 has performed the most effective reaction regarding n-hexane epoxide/CO2 polymerisation, it was less effective in the others. Complex 2 had different yield ranges and none yielded the optimum in any of the four processes. On the other side, complex 3 performed the best twice, once with styrene oxide and the other with propylene oxide polymerisation.
In similar conditions to the synthesis of complex 2, Supasitmongkol et al. [10] have synthesised AlCl(salenac)OH complex corresponding the Zn(salenac)OH complex, and has managed to use it in styrene oxide/CO2 copolymerisation reaction. Supasitmongkol et al. [10] report different reaction conditions for utilising the AlCl(salenac)OH complex in the copolymerisation reactions, a set with a co-catalyst and another without. From the data they provide, it can be concluded that the higher the reaction temperature and co-catalyst amount, the higher the reaction yield. By comparing with the closest reaction conditions to the experimental work that have been conducted utilising Zn(salenac)OH (80°C without co-catalyst for the AlCl(salenac)OH complex, and 70°C without co-catalyst for the later) it can be settled that AlCl(salenac)OH complex (in DCM) results with 48% yield cyclic carbonate whereas Zn(salenac)OH shows no clear results when carrying out the reaction with methanol, however it reported 22% yield polystyrene carbonate while conducting with DCM with much shorter reaction period. From the above comparison it can be stated that Zn(salenac)OH has more tendency towards forming polycarbonates than cyclic carbonates.
Cuesta-Aluja et al. [11] conveyed the synthesis of cyclic carbonate by different epoxides with CO2 utilising symmetric Cr(III) complexes without the aid of co-catalyst. The epoxide conversion and yield in cyclic carbonate were low (19–50% epoxide conversion and up to 15% cyclic carbonate yield). Comparing with the complex 3 (both Cr(III) and complex 3 are symmetric, yet not identical in structure, Cr(III) complexes reported only cyclic carbonates under 100°C, 170 bar, for 30 min. Due to the fact all the experimental work has been performed in Radelys curasel reactor and not an autoclave, 2 bar was the maximum CO2 pressure that can be reached accounting safety limits. Nonetheless, complex 3 reported between 15.3% (poly-hexane carbonates) and 90.9% (poly (cyclohexene carbonates) yield polymers. Again, Zn-salen complexes show more selectivity towards polycarbonate than cyclic carbonates, than Cr(III) complexes.
On the other hand, epoxide/CO2 copolymerisation by aluminum based catalysts along with tetrabutylammonium bromide has also been reported. Beattie et al. [12] conclude that the combination of the catalyst and co-catalyst in comparison to the catalyst alone results in an order of magnitude enhancement in over a temperature range of 25−100°C and a pressure range of 1−10 bar, where twenty-fold increase in the catalyst activity has been noticed. Furthermore, the higher the temperatures and pressures, the higher the catalytic activities seen towards the formation of cyclic carbonates.
Kumar et al. [13] have utilised similar amount of catalyst and epoxide for the polymerisation of CO2 with different epoxides as the conducted experimental work; however [K+ {PEG} Br−] complex has been used. The reaction conditions were close to the experimental conditions in the lab; stirring at 60°C for 4 hours. According to the literature, the product was extracted with diethyl ether, whereas micro filtration process has been applied to extract the produced polymers from the unreacted initial materials and catalyst. Utilising complex [K +{PEG}Br−] resulted in a high epoxide conversion percentages, hence high carbonate yields; from 94% cyclohexane and styrene cyclic carbonate and 96% yield n-hexane cyclic carbonates. Despite the fact that high yields have been obtained, the catalyst selectivity trends towards cyclic carbonate rather than polycarbonates. Similarly, Wei-Li et al. [14] reported the path of various catalysts where again all ended up producing cyclic carbonates; an example catalyst is [TMGC2H4NH2] Br.
Asymmetric, regio- and stereo selective alternating copolymerization of CO2 and various epoxides was the major focus of Lu et al. [15], it is revealed that the reactions proceed effectively under considerably low temperature and pressure via a binary catalyst system of a base cobalt complex [SalenCoIIIX] in combination with an ionic organic ammonium salt. According to Lu et al. [15], the main catalyst that has been utilised in the copolymerisation reaction is symmetric like complex 3 but with a hexane ring as complexes 4 and 5. In addition to different structures of extra bonds on the benzene rings on both sides, various configurations of SalcyCoIIIX complexes were synthesised. The copolymerisation reactions were performed in an autoclave at 25°C and a 1.5 MPa CO2 pressure. For reactions time periods between 1.5 – 2.0 hours, >99% polycarbonates selectivity were obtained from different epoxides, such as 1,2-BuO and cyclohexene oxide.
Much more intricate multi-chiral Co (III) complexes have been deliberated and conveyed by Ren et al. [16], completely stereoregular polycarbonate synthesis (polymers having a systematic arrangement of pendant groups lengthways the chain) was achieved with the use of unsymmetric multichiral cobaltbased complexes as catalyst for the copolymerization of aliphatic terminal epoxides/CO2 at moderately low pressures and temperatures. The Co (III) complex with sterically hindered substituent group was found to be more a stereoregular catalyst for the copolymerization of racemic propylene oxide/CO2; it afforded a perfectly regioregular poly (propylene carbonate) with >99% head-to-tail linkages and >99% carbonate linkages. The isotactic poly (propylene carbonate) reveals a heightened glass transition temperature of 47°C that is 10 – 12°C greater than that of the equivalent irregular polycarbonate. Although the reactions were conducted at 20°C, it required between 4 – 24 hours for them to be accomplished.
Copolymerization of cyclohexene oxide/CO2 in specific has been the main scope for Guo [17]; and for this particular reaction, a lysinebased (salen)CrIIICl catalyst was used. The novel natural lysine-based (salen)CrIIICl ((lys-salen)CrIIICl) complex was prepared and its catalytic activity for CO2/cyclohexene oxide copolymerization was described in combination with of a co-catalyst. The results indicated that the (lys-salen)CrIIICl complex could effectively catalyse the alternating copolymerization with >95% selectivity. Furthermore, it was found to be less sensitive to the temperature and the molar ratio of catalyst components, in comparison with that of the copolymerization catalysed by traditional salen–metal complexes. Nonetheless, and in contrast with 1-6 zn-based complexes and their unique role in the copolymerisation of CO2/cyclohexene; via proton-NMR test, no cyclic carbonate has been noticed in the product, the case that indicates complete selectivity towards polycarbonate formation; yet 88.2 - 96.9 polymer yield percentages was noted due to the existence of traces of the solvents (due to poor vacuum evaporation prior to NMR test) as well as traces of the starting materials (cyclohexene epoxide). Thus far, Cr-based salen complexes show the closest performance to Zn-based complexes; taking into consideration that no co-catalyst has been used in the experimental Zn-based salen copolymerisation reactions. In addition, higher pressure, temperature and reaction time period were applied for the symmetric (salen)CrIIICl catalyst. Likewise, cyclohexene oxide/CO2 copolymerisation has been explored by Xiao et al. [18]; variety of Mg (II) complexes was used. For 5 mol % catalyst at 60°C for 6 h, with/without additive, polymer yields in the range of 76 - >99% were obtained.
On the other hand, Fuchs et al. [19] investigated the catalytic possibilities of a first-hand aluminium complex relating a N2O2 ligand (amine and ester functions), less acknowledged than the well-known salen ligands (with phenol and aldimine functionalities). This new catalyst found to be very efficient for the reaction of epoxides with carbon dioxide. Fuchs et al. [19] also revealed that the use of a cocatalyst (an ionic co-catalyst in this case) is obligatory to get the most out of the system, although the halide anion nature appears to have no direct influence on the catalytic system efficiency. Depending on the chosen epoxides, polycarbonates/cyclic carbonates were found to be selectively isolated in extremely high yields. The poly (cyclohexene carbonate)s, for instance, were completely alternating; most remarkably, even under 2 bar pressure CO2, the screening tests result in completely alternating polycarbonates with 29% yield. In comparison, exploiting propylene oxide as substrate indicates selectively to 94% yields propylene carbonate.
A more sophisticated catalyst, bi-functional cobalt Salen complex containing a Lewis acid metal centre with two covalent bonded Lewis bases on the ligand, was designed and used by Li et al. [20] for the coupling of epoxide/CO2 under considerably low conditions. The complex exhibited exceptional and outstanding activity and >97% polymer formation selectivity in the copolymerization of propylene oxide (PO) and CO2 at a suitable combination of all variables. Yields of 99% were reached at higher epoxides to complex ratios, such as 6000:1.
Further investigation that has not been considered within this project is the terpolymerisation reaction; Li et al. [20] have utilised their co-based salen complex for the terpolymerization of CO2/PO/ cyclohexene oxide (CHO), where the complex was found to work satisfactorily without leading to the formation of cyclic carbonate or ether linkages to yield the polycarbonate. Both the experimental work that has been conducted utilising the six Zn-based complexes and Cosalen complex reported by Li et al. [20] indicate direct copolymerisation and terpolymerisation respectively. Moreover, Li et al. [20] noted that the higher the amount of cyclohexene carbonate content in the CO2/PO/CHO terpolymers, the more enhanced the thermal stability.
Conclusions
Carbon dioxide synthesis has made its own way in the growth of a new industrial lane. The syntheses of polymers from CO2 have been technologically advanced in the past few decades as a valuable, marketable method to minimise the increasing amounts of CO2 in the atmosphere. The copolymerisation of CO2 is found to be an efficient method to produce polycarbonate; polycarbonate has high impactresistance, low scratch-resistance, strong and usable over a great temperature range. It is also very transparent to visible light, and has better light transmission than many glass varieties, and most important is that polycarbonate can resist large plastic deformations without cracking or breaking. Firstly and as a major share of the project work, three homogenous Zn-based salen, mostly novel, catalysts have been formed. The first two complexes are quite similar in structure where both have been prepared using the same initial materials; however they performed differently in the copolymerisation reactions. Complex 3 is the only symmetric one among the six complexes. The three different complexes with different colours exhibited different acts during copolymerisation tests. The second major part of the work has been the copolymerisation reactions.
Few factors have played a major role in the products uncertainty; mainly is that it is typical for copolymerisation reactions to be held in an autoclave where properly sealed environment is initiated and no loss in the initial materials and solvents might occur; especially for the volatile materials with low boiling points like propylene oxide (with a boiling point of 34°C). It can be clearly noticed that the yield percentages for poly (propylene carbonates are the lowest among the other polymers. Furthermore, the maximum pressure Radleys curasel reactors can hold in the laboratory conditions provided to carry out the experimental work was 2 bar (counting safety limits), while reaction in an autoclave can hold much higher pressures. About 5% of error might have occurred where the samples might have been contaminated in any stage over the whole process.
Many sets of copolymerisation reactions of CO2 with four different epoxides have been carried out, the epoxides chosen were styrene oxide, cyclohexene oxide, n-hexane oxide and propylene oxide. The first three were picked for their high boiling points, which helps polymerisation in the reactor. The final was chosen as the polypropylene carbonate (PPC) product is commercially interesting. The different reactions were conducted under different reaction conditions for different time slots utilising different catalysts and different solvents. Styrene oxide/CO2 polymerisation with methanol revealed mostly high polymer yields (between 72-85.4%), however utilising complex 2 presented only 22% polymers, whereas symmetric complex 3 produced the highest. The same reaction, but with DCM as the solvent, showed considerably low yields (below 25%). Although high amount of papers and research demonstrate that for such reaction composition and conditions the trend is usually towards the formation of cyclic carbonate, none of experimental reactions has resulted in cyclic carbonate (according to the H-NMR test). With regard to nhexane epoxide/CO2 copolymerisation reactions, complexes 1 and 2 come first with polymer yields of up to 95% and 67.7% respectively, whereas the rest demonstrated low yields (between 12.2 and 33.6%).
Overall, high polymer yield is the case in cyclohexene/CO2 copolymerisation, where this time complex 6 targeted the highest polymer yield of 96%; again cyclic carbonate is the product for other salen metal centres complexes. Lastly, propylene epoxide and due to its low boiling point and the fact that the reactions have been carried out inside Radleys caurasel reactor, in the main low polymer yields have been noted. Nevertheless, complex 3 has performed more effective formation reaction than the others with polymer yield of 73.5%, whereas the rest recorded below 50% yields.
In summary, no single catalyst seems to dominate all over the four epoxides. Although catalyst 1 has performed the most effective reaction regarding n-hexane epoxide/CO2 polymerisation, it was less effective in the others. Complex 6 recorded a very high yield in the cyclohexene epoxide/CO2 polymerisation, whereas complex 2 had different yield ranges and none yielded the optimum in any of the four processes. On the other side, complex 3 performed the best twice, once with styrene oxide and the other with propylene oxide polymerisation.
No co-catalyst has been used over the reaction processes, unlike most of the literatures work. Moreover, and in comparison with previous work and research, the production of cyclic carbonates seem to dominate over the production of polycarbonates for different metalcentre salen complexes. However, Co-based salen complexes show a close performance to the Zn-based ones. It can also be concluded that no/traceable amounts of cyclic carbonate have been detected in the final product after micro filtration; the case that indicates the Zn-based catalysts selectivity trend towards the production of polycarbonates over cyclic carbonates. The wide range of low and high polymer yields can be contributed to samples being contaminated or other errors regarding the catalysts solubility and the produced polymers contain between 27-43% CO2 by weight. All the reaction products have been micro filtered and evaporated under vacuum, and then weights have been recorded. H-NMR test has been carried out for all the 30 products.
References
- Styring P, Jansen D (2011) Carbon capture and utilisation in the green economy using CO2 to manufacture fuel, chemicals and materials. CO2Chem Media & Publishing, UK, pp: 1-38.
- Miao CX, Wang JQ, He LN (2008) Catalytic processes for chemical conversion of carbon dioxide into cyclic carbonates and polycarbonates. The open organic chemistry journal 2: 68-82.
- North M (2012) Synthesis of cyclic carbonates from epoxides and carbon dioxide using bimetallic aluminium (salen) complexes. ARKIVOC 1: 610-628.
- Quadrelli EA, Centi G, Duplan JL, Perathoner S (2011) Carbon dioxide recycling: emerging large-scale technologies with industrial potential. ChemSusChem 4: 1194-1215.
- Yoo J, Na SJ, Park HC, Cyriac A, Lee BY (2010) Anion variation on a cobalt(III) complex of salen-type ligand tethered by four quaternary ammonium salts for CO2/epoxide copolymerization. Dalton Trans 39: 2622-2630.
- Phan NTS, Brown DH, Adams H, Spey SE, Styring P (2004) Solid-supported cross-coupling catalysts derived from homogeneous nickel and palladium coordination complexes. Dalton Trans 9: 1348-1357.
- Farnetti E, Di Monte R, Kaspar J (2012) Homogeneous and heterogeneous catalysis. Inorganic and bio-inorganic chemistry 2: 1-10.
- http://www.chem.ucla.edu/~harding/notes/notes_14C_nmr02.pdf
- Costes JP, Fernandez-Garcia MI (1988) Oxovanadium (IV) complexes of tetradentate unsymmetrical Schiff bases derived from 7-amino-4-methyl-5-aza-3-hepten-2-one. Transition Met Chem 13: 131-134.
- Supasitmongkol S, Styring P (2014) A single centre aluminium(III) catalyst and TBAB as an ionic organo-catalyst for the homogeneous catalytic synthesis of styrene carbonate. CatalSciTechnol 4: 1622-1630.
- Cuesta-Aluja L, Djoufak M, Aghmiz A, Rivasa R, Christ L, et al. (2014) Novel chromium (III) complexes with N4-donor ligands as catalystsfor the coupling of CO2and epoxides in supercritical CO2. J MolCatal A: Chem 381: 161-170.
- Beattie C, North M, Villuendas P, Young C (2013) Influence of temperature and pressure on cyclic carbonate synthesis catalyzed by bimetallic aluminum complexes and application to overall syn-Bis-hydroxylation of alkenes. J Org Chem 78: 419-426.
- Kumar S, Jain SL (2013) Polyethylene glycol wrapped potassium bromide assisted chemical fixation of carbon dioxide. IndEngChem Res 53: 541-546.
- Wei-Li D, Bi J, Sheng-Lian L, Xu-Biao L, Xin-Mana T, et al. (2013) Novel functionalized guanidinium ionic liquids: Efficient acid├ó┬?┬?base bi-functional catalysts for CO2 fixation with epoxides. J MolCatal A: Chem 378: 326-332.
- Lu X, Shi L, Wang Y, Zhang R, Zhang Y, et al. (2006) Design of highly active binary catalyst systems for CO2/ epoxide copolymerization: Polymer selectivity, enantioselectivity, and stereochemistry control. J Am ChemSoc 128: 1664-1674.
- Ren W, Liu Y, Wu G, Liu J, Lu X (2011) Stereoregular polycarbonate synthesis: alternating copolymerization of CO2 with aliphatic terminal epoxides catalyzed by multichiral cobalt(iii) complexes. Journal of polymer science part a: polymer chemistry, 49, 4894├ó┬?┬?4901.
- Guo L, Wang C, Zhao W, Li H, Sun W, et al. (2009) Copolymerization of CO2 and cyclohexene oxide using a lysine-based (salen) CrIIICl catalyst. Dalton Trans 27: 5406-5410.
- Xiao Y, Wang Z, Ding K (2006)Intramolecularlydinuclear magnesium complex catalyzed copolymerization of cyclohexene oxide with CO2 under ambient CO2 pressure: kinetics and mechanism. Macromolecules 39: 128-137.
- Fuchs MA, Altesleben C, Zevaco TA, Dinjus E (2013) An efficient homogeneous chloro├ó┬?┬?aluminum├ó┬?┬?[n2o2] catalyst for the coupling of epoxides with carbon dioxide. Eur J InorgChem 26: 4541-4545.
- Li H, Niu Y (2010)Bifunctional cobalt Salen complex: a highly selective catalyst for the coupling of CO2 and epoxides under mild conditions. ApplOrganometalChem 25: 424-428.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 14724
- [From(publication date):
June-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13570
- PDF downloads : 1154