Direct Paraffin Immunofluorescence: An Alternative for Renal Pathology Diagnosis
Received: 13-Aug-2021 / Accepted Date: 27-Aug-2021 / Published Date: 03-Sep-2021 DOI: 10.4172/2476-2024.s5.1000016
Abstract
The diagnosis of renal pathologies, especially glomerulopathies, requires a clinical suspicion and also the performance of a renal biopsy with its respective histopathological study. This includes light microscopy, electron microscopy and immunofluorescence; all these techniques help us to reach a conclusive diagnosis.
Renal immunofluorescence analysis is essential and not only shows the deposition of immunocomplexes, but also confirms the immunological mechanism involved in the disease.
The immunofluorescence technique on fresh, non-fixed, frozen kidney tissue (IF-F) has been the gold standard technique for more than 60 years; however, an alternative technique is immunofluorescence in fixed and paraffin- embedded sections (IF-P), which uses the principle of traditional immunohistochemistry, unmasking the antigens with heat or enzymatic digestion. IF-P has been successfully used as a salvage technique for renal biopsies and lately it is used as a valid technique for diagnosis, especially if the infrastructure, material, or fresh tissue is not available to carry out frozen sections.
The aim of this review is to learn about the handling of renal biopsies for immunofluorescence study, antigen retrieval methods and the usefulness of IF-P in the diagnosis of renal pathology.
Keywords: Kidney biopsy; Paraffin immunofluorescence; Antigen retrieval; Direct immunofluorescence; Kidney pathology
Introduction
In patients suffering from renal diseases, especially glomerulopathies, the objective of the nephrologist and the pathologist is to reach a definitive diagnosis, combining the clinical information and the complete histopathologic study [1,2].
Renal biopsy represents one of the most important advances in the field of nephrology [3,4].
Renal biopsies remain an irreplaceable tool, not only in the diagnosis of glomerulopathies, but also as a guide to treatment and in providing prognosis to patients [2,3,5].
This procedure is recommended for patients with persistent hematuria or proteinuria, with or without unexplained renal failure; it is a procedure that has been performed since 1961 [6].
The histomorphology study of the biopsy begins with the basic stains of hematoxylin eosin, PAS (Schiff’s periodic acid), Jones stain (silver methenamine) and trichrome, with which the morphologic pattern of the disease can be determined. However, there are other complementary techniques such as immunofluorescence that become indispensable in most cases, especially in patients in whom the histological pattern and the clinical pattern overlap, thus contributing to a more specific diagnosis [3,5,7].
Direct or indirect immunofluorescence is performed to detect the deposition of immunocomplexes on tissues; it is commonly used for the analysis of skin biopsies with bullous disease and of kidney tissue [8].
In the diagnosis of renal diseases, immunofluorescence has become necessary to verify that there is an immunological mechanism involved in their pathogenesis [1,3].
This is an indispensable technique in anatomic pathology departments; however, in many places the infrastructure and supplies to perform this technique are not available; access to it is limited by the cost, by the actual procedure of the biopsy, by the processing and technique factors, and finally, by the need for a microscope with fluorescent light for visualization [3].
Immunofluorescence in frozen sections (IF-F) requires adequate and rapid transport of the sample to the pathology laboratory, where a dissecting microscope is needed to assess the sufficiency of the biopsy (adequate number of glomeruli), and to section it for its various studies (light microscopy, electron microscopy and immunofluorescence).; then, a cryostat is needed for freezing and sectioning of the biopsy by technicians specialized in this type of sectioning, who later prepare the slide for its evaluation [9].
This is why IF-P is a good alternative to all these needs, by using an already fixed and paraffinized tissue block, which does not require the urgency of having the fresh sample, of counting glomeruli or of providing the freezing equipment. IF-P is a great technique when you do not have what is needed for IF-F, taking into account the limitations and benefits [10].
Renal Biopsy Handling for Immunofluorescence
Renal biopsy is a procedure that is not without risk and therefore the obtained material has to be handled with care [4]. Ideally, at least two cylinders of renal tissue of 1 cm in length and 1.2 mm in diameter should be sampled for biopsy [6].
It is usually better the sooner the sample arrives at the laboratory after collection; fresh tissue should be sent in gauze moistened with saline solution to avoid desiccation [7].
If the laboratory is located in another institution or another city, a preservation medium known as “Zeus medium” or “Michel’s solution” can be used, where the sample is preserved for up to 5 days at room temperature; this would help us to preserve the tissue antigens [8,9,11].
It should be emphasized that this solution is only for preserving and not for fixation. In addition, it is necessary to wash the sample with buffer solutions prior to processing. This preservation medium is not available in all countries or laboratories, which is a limitation for its use [8,11].
Once the biopsy is received, it must be subjected to stereomicroscopic or low magnification microscopic evaluation, where its viability will be determined. That is, at least 5 glomeruli to assess glomerular lesions, 6 to 10 for tubulointerstitial lesions, or 7 glomeruli for a transplant evaluation. It will then be split for immunofluorescence, electron microscopy and light microscopy [6,12].
The segment intended for light microscopy undergoes fixation with 10% buffered formalin (which is the best fixation medium for renal biopsies) for no longer than 48 hours [7,13]; It will later enter the process of paraffinization and block elaboration [9,14].
The segment allocated for immunofluorescence is frozen in a cryostat at -20°C to -35°C and sliced between 2 and 4 microns; then follow the process for IF-F, explained below.
It should be noted that if IF-P is performed, it is important not to exceed the fixation time, as this will cause artifacts when visualizing the IF-P [14-17].
It is necessary to have the paraffin block in order to be able to choose the IF-P technique. By performing IF-P the handling of the biopsy is simplified, since the sample is not divided and the same paraffin block can be used for immunofluorescence, light microscopy and even electron microscopy [14].
Finally, the slides will be analyzed by the pathologist under fluorescence microscopy [9].
Immunofluorescence Principles and Technique
The immunofluorescence technique is based on the specific binding of antigen and antibody [18]; with this technique it is possible to quantify and visualize this immunological reaction in any tissue [19].
This is done by marking the specific antibodies with a fluorochrome [20]; the most commonly used is FITC (fluorescein isocitrate), which absorbs UV light and is excited by emitting green visible light [19].
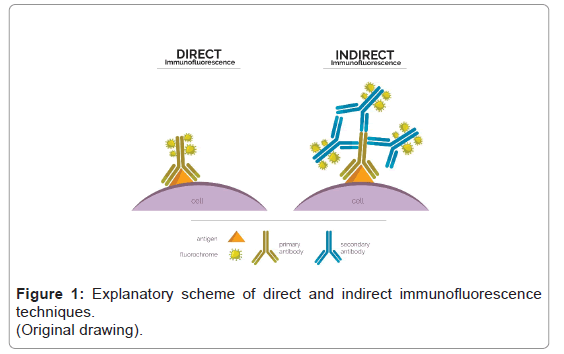
In direct immunofluorescence fluorochrome is directly conjugated to the primary antibody that reacts with the target epitope; whereas in indirect immunofluorescence two processes are involved: the first one where the antibody binds to the target epitope and the second one where the fluorochrome bound to a secondary antibody recognizes and binds to the primary antibody [20,21] (Figure 1).
In renal pathology, the direct method is preferred because it is faster and requires less equipment; however, the indirect technique is more sensitive and has a better amplification signal [21]. For the application of IF-P there is no advantage of one technique compared to the other [22].
The antibodies commonly deposited in most glomerulopathies and routinely used for the assessment of renal biopsies are IgG, IgM, IgA, C3, C1q, fibrinogen, kappa, and lambda; all are marked with fluorochrome [23].
The observation of the emitted fluorescent light requires the use of a fluorescence microscope, which is nothing more than a conventional optical microscope with a supplementary
Immunofluorescence in Frozen Sections (Gold Standard Technique)
IF in frozen sections is the gold standard technique for the study of renal biopsies and it has been used for more than 50 years in glomerular diseases mediated by immunological processes [5,24].
IF-F has a sensitivity of 87.9% and a specificity of 70.5% in the diagnosis of glomerulopathies and is therefore essential to avoid diagnostic errors. This technique is common practice in most pathology centers that handle this type of biopsies [3].
To perform this technique, it is necessary to have either a fresh renal tissue or one preserved in Michel’s medium (it has been observed that with this method the morphology is sometimes not well preserved.) [8,25]; subject it to freezing and slicing in a cryostat, with an approximate thickness of 2-5 microns [24]. Subsequently, the process of incubating the antibodies and mounting the slide for analysis is carried out [9] (Table 1).
| 1. Place the sample on a slide with cryogel, previously cooled to -25°C |
| 2. Leave it in the cryostat for 30 minutes at -25°C before slicing. |
| 3. Make slices of 2 to 4 microns and place on positively charged glass slides, preferably two levels per slide. |
| 4. Let dry in the room air for 30 minutes. |
| 5. Fix in 100% acetone for 10 minutes. |
| 6. Let dry in the room air for 10 minutes. |
| 7. Label the slides with the specific antibody that will be used. |
| 8. Wash the slides with phosphate buffered saline (PBS) for 5 min. |
| 9. Drain and wipe off excess buffer. |
| 10. Place the conjugated antibody (example: IgG/FITC, supplied by Bio SB) and incubate for 30 minutes at room temperature. |
| 11. Wash the slides with PBS performing three changes for 5 minutes each time. |
| 12. Dry the slides by tapping them on paper towels. |
| 13. Mount the slides in buffered glycerol using a coverslip. |
| 14. Store the slides at 4°C until further examination. |
Source: Technical instruction for performance immunofluorescence tests (Bio SB, Direct immunofluorescence Antiboby Specifications sheet).
Table 1: Steps for performing the direct immunofluorescence technique on frozen slices. Source: Technical instruction for performing immunofluorescence tests (Bio SB, Direct Immunofluorescence Antibody Specification sheet).
Steps for performing the direct immunofluorescence technique on frozen slices. Source: Technical instruction for performing immunofluorescence tests (Bio SB, Direct Immunofluorescence Antibody Specification sheet).
Immediate visualization and analysis of the slides is required to avoid fluorescence fading; they can be stored at 4°C until evaluation, but not for a long time [26].
Although it is the technique of choice because it preserves the antigenicity of the tissue, in many regions of the world it is restricted by its cost, the site where the biopsy is performed and the time it takes to reach the laboratory. Additional constraints are the difficulty of processing, small samples with few glomeruli and the need for a technician to adequately handle freeze cuts [3].
An alternative for transporting fresh samples is to snap-freeze them at minus 70°C, thus preserving the antigens, but this requires the proper equipment [11,26].
Despite the improvement of alternative methods with IF-P antigen retrieval and immunohistochemistry with immunoperoxidase, IF-F remains the gold standard and most widely used method [26].
Immunofluorescence on Paraffin-Embedded Sections
Fixation of tissues with formalin preserves their structure but causes covalent bonds to form between the aldehyde and the amino groups of tissues; such bonds denature the proteins and as a result antigenicity is lost [7,14]. Additionally, formaldehyde binds to cellular components forming intra-and intermolecular methylene cross-links reducing the penetration of large molecules such as antibodies and masking target epitopes. This whole process is known as masking [17,20].
To unmask the antigens so that they can be detected by immunofluorescence, an antigen retrieval process is required [16].
There are two methods for antigen retrieval: protease-induced epitope retrieval (PIER); and heat-induced epitope retrieval (HIER) [20].
PIER uses enzymatic digestion that attaches and breaks cross-links to restore antigenicity; enzymes such as proteinase K, trypsin, pepsin or pronase are used [27]. The drawback of using this method is that they are not specific enzymes and could destroy the morphology and antibodies of interest [20].
HIER achieves higher rates of antigen retrieval; heat and pressure are used to restore antigenicity; the mechanism of how it works is unknown, but it is believed to break cross-links and restore the secondary and tertiary structures of proteins so that the epitope is unmasked [20]. HIER better preserves the morphology and structure of the tissue [28]. In this process, the slide with the tissue is heated in a buffer solution that maintains the conformation of the proteins [13].
Buffer solutions are classified according to their pH (acidic, neutral or basic). The best solutions for IF-P are high pH (basic) solutions because they are more likely to bind to the tissue, often in association with pH-stabilizing agents or chelators such as Tris or EDTA [13,20].
For example, CC1 solution (cell conditioning 1, Roche-Ventana), a slightly basic Tris-based buffer (pH 8.5) that is used in antigen retrieval in the immunohistochemistry process and is available in most laboratories, can be used [7].
Heat recovery (HIER) can be performed with a variety of equipment, such as conventional microwave ovens, pressure cookers, automated staining equipment that heats the slide, or some other method of heating; the equipment to be used must take into account the temperature range, the volume of the buffer solution and that there is no evaporation or filtration of liquids [20].
After unmasking, the fluorescein-conjugated antibody is incubated, directly or indirectly, with subsequent mounting and slide reading [9] (Table 2).
| 1. Cut the paraffin block previously processed, 2 microns, serially and arrange them in positively charged slides. (6 to 8 slides) |
| 2. Deparaffine on a hot plate at 60 degrees for 60 minutes. |
| 3. Perform Xylol treatment for 3 to 5 minutes. |
| 4. Air dry |
| 5. Place the slides in a container filled with a buffer solution (Tris- based). (Cell Conditioning 1, CC1, Roche Ventana). |
| 6. Place the container in the autoclave (electric pressure cooker) with a little water in the base, 2 times during 45 minutes. |
| 7. Prepare antibodies with a 1/10 dilution. (90 UL of “Immunodetection Antigen blocker/ antibody diluent Bio SB” and 10 UL of Bio SB conjugated antibody). (Ex: IgG/FITC, of Bio SB). |
| 8. Keep the slides in the buffer (Cell Conditioning 1, CC1, Roche Ventana) until the antibody is placed on the slide. |
| 9. Place the slides in a dark chamber, in face up position. |
| 10. Place 100 UL of mixture of diluent and antibody. (Each antibody on each plate) |
| 11. Place an empty, loaded slide face down on top and incubate for 12 hours. |
| 12. Mount the slide with glycerin using coverslips and then observe under a fluorescence microscope. |
| 13. They can be stored at 4 degrees C, preferably up to 6 hours. |
Source: Technical instruction for performance immunofluorescence tests (Bio SB, Direct immunofluorescence Antiboby Specifications sheet).
Table 2: Protocol for handling renal biopsies for direct immunofluorescence. (Private communication, Vivar, N. Department of Anatomic Pathology. Syniab Ecuador).
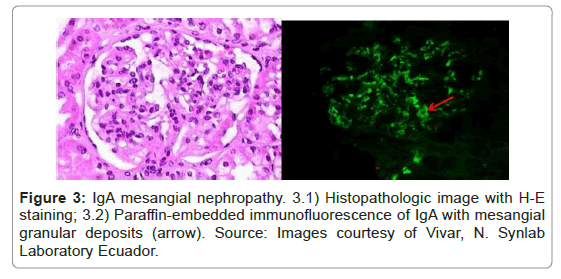
This technique has been described in papers since 1976 [10], with many variations in the detection and antigen retrieval methods. It is recognized as a hybrid technique between conventional immunohistochemistry and IF-F [22]; it is simple to perform in a pathology laboratory and is recognized as an excellent salvage technique for renal biopsies [24] (Figures 2 and 3).
Figure 2: Paraffin-embedded immunofluorescence process. 2.1) Deparaffinization of slides; 2.2 and 2.3) Xylol treatment; 2.4) Air dry; 2.5) Slices coated in buffer solution; 2.6 and 2.7) Antigen recovery with heat in pressure cookers; 2.8) Antibody preparation and dilution; 2.9) Incubation of antibodies in a dark chamber; 2.10) Slide mounting with glycerin; 2.11) Fluorescence microscope observation. Source: Images by courtesy of Synlab Laboratory Ecuador.
Constraints of Paraffin Immunofluorescence
IF-P still has some limitations and disadvantages compared to the IF-F technique; it depends a lot on the method used since this causes the results and therefore the success rate to vary [29].
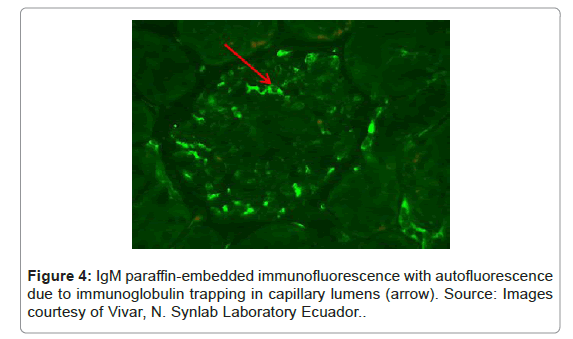
It must always be taken into account that the fixation process, in addition to masking the antigens, usually produces non-specific auto fluorescence of the tissue [22]. For example, some plasma proteins tend to fluoresce within glomerular capillaries and can be interpreted as glomerular deposits; other substances that can produce auto fluorescence are collagen, elastin and mucin [22] (Figure 4).
Some authors mention that complement antigens need more care in the unmasking process, since it has been seen that they usually do not fluoresce as in IF-F and can give false negatives[7]. Particularly C3 staining, which in IF-P is weaker. In addition, linear deposits of IgG in anti-basement membrane disease are not usually evident on IF-P [23,24].
In general, IgG, IgA, kappa, lambda staining is reported to be slightly weaker compared to IF-F [23].
IF-P is less sensitive than IF-F in some glomerular lesions such as primary membranous nephropathy, C3 glomerulonephritis and anti- glomerular basement membrane nephritis [23,24,29].
When evaluating biopsies that have undergone this technique, all these limitations must be taken into account, so that they do not lead to an incorrect diagnosis [24].
Advantages of Paraffin-Embedded Immunofluorescence
IF-F is not always possible, because fresh tissue is not always available [23].
In some pathology laboratories, which perform renal biopsies, IF-P has come to replace IF-F; these laboratories have been experimenting with several methods of antigen retrieval and improving the technique until it has become a routine procedure in surgical pathology [7].
By paraffinizing the tissue, preservation of the tissue is better, its morphology is better defined, and it is easier to handle than frozen tissue [15,18,30] The histological sections of the paraffin blocks can be thinner (2 to 3 microns) which improves the visualization of the structures and, since the same paraffin block is used for the remaining staining, the same light microscopy image can be correlated [31].
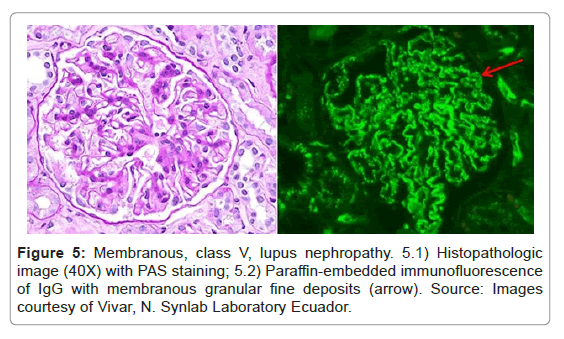
IF-F staining has a diffuse distribution of the antigen in some glomeruli; therefore, if we have few glomeruli, false negatives may occur [28]. However, in IF-P, staining is more homogeneous and all glomeruli are stained [23] (Figure 5).
Diagnostics That Could Be Achieved With IF-P Alone
Recent studies have been revealing new data in which IF-P is more sensitive than IF-F for the diagnosis of some renal lesions, where there is antigen masking, as in membranous glomerulopathy with masked IgG kappa deposits and in membranoproliferative glomerulonephritis with masked monotypic IgG deposits. In these two instances, IF-P is essential for diagnosis [23,24].
There are other pathologies with antigen masking that can benefit from the use of IF-P and in which its diagnostic usefulness is demonstrated; like in light chain deposition pathologies such as amyloidosis, light chain nephropathy, proximal light chain tubulopathy and light chain deposition disease [24].
When light microscopy and electron microscopy findings do not coincide with IF-F, the “masking” process should always be suspected, and IF-P should be performed [23].
Immunohistochemistry Using Immunoperoxidase
Due to the improvement of antigen retrieval techniques, the use of immunohistochemistry with immunoperoxidase has also been tested as an alternative to immunofluorescence in frozen sections, with some advantages over IF-P, such as not requiring a fluorescence microscope for its evaluation. In addition, fading and impermanence of the slide for archiving is avoided. A major limitation of this technique is the background staining it produces, but this has been gradually minimized, and as with IF-P, antigen retrieval methods have been refined [31].
Agreement between immunoperoxidase and IF-F is only 70% to 80%, with a sensitivity of 73% to 86% [28].
This technique is less tested than IF-P and depends on expertise, trial and error and the technique used in each laboratory and is another alternative technique to IF-F for the diagnosis of renal pathology.
Discussion
The immunofluorescence study is essential for the diagnosis of glomerular diseases; it is a way to show the deposition of immunoglobulins and complement in the renal tissue, corroborating the immunologic mechanism responsible for the damage [3]. Direct immunofluorescence has a sensitivity of 87% and specificity of 70.5% in the diagnosis of glomerulopathies [3].
IF has proven to be useful in the diagnosis of other diseases, such as bullous skin lesions; IF-P has even been tested in extrarenal tissues with masked deposits (extrarenal amyloidosis and duodenal macroglobulinemia), proving that it is a technique that does work, but with much research still to be done[27].
Immunofluorescence on paraffin sections is already an established technique as a salvage procedure for renal biopsies where the tissue was unsuitable for IF-F [24,27], and it has now also been shown to be diagnostically valid, demonstrating to be as sensitive and specific as IF-F [24].
Tissue fixation preserves the immunoreactivity of some epitopes while masking others [14,20]; for this reason, in the study by Messias et al. where they applied the IF-P technique to 304 cases, in 207 cases it was used as a salvage technique, and in 97 cases it was used to unmask immunoglobulins and light chains; as a conclusion they stated that IF-P helps in the diagnosis of rare cases where immune deposits are masked on IF-F and give false negatives; IF-P contributed to the diagnosis in more than 1⁄3 of the reviewed cases [24].
Gettika et al. conducted an observational, comparative study between IF-P with enzymatic digestion and IF-F, over a period of 5 years on a total of 214 biopsies. They conclude that IF-P was diagnostically useful in most cases, and that it is an easier technique to perform, with improved visualization of morphology, but not without its limitations [10].
Likewise, Solokani et al. performed a comparative study between IF-P and IF-F using 50 consecutive biopsies, which proved to be diagnostic in 92% of the cases compared to IF-F; it was proven again that it not only serves as a salvage method, but also as a diagnostic tool [31].
Mubarak et al. assessed 3 immunofluorescence techniques, in 40 cases of the same patients, to determine which is the best (HIER+TBS, Pronase and IF-F), with the most useful method being the use of HIER plus TBS (TRIS buffer saline), showing better antigen retrieval and better performance than the other techniques; they further concluded that IF-P is less sensitive and intense than IF-F, but was diagnostic in most cases [29].
Shi et al. uses microwave antigenic recovery, obtaining for IF-P a concordance of 98% for all immunoglobulins, and concludes that IF-P is not inferior to IF-F for detecting C3 and C1q [28].
Thus, there are many studies that show acceptable results and others that are unsuccessful.
The outcome depends on many variables, beginning with the pre-analytical handling of the sample, including proper fixation [14], deparaffinization, the antigen retrieval method used (HIER or PIER), the antibody concentration, and the primary antibody conjugation time [18,22]; For this reason, each laboratory should standardize its methodology and carry out comparative studies to validate the technique.
Conclusion
Pathologists have to choose which technique to perform, either IF-F or IF-P, based on their experience, their technical resources and the likely diagnosis. Both methods provide excellent data, and, in some cases, they can be complementary to each other in various clinical scenarios.
IF-P serves not only as a salvage technique but also is useful for diagnosis in more than 80% of cases; this performance varies depending on the technique that the laboratory is able to optimize.
IF-P is easy to perform, gives results comparable to those obtained in frozen tissue but always with some limitations that must be taken into account at the moment of its evaluation.
There are deposit pathologies in which the antigens are masked, and the use of IF-P is essential to identify them.
Despite the availability of improved antigen retrieval methods, better quality antibodies, and better detection systems, the frozen section immunofluorescence is still the most widely used and preferred by pathologists.
References
- Nasir H, Chaudhry S, Raza W, Moatasim A, Mamoon N, et al. (2012) Role of immunofluorescence in the diagnosis of glomerulonephritis. J Pak Med Assoc 62: 240-243.
- Abbas K, Mubarak M, Kazi JI, Muzaffar R (2009) Pattern of morphology in renal biopsies of nephrotic syndrome patients. Correlation with immunoglobulin and complement deposition and serology. J Pak Med Assoc 59: 540-543.
- Buch A, Sood S, Chandanwale S, Kumar H, Swapnil K, et al. (2015) Role of direct immunofluorescence in the diagnosis of glomerulonephritis. Med J Dr DY Patil Univ 8: 452.
- Muñoz AT, Valdez-Ortiz R, González-Parra C, Espinoza-Dávila E, Morales-Buenrostro LE, et al. (2011) Percutaneous renal biopsy of native kidneys: Efficiency, safety and risk factors associated with major complications. Arch Med Sci 7: 823-831.
- Agarwal SK, Sethi S, Dinda AK (2013) Basics of kidney biopsy: A nephrologist’s perspective. Indian J Nephrol 23: 243-252.
- Walker PD, Cavallo T, Bonsib SM (2004) Practice guidelines for the renal biopsy. Mod Pathol 17: 1555-1563.
- Michel B, Milner Y, David K (1972) Preservation of tissue-fixed immunoglobulins in skin biopsies of patients with lupus erythematosus and bullous diseases-preliminary report. J Invest Dermatol 49: 449-452.
- Merino IG (2017) Libro blanco de la anatomÃa patológica en España, 2017: Recomendaciones de los clubes para el diagnóstico anatomopatológico. 5th Edition.
- Singh G, Singh L, Ghosh R, Nath D, Dinda AK (2016) Immunofluorescence on paraffin embedded renal biopsies: Experience of a tertiary care center with review of literature. World J Nephrol 5: 461.
- Vodegel RM, de Jong MCJM, Meijer HJ, Weytingh MB, Pas HH, et al. (2004) Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol 4: 1-7.
- Hefter LG, Brennan GG (1981) Transillumination of renal biopsy specimens for rapid identification of glomeruli. Kidney Int 20: 411-415.
- Boenisch T (2001) Formalin-fixed and heat-retrieved tissue antigens: A comparison of their immunoreactivity in experimental antibody diluents. Appl Immunohistochem Mol Morphol 9:176-179.
- Oliver C, Jamur MC (1999) Fixation and embedding. Methods Mol Biol 115: 319-326.
- Parra-Medina R, Polo JF (2017) Inmunofluorescencia en tejidos fijados y preservados en parafina (IF-P). Una mirada desde la patologÃa quirúrgica. Repert Med y CirugÃa 26: 202-207.
- Boenisch T (2005) Effect of heat-induced antigen retrieval following inconsistent formalin fixation. Appl Immunohistochem Mol Morphol 13:283-286.
- Zaqout S, Becker LL, Kaindl AM (2020) Immunofluorescence staining of paraffin sections step by step. Front Neuroanat 14: 1-11.
- Aoki V, Sousa JX, Fukumori LMI, Périgo AM, Freitas EL, et al. (2010) Direct and indirect immunofluorescence Imunofluorescência direta e indireta. An Bras Dermatol 85.
- Im K, Mareninov S, Diaz MFP, Yong WH (2019) An introduction to performing immunofluorescence staining. In: Methods in Molecular Biology. Humana Press Inc.
- Becheva ZR, Gabrovska KI, Godjevargova TI (2018) Comparison between direct and indirect immunofluorescence method for determination of somatic cell count. Chem Pap 72: 1861-1867.
- Parra-Medina R, Polo JF (2017) Inmunofluorescencia en tejidos fijados y preservados en parafina (IF-P). Una mirada desde la patologÃa quirúrgica. Repert Med y CirugÃa 26: 202-207.
- Nasr SH, Fidler ME, Said SM (2018) Paraffin immunofluorescence: A valuable ancillary technique in renal pathology. Kidney Int Reports 3: 1260-1266.
- Messias NC, Walker PD, Larsen CP (2015) Paraffin immunofluorescence in the renal pathology laboratory: More than a salvage technique. Mod Pathol 28: 854-860.
- Yabuki A, Sawa M, Kohyama M, Hamamoto T, Yamato O (2017) Paraffin immunofluorescence for detection of immune complexes in renal biopsies: An efficient salvage technique for diagnosis of glomerulonephritis in dogs. BMC Vet Res 13: 1-6.
- Alwahaibi NY, Alsidiri RM, Alsinawi TA, Almalki WH, Alsinawi SS, et al. (2020) Immunoperoxidase and immunofluorescence on formalin-fixed, paraffin-embedded tissue sections versus immunofluorescence on frozen sections in the assessment of renal biopsies. Indian J Nephrol 30: 8-13.
- Singh G, Pradeep I, Agarwal S, Barwad A, Dinda A (2018) Paraffin immunofluorescence: A role beyond kidney biopsies.
- Shi S, Cheng Q, Zhang P, Wang N, Zheng Y, et al. (2013) Immunofluorescence with dual microwave retrieval of paraffin-embedded sections in the assessment of human renal biopsy specimens. Am J Clin Pathol 139: 71-78.
- Mubarak M, Kazi JI, Kulsoom U, Ishaque M (2012) Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathol 1: 91-100.
- Shibutani M, Uneyama C (2002) Methacarn: A fixation tool for multipurpose genetic analysis from paraffin-embedded tissues. Methods Enzymol 356: 114-125.
- Mölne J, Breimer ME, Svalander CT (2005) Immunoperoxidase versus immunofluorescence in the assessment of human renal biopsies. Am J Kidney Dis 45: 674-683.
- Solanki R, Solanki MK, Hemrajani D, Saha J (2019) A study of detection and comparison of immunofluorescence on formalin-fixed paraffin-embedded tissue with fresh frozen renal biopsy specimen. Saudi J Kidney Dis Transpl 30.
Citation: Vivar-Dávila MC (2021) Direct Parain Immunoluorescence: An Alternative for Renal Pathology Diagnosis. Diagnos Pathol Open 5: 016. DOI: 10.4172/2476-2024.s5.1000016
Copyright: © 2021 Vivar-Dávila MC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2668
- [From(publication date): 0-2021 - Sep 18, 2024]
- Breakdown by view type
- HTML page views: 2176
- PDF downloads: 492