Long-COVID Rates Vary Throughout the SARS-CoV-2 Pandemic
Received: 15-Dec-2022 / Manuscript No. JIDT-22-83697 / Editor assigned: 19-Dec-2022 / PreQC No. JIDT-22-83697 (PQ) / Reviewed: 02-Jan-2023 / QC No. JIDT-22-83697 / Revised: 09-Jan-2023 / Manuscript No. JIDT-22-83697 (R) / Published Date: 16-Jan-2023
Abstract
The infection with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) frequentlycauses a broad range of long-lasting symptoms. This condition, termed long-COVID, influences everyday life of affected individuals in many ways and causes a high economic burden. There is urgent need to obtain better understanding of the risk factors that contribute to the development of long-COVID. Aim of this study was to investigate the long-COVID rate of supposedly healthy adults during different phases of the pandemic. Therefore, 71,670 blood donations were screened for SARS-CoV-2 total anti-N antibodies between 5th June 2020 and 30th November 2022. 351 individuals could be recruited for our study to monitor long-COVID symptoms and their duration. Despite immense worldwide efforts to stop virus dissemination, our data reveal a constantly rising SARS-CoV-2 anti-N seroprevalence rate in Salzburg, Austria, peaking at 84.9% in October 2022. In addition, our data demonstrate varying rates of long-COVID in the course of the pandemic. While long-COVID rates were about 20% for the time span between March 2020 and August 2021, long-COVID was reported by 12% for infections from September 2021 to August 2022. This could be attributed to different virus variants, but also to increasing vaccination rates. We further found that long-COVID symptoms decline over time: while 18% of our study participants described persisting symptoms 3 months after the seropositive blood donation, 14% reported persisting symptoms 9 months afterwards and 3% after 18 months. In conclusion, our data reveal that long-COVID symptoms may persist for more than a year after a SARS-CoV-2 infection and that long-COVID rates are varying in the course of the SARS-CoV-2 pandemic.
Keywords: SARS-CoV-2; COVID-19; Long-COVID; Seroprevalence; SARS-CoV-2 anti-N antibodies; Blood donation
Abbreviations
COVID-19: Corona Virus Disease 2019 caused by SARS-CoV-2; PASC: Post-Acute Sequelae of COVID-19; PCS: Post COVID-19 Syndrome; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; VOC: Variants of Concern; WHO: World Health Organization
Introduction
In Since the first reported outbreak of Corona Virus Disease 2019 (COVID-19) in Wuhan, China several variants of the causative agent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) have been registered. Virus variants that were connected to significant situational changes on a global public health level based on features such as increased contagiousness and aggravated clinical picture were declared, “Variants of Concern” (VOC) [1]. After the wild type, five variants of SARS-CoV-2 have been declared to be VOC by the World Health Organization (WHO), causing remarkable health-associated, economic and social issues: alpha and beta (both since December 2020), gamma (since January 2021), delta (since May 2021) and the currently circulating omicron variant (since November 2021) [2]. According to the Austrian agency for health and food safety, all SARS-CoV-2 virus types were found in Austria as well, with only few cases of gamma infections. (https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus#c12422, accessed 02.12.2022).
The infection with SARS-CoV-2 frequently leads to late and long-term health issues, which are termed Post COVID-19 Syndrome (PCS), Post-Acute Sequelae of COVID-19 (PASC) or long-COVID. Long-COVID is defined by the World Health Organisation (WHO) as a health condition that usually occurs three months from the onset of COVID-19 with symptoms that last for at least two months and cannot be explained by an alternative diagnosis [3]. It is found both among patients that were hospitalized due to a severe disease course demanding intensive medical treatment and those presenting rather mild symptoms or even an asymptomatic course [4,5]. Symptoms characterizing long-COVID cover a broad range and feature respiratory, psycho-cognitive and physical fatigue-associated symptoms, among others [6-8].
The prevalence of long-COVID varies among different studies with estimates ranging from 5% to 50% [9-12]. According to recent publications, incidence, clinical picture and duration of long-COVID seem to differ depending on the causative virus variant: One study reports higher long-COVID rates for individuals infected with the wild type virus compared to individuals infected with the delta variant [13]. Another study describes lower long-COVID rates for the omicron variant [14]. Furthermore, it was found that the infection with SARS-CoV-2 variants may induce different long-COVID phenotypes displaying characteristic sets of symptoms with shortness of breath and chronic fatigue being common symptoms for all long-COVID patients [15]. Long-COVID is a matter of ongoing investigation and immense efforts are invested identifying the cause for this persisting health issue, putative treatment options and constant adjustments of official case numbers. Nevertheless, it is clear due to the sheer number of reported cases that long-COVID creates a substantial amount of chronic patients, causing multidimensional burden to the individuals affected, but also to economic and health systems worldwide. This poses an urgent need for better understanding of factors increasing the risk of the development of long-COVID. For an insight concerning the rather healthy adult population, we investigated blood donors as a representative group and observed the rate of long-COVID throughout the different pandemic waves.
Materials and Methods
Study design
The seroprevalence rate of SARS-CoV-2 total anti-N antibodies was determined by screening the serum samples of 71,670 voluntary, non-remunerated whole blood donations collected in Salzburg, Austria from 5th June 2020 until 30th November 2022. As already described in our previous studies [16,17], prior to donation all blood donors had a health screening and completed a written questionnaire, including an informed consent on pathogen screening as a standard part of the blood donation process. Children and teenagers (<18 years) and persons older than 70 years were not included, as individuals of these age groups are not admitted to regular blood donation. No further preselection of sample material was done. For a more detailed examination regarding the course of the SARS-CoV-2 infection, SARS-CoV-2 anti-N seropositive blood donors were invited to participate in our study. After signing informed consent, 351 SARS-CoV-2 seropositive blood donors willing to participate could be included in this study so far. Blood samples of study participants were collected 3, 6, 9 and 18 months after the initial seropositive blood donation, spanning the time of the first two years after a SARS-CoV-2 infection. In addition, the course of the SARS-CoV-2 infection and putative persisting symptoms (long-COVID) of each participants were monitored using online surveys.
Study design
The Elecsys Anti-SARS-CoV-2 (ACOV2) total antibody Electrochemiluminiscence Immunoassay (ECLIA, Roche Diagnostics, Basel, Switzerland) was used for the screening of SARS-CoV-2 anti-N total antibody (including IgM, IgG and IgA) on a cobas 8000-e801 device (Roche Diagnostics) according to manufacturer’s instructions. In this semi-quantitative assay, a recombinant viral Nucleocapsid (N) protein is used as antigen to determine antibodies against SARS-CoV-2 present in serum of blood donors and study participants. The results of this screening approach are based on the signal to cut-off ratio (values <1.0=negative result, values ≥ 1.0=positive result). According to the manufacturer, this assay detects but does not discriminate all known SARS-CoV-2 variants so far.
Online services
In addition to donated blood samples, all participants were asked to fill in online surveys including questions regarding the course of the SARS-CoV-2 infection, persisting symptoms, potential vaccination, SARS-CoV-2 reinfection and other health-associated issues (e.g. general health status and known comorbidities). These online surveys were to be completed 3, 9 and 18 months after the initial seropositive blood donation. The surveys were administered using Lime Survey.
Data analysis
Data were summarized descriptively, showing number of cases in percentages. The analysis concerning long-COVID rates was done using the full dataset including all participants who were willing to fill in the survey completely. 257 study participants provided a point in time for the infection with SARS-CoV-2. For the analysis of long-COVID over the time span of about 20 months after the first SARS-CoV-2 infection, study participants filling in all three online surveys completely by the end of November 2022 were included (n=120).
Results and Discussion
SARS-CoV-2 anti-N seroprevalence rates peak in October 2022
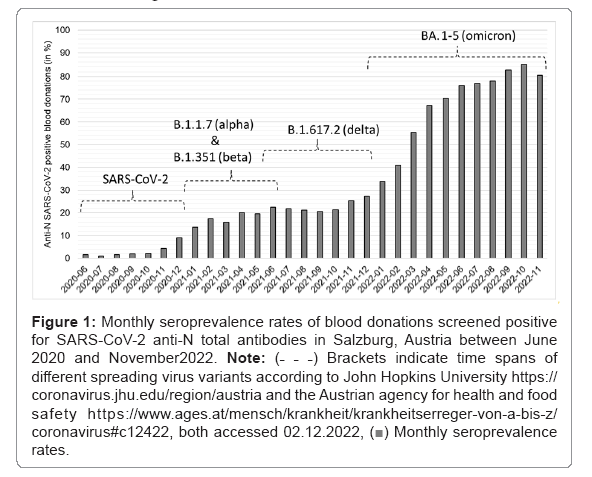
Despite immense efforts worldwide to stop virus dissemination including lock downs, social distancing and vaccination, our screening for SARS-CoV-2 anti-N total antibody of 71,670 voluntary blood donations in Salzburg, Austria revealed constantly rising seroprevalence rates between June 2020 and October 2022. It is important to note that anti-N antibodies are produced after an infection only, but not after vaccination. Thus, this screening approach monitors infected individuals only, irrespective of their vaccination status. The highest seroprevalence rate for SARS-CoV-2 anti-N detected was 84.9% in October 2022 (Figure 1).
Long-COVID rates are declining in the course of the pandemic
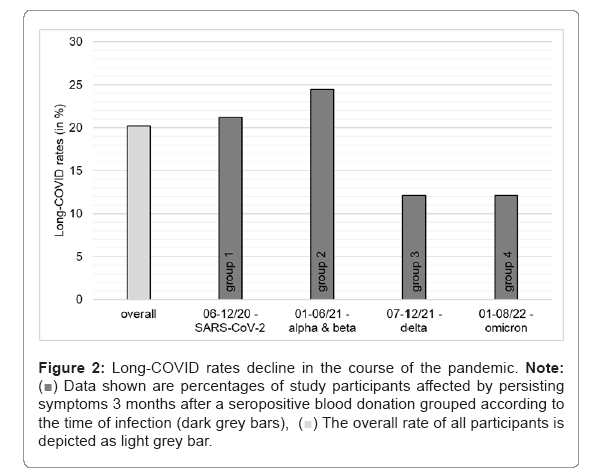
SARS-CoV-2 anti-N total antibody seropositive blood donors were asked to participate in our study. As all blood donors have a brief health screening prior donation and have to fulfill certain health-associated requirements prescribed by national and international authorities to be admitted to donate blood, the included study participants represent a supposedly healthy subgroup of the Austrian adult population. To date, 351 participants could be included with 257 individuals being able to provide the time of the SARS-CoV-2 infection. It is important to note that our study cohort mainly consists of individuals that experienced a rather mild course of SARS-CoV-2 infection, as no hospitalization due to COVID-19 was reported and they donated blood within 45 days on average after an infection [17]. For the current analysis, participants were grouped into four subgroups depending on the time of infection and thus depending on the virus variant spreading most dominantly in Austria at the particular point in time (Figure 1, based on public data from the Austrian agency for health and food safety, https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus#c12422, accessed 02.12.2022). Group1: SARS-CoV-2 wild type infection between 06/2020 and 12/2020 (SARS-CoV-2, n=146), group 2: infection between 01/2021 and 06/2021 (most widespread virus variants: B1.1.7 alpha and B.1.351 beta, n=45), group 3: infection between 07/2021 and 12/2021 (most widespread virus variant: B.1.617.2 delta, n=33) and group 4: infection between 01/2022 and 08/2022 (most widespread virus variant: BA.1-5 omicron, n=33). Three months after the seropositive blood donation, all study participants were asked about persisting symptoms, which were attributed to the previous SARS-CoV-2 infection (long-COVID). As shown in Figure 2, the overall rate for long-COVID within the whole study group (n=257) is 20%. Comparing the different groups according to the time of infection, we found higher rates of long-COVID in groups 1 and 2 (21% and 23%) compared to groups 3 and 4 (12% each), indicating lower rates for long-COVID after infections with delta and omicron variants (Figure 2).
Figure 1: Monthly seroprevalence rates of blood donations screened positive for SARS-CoV-2 anti-N total antibodies in Salzburg, Austria between June 2020 and November2022. Note: (˗ ˗ ˗) Brackets indicate time spans of different spreading virus variants according to John Hopkins University https://coronavirus.jhu.edu/region/austria and the Austrian agency for health and food safety https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus#c12422, both accessed 02.12.2022, Monthly seroprevalence rates.
Monthly seroprevalence rates.
Figure 2: Long-COVID rates decline in the course of the pandemic. Note:  Data shown are percentages of study participants affected by persisting symptoms 3 months after a seropositive blood donation grouped according to the time of infection (dark grey bars),
Data shown are percentages of study participants affected by persisting symptoms 3 months after a seropositive blood donation grouped according to the time of infection (dark grey bars),  The overall rate of all participants is depicted as light grey bar.
The overall rate of all participants is depicted as light grey bar.
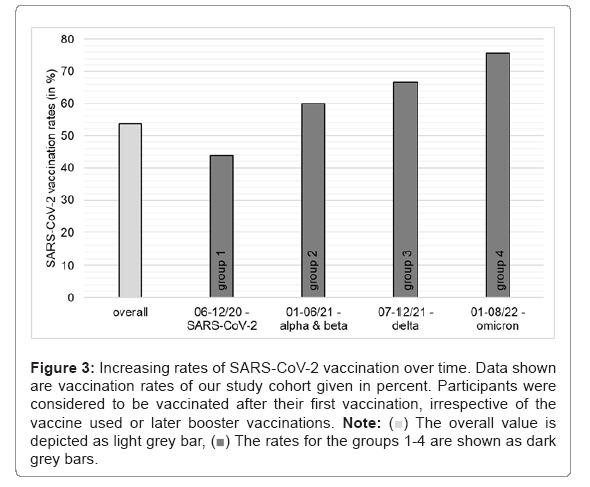
This finding is of particular interest, as it reveals that long-COVID rates vary in the course of the pandemic. This discovery could be explained by the effect of different virus variants, which induce varying immunologic and inflammatory reactions in the human body, as indicated by Spinicci, et al. [15]. Another explanation could be increasing rates of SARS-CoV-2 vaccinations, which constantly increased over time within our study cohorts indicating that vaccination might provide a protective effect and thus reduce the risk of developing long-COVID (Figure 3).
Figure 3: Increasing rates of SARS-CoV-2 vaccination over time. Data shown are vaccination rates of our study cohort given in percent. Participants were considered to be vaccinated after their first vaccination, irrespective of the vaccine used or later booster vaccinations. Note:  The overall value is depicted as light grey bar,
The overall value is depicted as light grey bar,  The rates for the groups 1-4 are shown as dark grey bars.
The rates for the groups 1-4 are shown as dark grey bars.
The study by Al-Aly et al. suggests that vaccination confers partial but not complete protection from developing long-COVID. Therefore, the authors suppose that the reliance on vaccination as the only mitigation strategy may not reduce the risk for developing long-COVID [18]. Independent of the time of infection and thus independent of the virus variant, the three most common persisting symptoms reported were shortness of breath, fatigue and hyposmia/dysgeusia. This is in line with the findings of a recently published study [15]. Further long-COVID symptoms described were nasal congestion, sore throat, reoccurring headache and chest pain. The symptoms reported correlate well our previous findings [17] and other studies [19-21].
Long-COVID persists up to 20 months after a SARS-CoV-2 infection
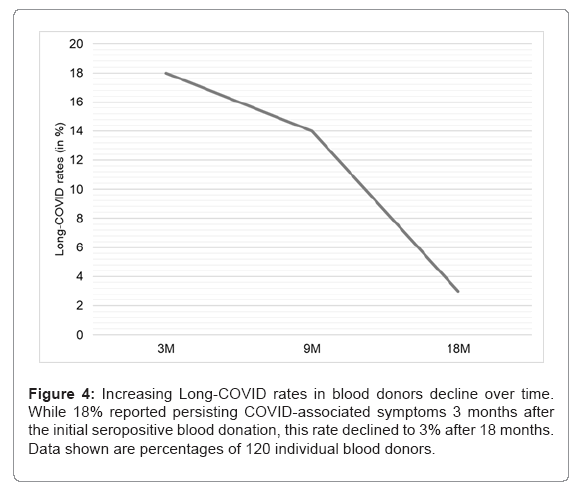
As shown in Figure 4, within a subgroup of 120 individuals that currently participate in the present study for more than 18 months, long-COVID symptoms strongly declined over time. While in this subgroup 3 months after the initial seropositive blood donation 18% reported persisting symptoms, the rate decreased to 14% after 9 months and to 3% after 18 months. Symptoms reported 18 months post donation, so about 20 months after the infection, include shortness of breath, fatigue and hyposmia and/or dysgeusia. It is important to note that one of the four individuals described persisting long-COVID symptoms 18 months after the initial blood donation also reported a reinfection with SARS-CoV-2 in October 2021. Even though three of the four individuals did not report reinfections with SARS-CoV-2, the levels of SARS-CoV-2 anti-N levels started to rise again between 9 and 18 months after the initial blood donation, indicating reinfections, which probably were unnoticed. Thus, the reported long-COVID symptoms at the time 18 months after the first SARS-CoV-2 infection might also be symptoms that can be attributed to later reinfections. Our data indicate that the majority of long-COVID cases last up to one year after a SARS-CoV-2 infection (Figure 4).
It should be noted that our findings might not be applicable to other age groups (children and people aged 70 and older), as our study cohort includes adults in legal blood donation age only. As blood donors represent a supposedly healthy subgroup of the population, our data cannot be extrapolated to adult individuals suffering from certain morbidities not permitting regular blood donations, such as cancer, autoimmune disorders and certain chronic diseases.
Conclusion
The investigation of more than 70,000 serum samples from June 2020 to November 2022 provide valuable insights into the course of SARS-CoV-2 infections in a representative cohort of adult blood donors in Central Europe. Our data show that the seroprevalence rate was raising constantly during the pandemic, despite of hygiene measures, social distancing and available vaccination. The highest seroprevalence rate obtained for Salzburg, Austria was 84.9% in October 2022. Furthermore, our data revealed varying rates for persisting symptoms associated with a SARS-CoV-2 infection. While the long-COVID rate was about 20% after SARS-CoV-2 infections between June 2020 and August 2021, later infections resulted in lower rates. This could be explained by different virus variants spreading at the times investigated, but also by increasing vaccination rates. Our analyses further showed that independent of the virus variant, the three mostly described long-COVID symptoms were shortness of breath, fatigue and hyposmia/dysgeusia. Long-COVID symptoms eased over time, with the majority of study participants being fully recovered about one year after the infection. However, our data do not allow drawing direct conclusions about the exact cause of the different long-COVID rates observed. Additional studies are urgently required to shed more light on the factors provoking long-COVID to find more efficient ways of treatment and therapy.
Author Contributions
ER and SLP designed the study. ADH, CG, NL, HN and VN collected blood samples. NH and SLP did serological measurements. GZ, NB and SLP performed data analysis and interpretation. VN and SLP wrote the manuscript. CJ, LW, ER and SLP reviewed and edited the manuscript. VN, ER, ADH, NB, TO, MF, JO and SLP designed the online questionnaires. All authors read and approved the final manuscript.
Acknowledgments
GZ gratefully acknowledges the support of the WISS 2025 project ‘IDA-Lab Salzburg’ (20204-WISS/225/197-2019 and 20102-F1901166-KZP).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the federal state of Salzburg, Austria (ethical votum number 1004/2021, date of approval: 18.02.2021). All participants signed written informed consent.
Conflict of Interests
The authors declare no conflict of interest.
References
- Thakur V, Bhola S, Thakur P, Patel SKS, Kulshrestha S, et al. (2022) Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection 50:309-325.
[Crossref] [Google Scholar] [PubMed]
- Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe.
- Crossref
- Adler L, Gazit S, Pinto Y, Perez G, Reuveni M, et al. (2022) Long-COVID in patients with a history of mild or asymptomatic SARS-CoV-2 infection: A nationwide cohort study. Scand J Prim Health Care 31:1-8.
[Crossref] [Google Scholar] [PubMed]
- Malkova A, Kudryavtsev I, Starshinova A, Kudlay D, Zinchenko Y, et al. (2021) Post COVID-19 syndrome in patients with asymptomatic/mild form. Pathogens 10:1408.
[Crossref] [Google Scholar] [PubMed]
- Briggs A, Vassall A (2021) Count the cost of disability caused by COVID-19. Nature 593:502-505.
[Crossref] [Google Scholar] [PubMed]
- Callard F, Perego E (2021) How and why patients made Long COVID. Soc Sci Med 268:113426
[Crossref] [Google Scholar] [PubMed]
- Deer RR, Rock MA, Vasilevsky N, Carmody L, Rando H, et al. (2021) Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine 74:103722.
[Crossref] [Google Scholar] [PubMed]
- Zimmermann P, Pittet LF, Curtis N (2022) The challenge of studying long COVID: an updated review. Pediatr Infect Dis J 41:424-426.
[Crossref] [Google Scholar] [PubMed]
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, et al. (2021) Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClin Med 38: 101019.
[Crossref] [Google Scholar] [PubMed]
- Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, et al. (2022) Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun 13:3528.
[Crossref] [Google Scholar] [PubMed]
- Ballering AV, Van Zon SKR, Olde Hartman TC, Rosmalen JGM (2022) Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 400:452-461.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-de-Las-Penas C, Cancela-Cilleruelo I, Rodriguez-Jimenez J, Gomez-Mayordomo V, Pellicer-Valero OJ, et al. (2022) Associated-Onset Symptoms and Post-COVID-19 Symptoms in Hospitalized COVID-19 Survivors Infected with Wuhan, Alpha or Delta SARS-CoV-2 Variant. Pathogens 11:725.
[Crossref] [Google Scholar] [PubMed]
- Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ (2022) Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 399:2263-2264.
[Crossref] [Google Scholar] [PubMed]
- Spinicci M, Graziani L, Tilli M, Nkurunziza J, Vellere I, et al. (2022) Infection with SARS-CoV-2 variants is associated with different long COVID phenotypes. Viruses 14.
[Crossref] [Google Scholar] [PubMed]
- Weidner L, Nunhofer V, Jungbauer C, Hoeggerl AD, Gruner L, et al. (2021) Seroprevalence of anti-SARS-CoV-2 total antibody is higher in younger Austrian blood donors. Infection 49:1187-1194.
[Crossref] [Google Scholar] [PubMed]
- Nunhofer V, Weidner L, Hoeggerl AD, Zimmermann G, Badstuber N, et al. (2022) Persistence of naturally acquired and functional SARS-CoV-2 antibodies in blood donors one year after infection. Viruses 14.
[Crossref] [Google Scholar] [PubMed]
- Al-Aly Z, Bowe B, Xie Y (2022) Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 28:1461-1467.
[Crossref] [Google Scholar] [PubMed]
- Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, et al. (2021) Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 18:e1003773.
[Crossref] [Google Scholar] [PubMed]
- Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, et al. (2022) Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 28 1706-1714.
[Crossref] [Google Scholar] [PubMed]
- Mantovani A, Morrone MC, Patrono C, Santoro MG, Schiaffino S, et al. Long COVID: where we stand and challenges ahead. Cell Death Differ 29:1891-1900.
[Crossref] [Google Scholar] [PubMed]
Citation: Nunhofer V, Hoegger AD, Weidner L, Zimmermann G, Badstuber N, et al. (2023) Differentiation of Long-COVID Rates during the SARS-CoV-2 Pandemic. J Infect Dis Ther 11:520.
Copyright: © 2023 Nunhofer V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1512
- [From(publication date): 0-2023 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 1306
- PDF downloads: 206