Dietary Polyphenols as Potential Therapeutics in Alzheimerâs Disease: Pleiotropic Effects and Toxicity Prediction Models
Received: 25-Mar-2022 / Manuscript No. JADP-22-58212 / Editor assigned: 28-Mar-2022 / PreQC No. JADP-22-58212 (PQ) / Reviewed: 11-Apr-2022 / QC No. JADP-22-58212 / Revised: 18-Apr-2022 / Manuscript No. JADP-22-58212 (R) / Published Date: 25-Apr-2022
Abstract
Individualized nutritional treatment with polyphenols has been proposed in the early stages of Alzheimer’s disease. However, it remains unclear at what doses polyphenols and their metabolites enter the brain tissue and whether they can act at sufficient concentrations. The present review provides useful insights into the multiple modes of actions of selected polyphenols as potential therapeutic tools at the pre-clinical stage of Alzheimer’s disease, as well as their ability to cross the blood-brain barrier and their detrimental effects after high dose consumptions. Several studies proposed flavonoids for their potential value in prevention and treatment of neurodegenerative diseases. The scarcity of clinical data though highlights the need for conducting metabolomics studies and well-designed clinical trials. Prolonged clinical trials with bioinformatics tools are needed to fully elucidate both neuroprotective effects and possible risks from polyphenol consumption at concentrated high doses.
Keywords
Polyphenols; Alzheimer’s disease; Neuroprotection; Cytotoxicity; Computer; Prediction; Safety
Abbrevations
3xTg-AD: Triple Transgenic AD Mouse; AD: Al- zheimer’s Disease; ABAD: Aβ-Binding Alcohol Dehydrogenase; AChE: Acetylcholinesterase; ADME: Absorption, Distribution, Metabolism, and Excretion; APP: Amyloid Precursor Protein; Base1: β-secretase; BDPP: Bioavailable Polyphenolic Preparation; BBΒ: Blood Brain Bar- rier; BChE: Butyrylcholinesterase; BDNF: Brain Derived Neurotrophic Factor; CAT: Catalase; CSF: Cerebrospinal Fluid; DOA: Decarboxy- methyl Oleuropein Aglycone; EGCG: Epigallocatechin Gallate; ECG: Epicatechin Gallate; EPIC: European Prospective Investigation into Cancer and Nutrition; ER: Endoplasmic Reticulum; EVOO: Extra Vir- gin Olive Oil; GDNF: Glial-Derived Neurotrophic Factor; GSH: Gluta- thione; GSK-3: Glycogen Synthase Kinase-3; GSSG: Glutathione Disul- phide; GSPE: Grape Seed Proanthocyanidin Extract; HMDB: Human Metabolome Database; HT: Hydrotyrosol; IL-1β: Interleukin-1β; INOS: Inducible Nitric Oxide Synthase; JNK: c-Jun NH2-terminal Kinase; MAPK: Mitogen Activated Protein Kinase; MDA: Malondialdehyde; MIND: Mediterranean DASH Intervention for Neurodegenerative Delay; MMP: Matrix Metalloproteinase; mPTP: mitochondrial Perme- ability Transition Pore; NGF: Nerve Growth Factor; NF-kB: Nuclear Factor-kappa B; NO: Nitric Oxide; Nrf2: Erythroid 2–related factor 2; OA: Oleuropein Aglycone; PKC: Protein Kinase C; PPARGC1α: Peroxi- some Proliferator-Activated Receptor Coactivator 1 Alpha; ppGalNAc- Ts: polypeptide N-acetyl-α-galactosaminyltransferase; QSAR: Quanti- tative Structure Activity Relationship; ROS: Reactive Oxygen Species; SMILES: Simplified Molecular Input Line Entry System; TETs: Ten Eleven Translocation methyl-cytosine dioxygenases; TNF-a: Tumor Necrosis Factor alpha; TTR: Transthyretin; TEDB: Toxic Exposome Database; T3DB: Toxin and Toxin Target Database
Introduction
The traditional Mediterranean diet and the MIND diet, a combination of the Mediterranean and the DASH dietary regimen, have been extensively studied as preventive non-pharmacological tools for ameliorating symptoms of Alzheimer ’s disease (AD), reducing the severity or inhibiting the disease progression. Dietary recommendations for the prevention of AD and the maintenance of brain health include a high consumption of polyphenol enriched foods such as fruits, vegetables, nuts, herbs, spices and beverages including red wine, tea and coffee [1,2].
Polyphenols are bioactive compounds that are spread in plant based products such as oils, seeds, spices, dried legumes, fruits, vegetables, cocoa and dark chocolate. More than 8000 are currently known in nature and more than 4000 species have been identified. Polyphenol intakes show heterogeneity in the European Prospective Investigation into Cancer and Nutrition (EPIC) study using different dietary assessment methods [3,4].
Animal studies or studies on cultured human cell lines revealed a protective role of polyphenol consumption in diabetes, cancer, cardiovascular diseases, osteoporosis and neurodegenerative diseases. Free radical scavenging, enhancing antioxidant defences, modulating of intracellular signalling pathways and gene expressions and inhibiting inflammatory response are a number of different mechanisms of actions. Polyphenols are classified according to their chemical structures, biological properties and their origin.
Dietary polyphenol subclasses include the flavonoids, phenolic acids, lignans and stilbenes. Five subclasses of flavonoids have been reported as indicated below [5-7].
-
Flavonols: Isorhamnetin, Kaempferol, Myricetin, Quercetin
-
Flavones: Apigenin, Luteolin
-
Flavanones: Eriodictyol, Hesperetin, Naringenin
-
Flavan-3-ols: (+)-Catechin, (+)-Gallocatechin, (-)-Epicatechin, (-)-Epigallocatechin, (-)-Epicatechin 3-gallate, (-)-Epigallocatechin 3-gallate, Theaflavin, Theaflavin 3-gallate, Theaflavin 3’-gallate, Theaflavin 3,3’-digallate, Thearubigins
-
Anthocyanidins: Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin
-
Isoflavones: Genistein, Daidzein
Literature Review
Polyphenols and blood-brain barrier permeability
Transmembrane diffusion, saturable transport, adsorptive endocytosis, and the extracellular pathways are some of the proposed mechanisms that BBB uses in order to allow and regulate the entry of critical substances with common physicochemical characteristics. The Blood-Brain Barrier (BBB) permeability of polyphenols and metabolites depends on their lipophilicity properties their polarity. Resveratrol as a lipophilic phenolic compound seems capable of penetrating the BBB. Using the high-performance liquid chromatography and the technique of solid phase extraction, different phenolics were measured in both plasma and CSF of patients with neurological disorders. According to the results, caffeic acid, homovanillic acid and 3-hydroxyphenyl acetic acid, derived from fermentation of quercetin-3-O-rutinoside (rutin) and quercetin by colonic microbiota have been detected in the majority of Cerebrospinal Fluid (CSF) samples, indicating their ability to cross the BBB in humans. The citrus flavonoid naringenin and other glucuronidated conjugates have the capacity to enter the brain endothelium and cross the BBB according to permeability studies and based on in vitro ECV304/C6 co-culture BBB model system. This permeability correlates with their lipophilicity that facilitates transcellular diffusion. In another study naringenin was determined in rat plasma and brain tissue at dosed 50 ng/ml and 0.4 μg/g, respectively, following i.v administration. Both naringenin and hesperetin interacts with the brain endothelial P-glycoprotein meaning a greater permeation through the BBB. Both epicatechin and epigallocatechin entered and localised within the brain tissue after oral administration. Grape polyphenols reached the brain at concentrations 0.1% to 1.7% of the dose, of the polyphenolic fractions. Taken all the data together, the majority of polyphenols are accumulated and distributed at different brain regions with no specific manner, making these compounds promising and suitable neutraceutical candidates for ameliorating severity of AD pathology [8-13].
Polyphenols as potential therapeutic regimen in Alzheimer’s disease
Dietary polyphenols have gained much attention regarding prevention and treatment of neurodegenerative diseases due to antiamyloidogenic, anti-inflammatory and antioxidant properties. Flavonoids are beneficial phytochemicals that exert chemoprotective, anti-allergic, anti-inflammatory, anti-viral, anti-proliferativea and anti- carcinogenic effects. Dietary flavonols seem capable of attenuating AD severity through reduction of b-amyloidosis, tauopathy, astrogliosis, microgliosis in amygdala and hippocampus regions. Certain flavonoids have been suggested as promising therapeutic compounds due to their capacity to disrupt the β amyloid aggregation through activation of α-secretase and deactivation of β-secretase. Furthermore, flavonoids possess neuroprotective effects against tau phosphorylation and downregulate the expression of Glycogen synthase kinase-3 (GSK- 3). A community based prospective cohort study with recruited 921 dementia free participants from Rush Memory and Aging Project, showed that highest intake of flavonols was associated with reduced risk for Alzheimer dementia [14-17].
Bioactive polyphenolic compounds are also considered as modifiers of gene expression in the hippocampus and modulators of epigenetic pathways of AD. Particularly, administration of a standardized Bioavailable Polyphenolic Preparation (BDPP) containing a mixture of concord grape juice, grape seed extract, and resveratrol seems capable of regulating the transcription DNA methyl transferases and the Ten Eleven Translocation methyl-cytosine dioxygenases (TETs) that are involved in synaptic plasticity and might be involved in the AD pathogenesis. The safety and absorption of this polyphenol rich nutraceutical combination in CSF will be assessed in ongoing clinical trial. An FDA-approved functional food supplement known as Oligonol containing a combination of catechin monomers and oligomeric proanthocyanidins derived mainly from grapes and apples, showed both chemoprotective and neuroprotective effects in animal and cell line studies. This particular neutraceutical consists of 15%- 20% monomers, 8%-12% dimers and 5%-10% trimers and it approved to be capable of counteracting cognitive impairment and pathological markers after administered for 12 months to a triple transgenic mouse model of AD. Regulating cellular proteasomes, restoring mitochondrial dysfunctions and ER stress are some of the proposed mechanisms of Oligonol that are involved in depletion of AD pathology [18-21].
Grape-derived polyphenols play a regulatory role in oxidative stress, protein misfolding, inflammation and synaptic dysfunction. The most abundant flavonol in white grapes is quercetin and myricetin in red grapes in the form of glucosides, galactosides, rytinosides, rhamnosides and glucuronides. Polyphenol-rich sesame lignans and cinnamon helps eliminate the APP activity. Other dietary phenolic compounds such as (−)-Epicatechin, quercetin and myricetin found in tea, berries and herbs inhibit the activity of BACE1. Similar antiamyloidogenic properties are present in the flavonone nariturin found in citrus fruit and juices [22-25].
Quercetin: Quercetin (3,5,7,3′,4′-pentahydroxyflavone) that is found abundantly in tea, apples, onions, dark chocolate and red wine, has been suggested as neutraceutical agent due to antioxidant, anti-inflammatory and antiamyloidogenic properties. Quercetin administration on aged triple transgenic AD model (3xTg-AD) mice resulted in a significant reduction in the Paired Helical Filament (PHF), β-amyloid 1–40 and 1–42 levels and inhibition of APP cleavage by β-secretase (BASE 1). Quercetin also improved performance on learning and spatial memory tasks and displayed efficacy against β-amyloidosis, tauopathy, strogliosis, microgliosis in amygdala and hippocampus regions. Quercetin administration has shown capacity to inhibit neuroinflammation through downregulation of INOS gene expression in microglia. However, as indicated at the HMDB, U.S. Food and Drug Administration has not approved any health declaration for quercetin, due to a limited number of intervention studies with flavonoid rich foods and extracts. Data for quercetin at HMDB is available at https://hmdb.ca/metabolites/HMDB0005794 [26-28].
(-)-Epigallocatechin 3-O-gallate: Epigallocatechin Gallate (EGCG), the major catechin in green tea, is a well-studied antioxidant compound effective in cancer chemoprevention. The Aβ accumulation in brain mitochondria targets a series of enzymes involved in Krebs cycle, respiratory chain enzymes, mitochondrial Permeability Transition Pore (mPTP) and the mitochondrial matrix protein Aβ-binding alcohol dehydrogenase, leading to mitochondrial dysfunction and cellular stress. EGCG treatment remodels mature amyloid fibrils preventing the aggregation procedure and forming nontoxic amorphous species. EGCG could inhibit permeabilization of mitochondrial membranes caused by Aβ42, α-sinuclein and tau complexes and could also prevent amyloid aggregation through binding to oligomeric peptide with high affinity. A specific NMR technique, named 1H-N Heteronuclear Single Quantum Coherence (1H-15N HSQC) spectra indicated a chemical shift change in Aβ40 protofibrils upon catechin addition. Apart from Aβ, it interacts with other numerous proteins leading to protein misfolding and amyloidogenicity such as tau, α-sinuclein and transthyretin [29-33].
MMPs are cysteine and serine-proteinases that may play an important role in the AD pathogenesis and progression of human brain tumors. Levels of MMP2 in CSF have shown a significant association with p-tau and levels of MMP10 with tau and p-tau. Green tea catehins’efficacy in inhibiting Matrix Metalloproteinase (MMP)-2, MMP-9 and MMP-12 activities was assessed using substrate gel zymography combined with electrophoresis technique and fluorometric assays. The activities of MMP-2 and MMP-9 were significantly suppressed by Epigallocatechin Gallate (EGCG) and Epicatechin Gallate (ECG) [34-36].
Resveratrol: Major biological properties of resveratrol (3, 4′, 5-trihydroxystilbene) include antioxidant, anti-inflammatory, neuroprotective, cardioprotective and anticancer mechanisms. Resveratrol (RV) found predominantly in grape skins and red wine, show protection from neurodegenerative diseases such as Alzheimer’s disease, through antioxidant activity and by stimulating the proteins SIRT1and AMPK leading to Aβ aggregation clearance. Sirtuin1 is a NAD+-dependent histone deacylase demonstrating neuroprotection against AD. Furthermore, in Huntington’s disease models, resveratrol regulates the expression of FOXO proteins, promoting neuronal survival. Resveratrol (30 mg/kg/day for 8 weeks)-induced Sirt1 activation reduced phosphorylated tau levels in the hippocampus region, as indicated in a rat AD model. After a 52-week treatment with resveratrol, a decline of proinflammatory cytokines such as IL-IR4, IL- 12P40, IL-12P70, TNF-α and chemokines has been observed. Finally, resveratrol reduced the activity of the Glycogen Synthase Kinase-3 (GSK3B), inhibited Endoplasmic Reticulum (ER) stress in the mice hippocampus, reduced APP expression and involved in the regulation of synaptic plasticity, programmed cell death and cell survival [37-40].
Luteolin: Luteolin is a plant flavonoid found in celery, chamomile, olive oil, carrots, spinach, oregano and rosemary. Luteolin, and genistein enhanced the secretion of neurotrophic factors, including Nerve Growth Factor (NGF), Glial Derived Neurotrophic Factor (GDNF), and Brain Derived Neurotrophic Factor (BDNF) in cultured rat astrocytes. These neurotrophic factors are promoters of neuronal survival, neurogenesis and synaptic plasticity. Luteolin also reduced the amyloid production through inhibition of polypeptide N-acetyl-α- Galactosaminyl Transferase ( ppGalNAc-Ts) isoforms in vitro and in cells. These enzymes initiate the process of O-GalNAc glycosylation of APP leading to AD pathogenesis [41-43].
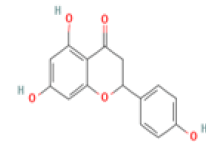
Myricetin: Myricetin (3,5,7,3′,4′,5′-hexahydroxyflavonol) has been indicated as a potent therapeutic flavonoid in many diseases including inflammatory diseases, atherosclerosis, cerebral ischemia, diabetes and Alzheimer’s disease through immunomodulatory actions and suppression of inflammatory cytokine overproduction. Myricetin also increased the expression of BDNF in the hippocampus and improved memory impairment in mice and efficiently reduced beta-site amyloid precursor protein-cleaving enzyme 1 activity, involving in AD treatment [44-46].
Rutin (quercetin-3-O-rutinoside): Rutin, a flavonoid found mainly in asparagus, apples, berries, grapes and blackcurrant is a glycoside containing flavonoid aglycone quercetin alongside with disaccharide. Rutin has the capacity to increase nuclear factor erythroid 2–related factor 2 (Nrf2) levels, as well as cellular antioxidant defence system causing an increase in Catalase (CAT), Superoxide Dismutase (SOD) and Glutathione (GSH) levels. Rutin has demonstrated a protective role in AD, due to its ability to cross BBB and its physicochemical characteristics. Inhibition of neuroinflammation, Amyloid beta (Aβ) processing and aggregation, metal chelation and antioxidant activity are some of the proposed mechanisms. Finally, rutin reduces the Nitric Oxide (NO) production, downregulates the expression of NF-κB and NFκB-dependent pro-inflammatory cytokines TNF-a, IL-6 and CRP in a mouse model study of AD [47,48].
Caffeic acid: Coffee consumption has negatively been associated with the onset and progression of neurodegenerative diseases including dementia, Alzheimer’s disease and Parkinson’s disease and other chronic metabolic disorders such as cardiovascular, diabetes, stroke, liver diseases and cancer. Caffeic acid and chlorogenic acid are the two major bioactive compounds of coffee beverage with chemoprotective and pleiotropic neuroprotective properties that have been extensively analysed in several epidemiological and clinical studies. One cup (200 ml) of coffee contains 70-350 mg chlorogenic acid and 35-175 mg caffeic acid [49-51]. The Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study highlightened the protective role of coffee consumption during midlife against dementia/AD later in life after adjusting for demographic, lifestyle and vascular factors, apolipoprotein E epsilon4 allele and depressive symptoms [52]. Caffeic acid showed disaggregating activity in mature fibrils, enhanced the hippocampal expression of synaptophysin, reduced cerebral damage and inhibited catalase and Glutathione (GSH) activity in a model of AD. In the same study, suppression of inflammation via p53 and phosphorylated p38 MAPK signaling pathway, nuclear factor-κB-, p65 protein expression and caspase-3 were observed after caffeic acid treatment [53,54].
Chlorogenic acid: Mean daily intake of chlorogenic acid can be provided through consumption of coffee, nectarine, apple and apple juice, oregano, sunflower seed, potato, chicory and artichoke heads. Chlorogenic acid is a cinnamate ester of coffee acid and quinic acid and it is metabolised to hippuric acid through colonic microflora before entering the circulation [55]. It is worth mentioning that among older people, low plasma levels of hippuric acid, an index of low intake of polyphenol enriched fruits and vegetables, are significantly associated with a high risk of frailty after four years. Frailty predicts the risk of cognitive impairment and dementia independently of familiar effects. Both in vitro and in vivo animal studies revealed the ability of chlorogenic acid to inhibit AChE and BChE activity that are implicated in the pathogenesis of AD [56-58].
Rosmarinic acid (RA): Neuroprotective properties of rosmarinic acid have been demonstrated in many research papers focusing on antioxidant and metal chelation mechanisms. Rosmarinic acid has the capacity to interfere with the toxic amyloid β-Cu (II) complex, attenuating Aβ accumulation and ROS generation. Safety and tolerability of a 6-month intake of Melissa officinalis extract (500 mg daily), rich in rosmarinic acid, in patients with Alzheimer’s disease have been evaluated in double-blind placebo controlled clinical trial. RA demonstrated a good tolerability profile for 48 weeks, in patients with mild dementia due to probable AD. Although no significant differences observed in AD biomarkers (11C-PiB PET, 18F-FDG PET, volumetric MRI, CSFAβ1-42, tau, ptau181), the treated group achieved an improved mean score at Neuropsychiatric Inventory Questionnaire (NPI-Q), compared to controls. According to the study, RA can be proposed as a potential agent for the prevention of deterioration of AD symptoms [59,60].
Cinnamaldehyde (CNA): Cinnamomum species are well studied plant species used as spice in food, food additives and essential oils. Cinnamaldehyde (CNA) is a volatile compound be utilized as sensory and cognition stimulator. Neuroprotective potentials of cinnamon extract derived from Cinnamomum zeylanicum has been proposed by several in vitro studies and can be attributed to cinnamic aldehyde, the main bioactive ingredient. Cinnamaldehyde (CNA) showed a potential to deactivate pro-inflammatory nuclear transcription factor NF-kappaB via NIK/IKK, ERK, and p38 MAPK signal transduction pathways. Moreover, it enhances sirt1 activity in rat C6 glioma cells by Qin, et al. and downregulates the secretion of proinflammatory cytokines IL-1β and TNF-a that are implicated in neuronal toxicity and death [61-63].
Naringenin: Naringenin belongs to flavonones subclass and it is found in tomatoes, dried Mexican oregano, bergamot, pure juice of grapefruit and in several citrus fruits. Extracted from citrus junos, showed a dose-related neuroprotection through inhibition of AchE acitivity. When this flavonone administered to mice (4.5 mg/kg body weight) with scopolamine-induced amnesia resulted in amelioration of symptoms. In another Alzheimer’s disease rat model study, exposure of Aβ-injected Wistar rats to naringenin (100 mg/kg) improved learning and memory and in parallel reduced levels of hippocampal Malondialdehyde (MDA) indicating a remission of lipid peroxidation. Naringenin is capable of inhibiting neuroinflammation pathways through suppression of cytokine signaling 3 expression [64-66].
Oleuropein aglycone (OA) and Hydroxytyrosol: Hydroxytyrosol is derived from hydrolysis of oleuropein found mainly in extra virgin olive oil and black olives. A diet rich in EVOO polyphenols could prevent cognitive decline through inhibition of tau aggregation and upregulation of nerve growth factor receptor expression in mouse brain. HT enhances peroxisome proliferator-activated receptor coactivator 1 alpha (PPARGC1α) and Nuclear factor-E2-related factor-2 (Nrf2) in cultured ARPE-19 human retinal pigment epithelial cells, leading to mitochondrial biogenesis. Similar results obtained from cell line study, since HT treatment restored mitochondrial energy deficit. Other proposed mechanisms are restoration of the GSH/GSSG ratio and JNK/ MAPK signaling in animal AD model study and reservation of the survival genes downscale mediated by amyloid. For these reasons, HT and oleuropein could be considered as promising therapeutic agents in neurological and neurodegenerative disorders [67-71].
Curcumin: Curcumin, an active polyphenol derived from Curcuma Longa exerts beneficial effects through its antioxidant, anti- inflammatory, chemoprotective and neuroprotective mechanisms. Curcumin’s ability to fight neuroinflammation has been displayed in in vitro and in vivo studies through inhibition of Nuclear Factor kappa B (NF-κB), Cyclooxygenase-2 (COX-2) and proinflammatory cytokines (TNF-a., IL-1, -2, -6, -8, and -12) [72]. It also scavenges Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) and reduces lipid peroxidation in aged rat brain regions including cerebral cortex, hippocampus, cerebellum and medulla [73]. In animals studies, curcumin administration ended up with restoration of acetyl cholinesterase activity and upregulation of Brain Derived Neurotrophic Factor (BDNF) expression. Finally, curcumin binds more effectively with redox-active metals such as iron and copper than the redox- inactive zinc, reducing metal neurotoxicity and amyloid aggregation [74-76]. Further investigation is needed to assess curcumin’s efficacy against AD due to its low bioavailability and limited human studies (Table 1).
| Polyphenolic compounds | Chemical structure | Mechanisms of action | Toxicity prediction by softwares | Blood Brain Barrier (BBB) permeability (ToxDP2 Database) |
|---|---|---|---|---|
(-)-Epigallocatechin gallate C22H18O11 Green and black tea, pecan  |
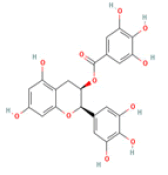 |
PKC–dependent formation of non-amyloidogenic soluble APP binding to oligomeric peptide with high affinity; Interaction with tau and TTR | Hepatotoxicity (ToxDP2 Database); Predicted LD50: 1000 mg/kg Predicted Toxicity Class: 4 (ProTox-II) | Undefined |
Resveratrol C14H12O3 Muscadine grape, grape juice, red wine, Lingonberry, peanuts  |
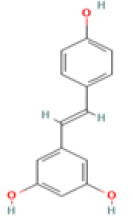 |
Induction of Amyloid clearance; Reduction of hippocampal ER stress; Enhancement of mitochondrial biogenesis | Predicted LD50: 1560 mg/kg Predicted Toxicity Class: 4(ProTox-II) | Medium |
Quercetin C15H10O7 Tea, apples, onions, dark chocolate, red wine  |
 |
Inhibition of neuro inflammation | Changes in prostate, Hepatotoxicity, no mutagenicity, carcinogenicity (ToxDP2 Database) Predicted LD50: 159 mg/kg Predicted Toxicity Class: 3(ProTox-II) | Undefined |
Caffeic acid C9H8O4 Whole grain flour, sunflower seed, Black chokeberry, date, prune juice, dried oregano, thyme, sage, rosemary, spices (Ceylan Cinnamon, cymin, ginger)  |
 |
Inhibition of AChE activity and nitrite synthesis; Suppression of oxidative stress; Suppression of inflammation via p53 and p38 MAPK signaling pathway, nuclear factor‑κB‑, p65 protein expression and caspase‑3 activity | Absence of hepatotoxicity/ carcinogenicity (ToxDP2 Database), Predicted LD50: 2980 mg/kg Predicted Toxicity Class: 5(ProTox-II) | Low |
Chlorogenic acid C16H18O9 coffee, black tea, plum, blueberry, apricot  |
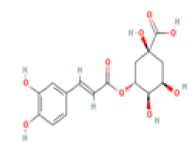 |
Inhibition of AChE and BChE activity | Absence of hepatotoxicity/ carcinogenicity (ToxDP2 Database); Predicted LD50: 5000 mg/kg Predicted Toxicity Class: 5(ProTox-II) | No available data |
Luteolin C15H10O6 Black olive, artichoke, broccoli, oregano, fresh sage, fresh thyme  |
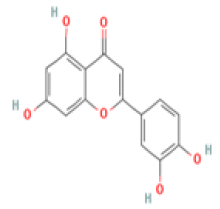 |
Upregulation of the expression and the activity of BDNF; Regulation of microglia neuroinflammation in aged mice; Reduction of Aβ and GSK-3 activity in a Tg2576 mouse model | Hepatotoxicity, carcinogenicity, non-mutagenicity (ToxDP2 Database) Predicted LD50: 3919 mg/kg Predicted Toxicity Class: 5(ProTox-II) | Undefined |
Rosmarinic acid C18H16O8 Dried herbs: Rosemary, thyme, sage, peppermint, oregano  |
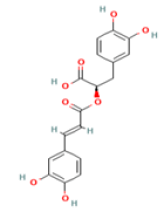 |
Inhibition of ab aggregation through RA–Cu(II) –Aβ complex | No hepatotoxicity, no carcinogenicity Predicted LD50: 5000 mg/kg Predicted Toxicity Class: 5(ProTox-II) | Undefined |
Rutin C27H30O16 Asparagus, apples, berries, grapes, blackcurrant, lemons,  |
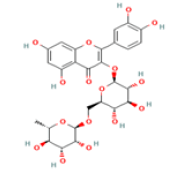 |
Inhibition of tau aggregation; Suppression of proinflammatory cytokines IL-1β and TNF-α; Deactivation of the nuclear factor-kappa B (NF-kB) pathway | Predicted LD50: 5000 mg/kg Predicted Toxicity Class: 5(ProTox-II) | No available data |
Naringenin C15H12O5 Orange, Mexican oregano, grapefruit juice  |
 |
Suppression of neuroinflammation; Inhibition of AChe activity in a dose-dependent manner | Hepatotoxicity, carcinogenicity, non mutagenicity reported (ToxDP2 Database) Predicted LD50: 2000 mg/kg Predicted Toxicity Class: 4(ProTox-II) | Low |
Myricetin C15H10O8 Chinese, bayberry, tea, wine, kale, berries, oranges, tomatoes, honey  |
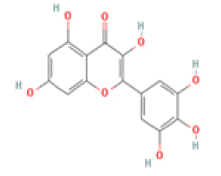 |
Inhibition of BACE1 activity; Enhancement of BDNF expression | Hepatotoxicity, carcinogenicity, mutagenicity reported (ToxDP2 Database), Predicted LD50: 159 mg/kg Predicted Toxicity Class: 3(ProTox-II) | Undefined |
|
Cinnamaldehyde C9H8O Cinnamomum plant |
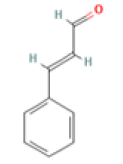 |
Inhibition of tau aggregation and filament formation; Downregulation of of NF-kappaB activation | Predicted LD50: 1850 mg/kg Predicted Toxicity Class: 4(ProTox-II) | No available data |
Oleuropein aglycone (OA) C19H22O8 Extra virgin olive oil, black olives  |
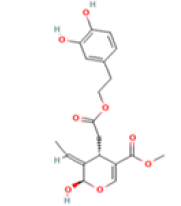 |
Autophagy and αβ amyloid deposition clearance; Destabilization of the whole amyloid fibril | Predicted LD50: 500 mg/kg Predicted Toxicity Class: 4(ProTox-II) | No available data |
| Hydroxytyrosol C8H10O3 Extra virgin olive oil, olives, wine |
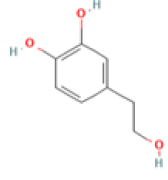 |
Inhibition of tau aggregation ; Restoration of mitochondrial energy deficit ; Restoration of the GSH/GSSG ratio and JNK/MAPK signaling | NOAEL=500 mg/kg/d, Predicted LD50: 2820 mg/kg Predicted Toxicity Class: 5(ProTox-II) | No available data |
|
Curcumin C21H20O6 Turmeric, curry
|
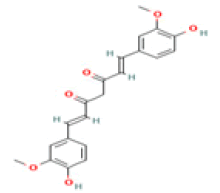 |
Induction of BASE-1; Suppression of amyloid; Metal chelation; Restoration of acetylcholinesterase activity; Reduction of lipid peroxidation in aged rat brain regions | Predicted LD50: 2000 mg/kg Predicted Toxicity Class: 4(ProTox-II) | No available data |
Table 1: Summary of major polyphenolic compounds with their dietary sources, proposed neuroprotective activities, toxicity prediction and BBB permeation.
Summary of major polyphenolic compounds with their dietary sources, proposed neuroprotective activities, potential toxicity and BBB permeation. Adapted from http://phenol-explorer.eu/, http://ctf. iitrindia.org/toxdpp/, https://tox-new.charite.de/protox_II/. LD50, Lethal Dose, the amount of a chemical which causes the death of 50% of a population of test animals via oral, dermal, inhalation or intravenous; ToxDP2 Database, Toxicity Prediction of Dietary Polyphenols, ProTox- II, prediction of toxicity of chemicals.
Pro-oxidant activity and toxicity of polyphenolic compounds
Polyphenols oxidize in cell culture media producing a range of free radicals including O2•–H2O2, semiquinones and quinones with potential cytotoxic effects. Antioxidant agents such as dietary polyphenols may act, in parallel, as pro-oxidant compounds through their capacity to chelate metals. Grape Seed Proanthocyanidin Extract (GSPE), at high doses of 500 micrograms/mL, acted as a ROS inducer and caused apoptotic cell injury in cardiomyocytes, through upregulation of Caspase-3 activity. In animal rat study, under Fe3+-EDTA conditions, both myricetin and quercetin accelerated the formation of hydroxyl radicals (•OH) and DNA damage in rat liver microsomes. However, it is unlikely that myricetin might possess human health risks when used in trace concentration [77-80].
Caffeic acid (CA) seems to act as a promoter of lipid peroxidation after chelating Copper (Cu) and forms a CA-Cu(II) complex with a high electron transfer activity resulting in the genesis of semiquinone radical anion with Cu(I). CA dissociates or deprotonates to form a phenoxide (a salt of a phenol), where the Cu(I) ion will be bound as a chelating binder. The reaction of oxygen (O2) with Cu(I) gives rise to hydrogen peroxide which is catalysed by Fenton reaction to the free hydroxyl radical (−OH) [81]. Copper and iron, as transition metals, catalyse the redox process of phenolics when oxygen is available, resulting in the upregulation of ROS and phenoxyl radicals. These pro-oxidant phenoxyl radicals can oxidize either GSH or NADH with further formation of reactive oxygen radicals. Moreover, phenol rings in dietary polyphenols are capable of inducing erythrocyte hemolysis from oxidation of erythrocyte oxyhemoglobin [82].
A recent systematic review based on toxicological effects of catechin enriched green tea extracts showed that hepatotoxicity was associated with (–)-Epigallocatechin-3-gallate, consumption at doses from 140 mg to 1,000 mg/day. Genetic susceptibility and overall liver health are contributors to these side effects [83]. Interventional clinical studies reported that EGCG in the form of supplement, when given at doses above 800 mg EGCG/day, for more than 4 months significantly elevated plasma transaminases (ALT, AST). The mean daily intake of EGCG from green tea infusions, in Europe ranges from 90-300 mg/day whereas daily intakes for high consumers can reach high exposure levels equal to 300–866 mg/day. Although Tolerable Upper Intake Levels (ULs) for polyphenols have not yet been established, traditionally prepared green tea beverages (up to 10 cups/day) have been reported as a safe way of EGCG administration, whereas herbal extracts and catechin supplements should be used with caution due to possible cytotoxicity [84]. Overall toxicity data shows that polyphenolics with a phenol ring in their structure demonstrate a higher pro-oxidant action with several deleterious impacts than polyphenolics containing a catechol ring [85].
Toxicity prediction models of dietary polyphenols
The Toxin and Toxin Target Database (T3DB) or the Toxic Exposome Database (TEDB) is a comprehensive bioinformatics resource that provides data on 42,374 toxin and 33,000 toxin-target associations. Data are extracted from scientific literature as well as from DrugDatabases (DrugBank, Therapeutic Target DB, PharmGKB), metabolic pathways Databases (KEGG, HumanCyc), compound-specific databases (FooDB, Phenol-Explorer, PubChem, The Toxic Exposome Database (TEDB) and The Human Metabolome Database (HMDB). For each toxin, T3DB provide information to the public such as chemical properties, toxicity values, mechanism of toxicity, toxin-drug interactions, molecular and cellular interactions [86]. Toxicity prediction of dietary polyphenols (ToxDP2 Database) is another data-driven approach that provides knowledge about safety of polyphenolic bioactive compounds, exploring toxicity endpoints, physicochemical properties and pharmacokinetics characteristics including ADME (Absorption, Distribution, Metabolism, and Excretion).The physicochemical properties and potential harmful effects from acute or chronic exposure mainly from supplement/pharmaceutical products, are associated to molecular structure. The QSAR studies correlate biological end points such as pharmacokinetics and pharmacodynamics assessments with chemical properties at atomic/molecular level (Hansch analysis) or with structural characteristics (Free-Wilson analysis). Finally, the Pro Tox-II is a webserver that provides in silico toxicity prediction based on data from both in vitro assays (cytotoxicity assays, hepatic and cytotoxicity assays) and in vivo cases (hepatotoxicity, carcinogenicity).The in-silico toxicity model is a considerably less time consuming technique that minimizes the cost and the need for animal testing. In the ProTox-II platform, the input data refers to information (name of the selected molecule, SMILES format, chemical structure drawing) provided by the user. The output information refers to the predicted median lethal dose (LD50) in mg/kg weight, the toxicity class and the prediction accuracy. These computer-aided prediction toxicity models that predict carcinogenicity, mutagenicity, hepatotoxicity, developmental toxicity, are based on machine learning algorithms (example: a Random Forest algorithm) and then are appropriately validated for accuracy [87].
Conclusion and Future Perspective
Plant flavonoids’ capacity to induce amyloid aggregation clearance, inhibit the AChE and BChE activity, relieve ER stress, scavenge free radicals, downregulate inflammatory mediators and restore mitochondrial energy deficit have been proposed as potential neuroprotective actions in several in vitro and in vivo animal studies. Although the majority of polyphenols studied, showed promising findings against AD-induced neurotoxicity, when administered at high doses, they may exhibit pro-oxidant status and cytotoxicity in humans. These detrimental effects are worth health concerning and should be considered before dietary recommendations are made. Under these conditions, toxicity prediction tools are crucial steps in the identification of novel polyphenolic ingredient candidates in AD neutraceutical discovery field. Omics studies and well-designed clinical trials combined with data-driven toxicity prediction approaches, are needed to establish the therapeutic efficacy in AD and safety among different species of polyphenolic compounds.
Acknowledgement
Sincere thanks to the staff of the Bioinformatics and Human Electrophysiology Laboratory (BiHeLab) at the Ionian University for the discussions and support to our work mentioned in this review.
Conflict of Interest Disclosure
The authors have no conflicts of interest to declare.
References
- Cherian L, Wang Y, Fakuda K, Leurgans S, Aggarwal N, et al. (2019) Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis 6: 267-273.
- Liu X, Morris MC, Dhana K, Ventrelle J, Johnson K, et al. (2021) Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: Rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials 102: 106270.
- Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2: 270-278.
- Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, et al. (2016) Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 55: 1359-1375.
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45: 287-306.
- Choi DY, Lee YJ, Hong JT, Lee HJ (2012) Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res Bull 87: 144-153.
- Woodward KA, Draijer R, Thijssen DH, Low DA (2018) Polyphenols and microvascular function in humans: A systematic review. Curr Pharm Des 24: 203-226.
- Banks WA (2009) Characteristics of compounds that cross the blood-brain barrier. BMC neurology 9: 1-5.
- Jaganath IB, Mullen W, Lean ME, Edwards CA, Crozier A (2009) In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic Biol Med 47: 1180-1189.
- Grabska-Kobylecka I, Kaczmarek-Bak J, Figlus M, Prymont-Przyminska A, Zwolinska A, et al. (2020) The presence of caffeic acid in cerebrospinal fluid: Evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients 12: 1531.
- Peng Y, Hou C, Yang Z, Li C, Jia L, et al. (2016) Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol Nutr Food Res 60: 2331-2342.
- Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, et al. (2003) Interaction between flavonoids and the blood–brain barrier: In vitro studies. J Neurochem 85: 180-192.
- Janle EM, Lila MA, Grannan M, Wood L, Higgins A, et al. (2010) Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and the central nervous system following oral administration. J Med Food 13: 926-933.
- El Gaamouch F, Liu K, Lin HY, Wu C,Wang J. (2021) Development of grape polyphenols as multi-targeting strategies for Alzheimer’s disease. Neurochem Int 147: 105046.
- Birt DF, Hendrich S, Wang W. (2001) Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol Ther 90: 157-177.
- Sabogal-Guáqueta AM, Munoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, et al. (2015) The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology 93: 134-145.
- Holland TM, Agarwal P, Wang Y, Leurgans SE, Bennett DA et al. (2020) Dietary flavonols and risk of Alzheimer dementia. Neurology 94: e1749-e1756.
- Frolinger T, Herman F, Sharma A, Sims S, Wang J, et al. (2018) Epigenetic modifications by polyphenolic compounds alter gene expression in the hippocampus. Biology open 7: Bio035196.
- Li L, Miao M, Chen J, Liu Z, Li W, et al. (2021) Role of Ten eleven translocation‐2 (Tet2) in modulating neuronal morphology and cognition in a mouse model of Alzheimer's disease. J Neurochem.
- Aruoma OI, Sun B, Fujii H, Neergheen VS, Bahorun T, et al. (2006) Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors 27: 245-265.
- Chen X, Xu B, Nie L, He K, Zhou L, et al. (2021) Flavanol-rich lychee fruit extract substantially reduces progressive cognitive and molecular deficits in a triple-transgenic animal model of Alzheimer disease. Nutr Neurosci 24: 720-734.
- Mattivi F, Guzzon R, Vrhovsek U, Stefanini M and Velasco R (2006) Metabolite profiling of grape: Flavonols and anthocyanins. J Agric Food Chem 54: 7692-7702.
- Koyama K, Ikeda H, Poudel PR and Goto-Yamamoto N (2012) Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 78: 54-64.
- Katayama S, Sugiyama H, Kushimoto S, Uchiyama Y, Hirano M, et al. (2016) Effects of sesaminol feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8. J Agric Food Chem 64: 4908-4913.
- Chakraborty S, Basu S. (2017) Multi-functional activities of citrus flavonoid narirutin in Alzheimer’s disease therapeutics: An integrated screening approach and in vitro validation. Int J Biol Macromol 103: 733-743.
- Sabogal-Guáqueta AM, Munoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, et al. (2015) The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology 93: 134-145.
- Kao TK, Ou YC, Raung SL, Lai CY, Liao SL, et al. (2010) Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci 86: 315-321. [Crossref]
- TMIC. (2022) The metabolomics innovation centre. HMBD.
- Fujiki H, Suganuma M (2012) Green tea: An effective synergist with anticancer drugs for tertiary cancer prevention. Can Cancer Lett 324: 119-125.
- Palhano FL, Lee J, Grimster NP, Kelly JW (2013) Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J Am Chem Soc 135: 7503-7510.
- Henríquez G, Gomez A, Guerrero E, Narayan M (2020) Potential role of natural polyphenols against protein aggregation toxicity: in vitro, in vivo, and clinical studies. ACS Chem Neurosci 11: 2915-2934.
- Sonawane SK, Chidambaram H, Boral D, Gorantla NV, Balmik AA, et al. (2020) EGCG impedes human Tau aggregation and interacts with Tau. Sci Rep 10: 12579.
- Ferreira N, Cardoso I, Domingues MR, Vitorino R, Bastos M, et al. (2009) Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett 583: 3569-3576.
- Rooprai HK, McCormick D (1997) Proteases and their inhibitors in human brain tumours: a review. Anticancer Res 17: 4151-4162.
- Duits FH, Hernandez-Guillamon M, Montaner J, Goos JD, Montañola A, et al. (2015) Matrix metalloproteinases in Alzheimer’s disease and concurrent cerebral microbleeds. J Alzheimers Dis 48: 711-720.
- Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R (2000) Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta (BBA)-Prot Struc and Mol Enzym 1478: 51-60.
- Ma T, Tan MS, Yu JT, Tan L (2014) Resveratrol as a therapeutic agent for Alzheimer’s disease. BioMed Res Int.
- Markus MA, Morris BJ (2008) Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging 3: 331.
- Simão F, Matte A, Pagnussat AS, Netto CA, Salbego CG (2012) Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK‐3β and CREB through PI3‐K/Akt pathways. Eur J Neurosci 36: 2899-2905.
- Ahmadi A, Hayes AW, Karimi G (2021) Resveratrol and endoplasmic reticulum stress: A review of the potential protective mechanisms of the polyphenol. Phytother Res 35: 5564-5583
- Xu SL, Bi CW, Choi RC, Zhu KY, Miernisha A, et al. (2013) Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: A signalling response mediated by estrogen receptor. Evid Based Complement Alternat Med.
- Baik J (2020) Neurotrophins and neuropathic pain in patients with traumatic brain injury. The Korean Journal of Pain 33: 1-2.
- Kitazume S, Tachida Y, Kato M, Yamaguchi Y, Honda T, et al. (2010) Brain endothelial cells produce amyloid β from amyloid precursor protein 770 and preferentially secrete the O-glycosylated form. J Biol Chem 285: 40097-40103.
- Song X, Tan L, Wang M, Ren C, Guo C, et al. (2021) Myricetin: A review of the most recent research. Biomed Pharmacother 134: 111017.
- Wang QM, Wang GL, Ma ZG (2016) Protective effects of myricetin on chronic stress-induced cognitive deficits. Neuroreport 27: 652-658.
- Naushad M, Durairajan SSK, Bera AK, Senapati S, Li M (2019) Natural compounds with anti-BACE1 activity as promising therapeutic drugs for treating Alzheimerʼs disease. Planta medica 85: 1316-1325.
- Hassan H, Rawlinson C, Morgan D, Chen R (2020) Pharmacological properties of rutin and its potential uses for Alzheimer’s disease. J Exp Stroke and Trans Med.
- Sun XY, Li LJ, Dong QX, Zhu J, Huang YR, et al. (2021) Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation 18: 1-14.
- Van Dam RM, Dekker JM, Nijpels G, Stehouwer CDA, Bouter LM, et al. (2004) Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia 47: 2152-2159.
- Kim B, Nam Y, Kim J, Choi H, Won C (2012) Coffee consumption and stroke risk: A meta-analysis of epidemiologic studies. Korean J Fam Med 33: 356.
- Socała K, Szopa A, Serefko A, Poleszak E, Wlaź P (2020) Neuroprotective effects of coffee bioactive compounds: a review. Int J Mol Sci 22: 107.
- Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J et al. (2014) Heart diseases and long-term risk of dementia and Alzheimer's disease: a population-based CAIDE study. J Alzheimers Dis 42: 183-191.
- Andrade S, Loureiro JA, Pereira MC (2021) Caffeic acid for the prevention and treatment of Alzheimer's disease: The effect of lipid membranes on the inhibition of aggregation and disruption of Aβ fibrils. Int J Biol Macromol 190: 853-861.
- Wang Y, Wang Y, Li J, Hua L, Han B, et al. (2016) Effects of caffeic acid on learning deficits in a model of Alzheimer's disease. Int J Mol Med 38: 869-875.
- Olthof MR, Hollman PC, Buijsman MN, Van Amelsvoort JM, Katan MB (2003) Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J nutr 133: 1806-1814.
- Bai G, Wang Y, Kuja-Halkola R, Li X, Tomata Y, et al. (2021) Frailty and the risk of dementia: The association explained by shared environmental and genetic factors? BMC medicine 19: 1-12.
- Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, et al. (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol 649: 210-217.
- Nazir N, Zahoor M, Nisar M, Karim N, Latif A, et al. (2020) Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complement Med Ther 20: 1-17.
- Kola A, Hecel A, Lamponi S, Valensin D (2020) Novel perspective on Alzheimer’s disease treatment: Rosmarinic acid molecular interplay with copper (II) and amyloid β. Life 10: 118.
- Noguchi-Shinohara M, Ono K, Hamaguchi T, Nagai T, Kobayashi S, et al. (2020) Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci rep 10: 1-10.
- Peterson DW, George RC, Scaramozzino F, LaPointe NE, Anderson RA, et al. (2009) Cinnamon extract inhibits tau aggregation associated with Alzheimer's disease in vitro. J Alzheimers Dis 17: 585-597.
- Kim DH, Kim CH, Kim MS, Kim JY, Jung KJ, et al. (2007) Suppression of age-related inflammatory NF-κB activation by cinnamaldehyde. Biogerontology 8: 545-554.
- Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, et al. (2008) Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol 46: 220-231.
- Heo HJ. Kim MJ, Lee JM, Choi SJ, Cho HY, et al. (2004) Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement Geriatr Cogn Disord 17: 151-157.
- Ghofrani S, Joghataei MT, Mohseni S, Baluchnejadmojarad T, Bagheri M, et al. (2015) Naringenin improves learning and memory in an Alzheimer's disease rat model: Insights into the underlying mechanisms. Eur J Pharmacol 764: 195-201.
- Wu LH, Lin C, Lin HY, Liu YS, Wu CYJ, et al. (2016) Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol Neurobiol 53: 1080-1091.
- Daccache A, Lion C, Sibille N, Gerard M, Slomianny C, et al. (2011) Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem Int 58: 700-707.
- Luceri C, Bigagli E, Pitozzi V, Giovannelli L (2017) A nutrigenomics approach for the study of anti-aging interventions: olive oil phenols and the modulation of gene and microRNA expression profiles in mouse brain. Eur J Nutr 56: 865-877.
- Zhu L, Liu Z, Feng Z, Hao J, Shen W, et al. (2010) Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J Nutr Biochem 21: 1089-1098.
- Visioli F, Rodríguez-Pérez M, Gómez-Torres Ó, Pintado-Losa C, Burgos-Ramos E (2020) Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer’s disease. Nutr Neurosci.
- Peng HW, Cheng FC, Huang YT, Chen CF, Tsai TH (1998) Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 714: 369-374.
- Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, et al. (2005) Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol 174: 8116-8124.
- Bala K, Tripathy BC, Sharma D (2006) Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology 7: 81-89.
- Awasthi H, Tota S, Hanif K, Nath C, Shukla R (2010) Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life sci 86: 87-94.
- Nam SM, Choi JH, Yoo DY, Kim W, Jung HY, et al. (2014) Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food 17: 641-649.
- Baum L , Ng A (2004) Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimer's dis 6: 367-377.
- Halliwell B. (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476: 107-112.
- Shao ZH, Hoek TLV, Xie J, Wojcik K, Chan KC, et al. (2003) Grape seed proanthocyanidins induce pro-oxidant toxicity in cardiomyocytes. Cardiovasc toxicol 3: 331-339.
- Laughton MJ, Halliwell B, Evans PJ, Robin J, Hoult S (1989) Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem pharmacol 38: 2859-2865.
- Hobbs CA, Swartz C, Maronpot R, Davis J, Recio L, et al. (2015) Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin. Food Chem Toxicol 83: 283-292.
- Espíndola KMM, Ferreira RG, Narvaez LEM, Silva Rosario ACR, da Silva AHM, et al. (2019) Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front oncol 9: 541.
- Han X, Shen T, Lou H (2007) Dietary polyphenols and their biological significance. Int J M Sci 8: 950-988.
- Oketch-Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, et al. (2020) United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep 7: 386-402.
- Yates A.A, Erdman Jr JW, Shao A, Dolan LC, Griffiths JC (2017) Bioactive nutrients-time for tolerable upper intake levels to address safety. Reg Toxicol Pharmacol 84: 94-101.
- Galati G, Sabzevari O, Wilson JX, O'Brien PJ (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicol 177: 91-104.
- Wishart D, Arndt D, Pon A, Sajed T, Guo AC, et al. (2015) T3DB: the toxic exposome database. Nucleic acids Res 43: D928-D934.
- Banerjee P, Eckert AO, Schrey AK, Preissner R (2018) ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic acids Res 46: W257-W263.
Citation: Kalli E, Panayiotis V (2022) Dietary Polyphenols as Potential Therapeutics in Alzheimer’s Disease: Pleiotropic Effects and Toxicity Prediction Models. J Alzheimers Dis Parkinsonism 12: 540.
Copyright: © 2022 Kalli E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2875
- [From(publication date): 0-2022 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 2350
- PDF downloads: 525


