Dietary Long-Term Exposures to Fipronil Alter the Expression of Catalase in Lung and Serum
Received: 01-Oct-2022 / Manuscript No. cmb-22-76372 / Editor assigned: 03-Oct-2022 / PreQC No. cmb-22-76372(PQ) / Reviewed: 15-Oct-2022 / QC No. cmb-22- 76372 / Revised: 20-Oct-2022 / Manuscript No. cmb-22-76372(R) / Accepted Date: 27-Oct-2022 / Published Date: 27-Oct-2022 DOI: 10.4172/1165-158X.1000245 QI No. / cmb-22- 76372
Abstract
Fipronil is a broad-spectrum insecticide that belongs to the phenylpyrazole chemical family. We have earlier reported that long term dietary exposures to fipronil cause lung inflammation. Endotoxins are frequently prevalent among agricultural settings and have also been associated with lung damage. However, the mechanism of fipronil induced lung damage with or without endotoxin remains to be elucidated. The present investigations included male Swiss albino mice (N=36) aging 6-8 weeks to estimate catalase expression in lung and serum following exposure to fipronil with or without LPS. Animals were divided into two treatments and one control group (N=12, each). Treatment groups were orally administered high (1/10th of LD50) and low (1/20th of LD50) dose of fipronil dissolved in corn oil for 90 days followed by LPS (80 μl/animal) or NSS challenge via intranasal route. Low dose of fipronil along with LPS resulted 3.75 folds increase in the mRNA expression of catalase in the lung. High and low dose of fipronil alone or in combination with LPS showed strong immunopositive reactivity for catalase in the airways and bronchial epithelial cells. There was a significant increase in the number of immunopositive cells for catalase following exposure to individual high or low dose of fipronil or in combination with LPS as compared to control and LPS group. High dose of fipronil significantly (p<0.05) increased the protein concentration of catalase as compared to control group suggesting dose dependent dysregulation of catalase in serum. The data taken together suggest that exposures to fipronil with or without LPS altered the pulmonary expression of key genes associated with oxidative stress.
Keywords
Fipronil; Lung; Catalase; Oxidative Stress
Introduction
Pesticides are used for destroying harmful insects or other organisms and among these fipronil is used on a very large scale [1]. It has been reported to cause various toxic effects on target and non-target organisms including human by acting as antagonism of Gamma-Amino Butyric Acid/GABA [2]. Endotoxin is a Lipo Poly Saccharide (LPS) molecule derived from Gram-negative bacteria and endotoxin exposure has been related with pulmonary dysfunction [3]. Our previous reports indicate that endotoxin interact with various pesticides to alter the pulmonary responses during pesticide induced lung damage [4-9]. Further long term exposure to fipronil with or without endotoxin cause lung damage and dysregulate the key genes of Wnt pathway in mice [8].
Pesticides increase the production of Reactive Oxygen Species (ROS) resulting tissue damage and oxidative stress [9]. Catalase is a common antioxidant enzyme that uses either iron or manganese as a cofactor and catalyzes the degradation or reduction of H2O2 to water and molecular oxygen [10]. It is present almost in all living tissues such as liver, kidney and erythrocytes [11] and plays an essential role in cell defence against oxidative stress in these organs [12]. Catalase is also expressed in the lung during the later stages of development and is constitutively expressed in the airway, alveolar epithelial cells and macrophages to play an important role in the endogenous antioxidant defence system [12]. Targeting of catalase directly to the mitochondria in lung epithelial cells protects the cells from H2O2-induced apoptosis [13] and deficiency in catalase activity in the lungs predisposes the lung to worsening lung inflammation and subsequent fibrosis [14]. Overexpression of catalase prevent ROS induced damage including initiation of apoptosis and suppress age-related DNA oxidation in skeletal muscle [15].
However, there is no report on the expression of catalase during fipronil and/or LPS induced lung damage. Hence, the present study was conducted and we report the first data on the mRNA expression of catalase in the lung and its serum concentration following exposure to fipronil alone or in combination with LPS.
Material and Methods
Experimental design
Healthy male Swiss albino mice aging 6-7 weeks (N=36) were randomly divided into three groups viz two treatments and one control group (n=12 each group). Treatment group I and II were orally administered fipronil @ 1/10th and 1/20th of LD50, respectively for 90 days. Fipronil was dissolved in corn oil. Control group was administered with corn oil only. At the end of experiment half animals from all groups (N=6) were anaesthetized with xylazine ketamine combination anesthesia and challenged intranasally with LPS @ 80 μl/animal. The remaining animals of each group were administered same amount of Normal Saline Solution (NSS) intranasally. The animals were sacrifices after 9 hr of LPS or NSS challenge and lung samples were collected in RNA Later solution and paraformaldehyde for quantitative real time PCR analysis and immunohistochemistry, respectively. Blood samples were also collected to estimate serum concentration of catalase in all the groups.
Quantitative Real Time PCR (qPCR) Right lung from each animal stored in RNA later solution at -80°C was used for detection of expression of catalase mRNA by qPCR. About 50 mg of frozen lung from all the samples was homogenized using Qiagen Tissue Ruptor II (Cat No: Invitrogen 9002755). Total RNA was extracted by using Trizol (Ambion, Life Technologies, USA) method and the quality as well quantity of the resulting RNA was assessed by spectrophotometrically by Nanodrop (Thermo Fisher) and also by visualizing via agarose gel electrophoresis. The concentration of total RNA varied between 100-700 ng/ml in different samples. The amount of total RNA used for cDNA synthesis was adjusted to 100 ng/μl for each sample. Total RNA was reversed transcribed into cDNA using a Revert aid cDNA synthesis kit (Thermo Scientific) according to the manufacturer’s instruction. qPCR was performed using Syber green chemistry. Primer sequences used for amplification of MYCN gene was F 5’- GCGTCCAGTGCGCTGTAGA -3’ and R 5’- TCAGGGTGGACGTCAGTGAA -3’ [16]. Further, β-actin (F5’-CTGTCCCTGTATGCCTCTG -3’ and R5’- ATGTCACGCACGATTTCC -3 was used as an endogenous control. Transcript levels were normalized by comparison with β-actin.
Immunohistochemistry
Processed paraffin blocks were used to obtain 5μm thick paraffin sections using a rotary microtome. Paraffin sections were subjected to immunohistochemical staining as described earlier [17] to localize immunopositive catalase cells in the lungs of various groups. The sections were processed and incubated with primary antibody against catalase (Goat polyclonal antibody anti catalase; Elabscience, Dilution 1:25) for one hour followed by incubation with secondary antibody (anti goat, dilution 1:400) for 30 minutes. Colour development was done with a commercial kit (SK4100; Vector Laboratories, USA) followed by counter staining with haematoxylin.
Enzyme-Linked Immuno Sorbent Assay (ELISA)
Sandwich ELISA was conducted to estimate serum concentrations of catalase in different groups by using mouse catalase kit (Immunotag, USA range: 0.2 ng/ml-70 ng/ml and sensitivity 0.093 ng/ml). Standards of different concentrations were prepared such as Standard 1 (2.5ng/ ml), Standard 2 (5.0ng/ml), Standard 3 (10.0ng/ml), Standard 4 (20.0ng/ ml) and Standard 5 (40.0ng/ml) as per the manufacturer’s instructions. About 40 μl of serum samples from all the groups were diluted (1:10) in phosphate buffer saline and were added to the flat bottom polystyrene pre-coated plates. A control consisting of Standard Diluent and Blank (PBS) were also incorporated in the plates. About 50 μl standard solution were added to the wells designated as standard wells. Then 10 μl antibody against the catalase was added to the corresponding sample wells. Standard solution already contains biotinylated antibody. So, antibodies were not added separately to the standard wells. Next, 50 μl streptavidin-HRP conjugate was added to the sample wells and standard wells (not blank) and mixed well. The plates were covered with sealer and incubated for 60 minutes at 37°C. After incubation, the plates were washed five times with 300 μl wash buffer at room temperature for 5 minutes each to remove any unbound antigen. Finally, 50 μl substrate solution A followed by 50 μl substrate solution B was added to each well and the plates were incubated undisturbed at 37°C in the dark for 10 minutes till color was developed. The color reaction was stopped by adding 50μl stop solution into each well. The Absorbances (OD) were measured at a wavelength of 450 nm on ELISA reader. The Absorbance (OD) of unknown sample were inter plotted against the exponential curve to determine serum concentration of catalase in different groups of mice.
Statistical analysis
Data were presented as mean (± SE) and analysed by single analyses of variance (ANOVA) using GraphPad Prism 7 software followed by group comparisons with post-hoc tests. The significance was accepted at P<0.05.
Result and Discussion
Pulmonary expression of catalase mRNA
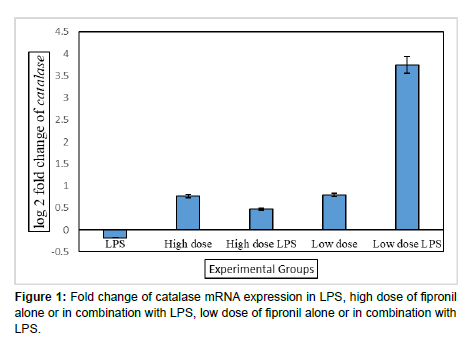
In the present study, the pulmonary mRNA expression of catalase was evaluated by using real time PCR in the lung samples of all the groups following long term exposures to fipronil with or without LPS. Catalase plays a central role in the antioxidant system of the lungs due to its ability to convert H2O2 to oxygen and water. Catalase is the most abundant antioxidant gene among the 22 oxidative and metabolic stressrelated genes and is expressed in bronchiolar epithelium in normal adult lung [18]. In the present observations, LPS or both individual doses of fipronil did not alter the mRNA expression of catalase (Figure 1). There was downregulation of catalase activity during bleomycin-induced inflammation and fibrosis in the lung of mice [19]. Catalase handles the intracellular accumulation of H2O2 and its toxic derivatives, however hydrogen peroxide reversely downregulates catalase expression [20]. Low levels of catalase expression are correlated with high H2O2 production and activation of signaling pathways to cause proliferation, migration and invasion of cancer cells [21]. Decreased catalase elevates H2O2 which activate signal transduction pathways for the formation of squamous cell carcinomas [22]. Similarly [23] observed that the immortalization and transformation of mouse liver cells with SV40 (Simian virus 40) decreased catalase expression resulting oncogenesis via increasing the levels of ROS in transformed cells as cancer cells require higher ROS amounts than healthy cells [24].
In the present investigations, low dose of fipronil along with LPS resulted 3.75 folds increase in the mRNA expression of catalase in the lung (Figure 1). Catalase expression increases after initial smoke exposure in lungs of mice [24] reported that catalase overexpression (7- fold) in MCF-7 cells impaired the proliferation and migration capacities of MCF-7 cells and suggested that changes in the expression of catalase may be a mechanism of resistance of cancer cells towards redox-based chemotherapeutic drugs. Similarly, [22] observed that catalase plays an important role as an antioxidant to maintain the redox state during the progression step of carcinogenesis.
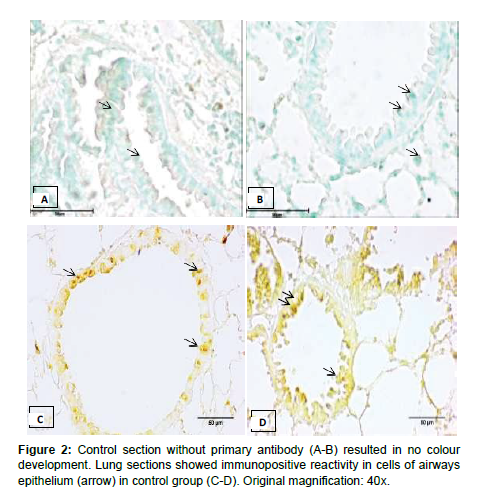
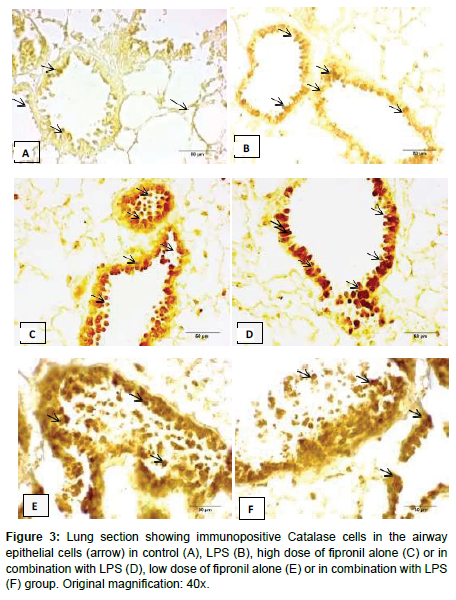
In the present study, the lung sections incubated without primary antibody did not show any colour development in control group (Figure 2). There was moderate catalase immunopositive reactivity in the cells of airways and bronchium following exposure to LPS (Figure 3). Further, LPS did not alter the number of immunopositive cells (Table 1). High and low dose of fipronil alone or in combination with LPS showed strong immunopositive reactivity for catalase in the airways and bronchial epithelial cells (Figure 2). There was a significant increase in the number of immunopositive cells for catalase following exposure to individual high or low dose of fipronil or in combination with LPS as compared to control and LPS group (Table 1). Catalase is expressed in later stages of pulmonary development and is constitutively expressed in airways and alveolar epithelial cells and macrophages [19].
| Groups | Number of immunopositive cells | Serum concentration (ng/mL) |
|---|---|---|
| Control | 199.16 ± 9.19 | 30.32a ± 0.92 |
| LPS | 78.00a ± 6.42 | 31.45a ± 2.00 |
| High dose | 80.66c ± 9.62 | 78.74b ± 6.68 |
| High dose + LPS | 85.50b ± 8.76 | 29.53a ± 1.32 |
| Low dose | 99.33b ± 12.63 | 29.81a ± 2.56 |
| Low dose + LPS | 277.50c ± 14.97 | 30.36a ± 4.97 |
| Values are Mean ± SE. Means bearing superscript ab differ significantly at p ≤ 0.05. | ||
Table 1: Immunopositive Score and serum concentration of catalase in various groups.
Catalase expression in serum
In the present study, individual LPS or low dose of fipronil did not alter the serum concentration of catalase (Table 1). However high dose of fipronil significantly (p<0.05) increased the protein concentration of catalase as compared to control group suggesting dose dependent dysregulation of catalase in serum (Table 1). Increased serum catalase activity alter immune function, viral replication and/or repair processes [25]. Catalase has both beneficial and deleterious effects as addition of catalase protected cultured endothelial cells against H202 induced damage, while the same concentrations did not effect the neutrophil bactericidal activity or mononuclear cell cytotoxicity in vitro [26]. Catalase expression increases following inhibition of the PI3K/Akt signaling pathway in vascular smooth muscle of rat and human MCF-7 cancer cells [25-29].
Acknowledgement
None
Conflict of Interest
None
References
- Qian J, Liu L, Chen L, Lu X and Zhu C (2015) Increased toll like receptor 9 expression is associated with the severity of paraquat-induced lung injury in mice. Hum Exp Toxicol 34: 430-438.
- Godinho AF, Oliveira Souza AC, Carvalho CC, Horta DF, Fraia DD, et al. (2016) Memory impairment due to fipronil pesticide exposure occurs at the GABAA receptor level, in rats. Physiol Behav 165: 28-34.
- Singh J, Schwartz D (2005) Endotoxin and the lung: Insight into the host-environment interaction. Environmental and occupational respiratory disorders. J Allergy and Clin Immunol 12: 330-333.
- Kaur S, Mukhopadhyay CS, Sethi RS (2016) Chronic exposure to indoxacarb and pulmonary expression of toll-like receptor-9 in mice. Vet World 9: 1282-1286.
- Merkowsky K, Sethi RS, Gill JPS, Gill BS (2016) Fipronil induces lung inflammation in vivo and cell death in vitro. J Occup Med Toxicol 11: 1-10.
- Pandit AA, Choudhary S, Verma R, Singh B, Sethi RS (2016) Imidacloprid induced histomorphology changes and expression of TLR-4 and TNFα in lung. Pestic Biochem Physiol 131: 9-17.
- Tewari A, Sethi RS, Banga HS, Singh B, Gill J (2017) Concomitant effect of low dose of lindane and intranasal lipopolysaccharide on respiratory system of mice. Hum Exp Toxicol 36: 1201-1211.
- Pandit AA, Sethi RS (2019) Pulmonary expression of Pla2g5 during lung damage in mice induced by fipronil and lipopolysaccharide interaction. J Appl Nat Sci 11: 285-290.
- Verma G, Mukhopadhyay CS, Verma R, Singh B, Sethi RS (2019) Long-term exposures to ethion and endotoxin cause lung inflammation and induce genotoxicity in mice. Cell and Tissue Res 15: 1-13.
- Pandit A, Kumar R, Mukhopadhyay CS, Verma R, Sethi RS (2019) Transcriptome analysis reveals the role of the PCP pathway in fipronil and endotoxin-induced lung damage. Respir Res 20: 24.
- Verma N, Khosa RL, Pathak AK (2008) Antioxidant and free radical scavenging activity of fruits of Ficus bengalensis linn. Pharmacology online 3: 206-215.
- Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61: 192-208.
- Zamocky M, Furtmüller PG, Obinger C (2008) Evolution of catalases from bacteria to humans. Antioxid and Redox Signal 10: 1527-1548.
- Nishikawa, Hashida M, Takakura Y (2009) Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv Drug Deliv Rev 61: 319-326.
- Sethi RS, Schneberger D, Singh B (2013) Characterization of the lung epithelium of wild-type and TLR9 mice after single and repeated exposures to chicken barn air. Exp Toxicol Pathol 65: 357-364.
- Arita Y, Harkness SH, Kazzaz JA, Koo HC, Joseph A, et al. (2006) Mitochondrial localization of catalase provides optimal protection from H2O2-induced cell death in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 290: L978-L986.
- Raza Y, Khan A, Farooqui A, Mubarak M, Facista, et al. (2014) Oxidative DNA damage as a potential early biomarker of Helicobacter pylori associated carcinogenesis. Pathol Oncol Res 20: 839-846.
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, et al. (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909-1911.
- Wang X, Phelan S, Forsman S, Kristina T, Petros E, et al. (2003) Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278: 25179-25190.
- Betsuyaku T, Fuke S, Inomata T, Kaga K, Morikawa T, et al. (2013) Regulation of bronchiolar catalase in COPD depends on the duration of cigarette smoke exposure. European Respiratory Journal 42: 42-53.
- Odajima N, Betsuyaku T, Nagai K (2010) The role of catalase in pulmonary fibrosis. Respir Res 11: 183.
- Goth L, Rass P and Pay A (2004) Catalase enzyme mutations and their association with diseases. Mol Diagn 8: 141-149.
- Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB (2015) Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med 87: 84-97.
- Kwei KA, Finch JS, Thompson EJ, Bowden GT (2004) Transcriptional repression of catalase in mouse skin tumor progression. Neoplasia (New York, NY) 6: 440.
- Glorieux C, Marcelo J, Dejeans N, Sandrine N, Khadija B, et al. (2018) Evaluation of Potential Mechanisms Controlling the Catalase Expression in Breast Cancer Cells. Oxid Med Cell Longev 2018: 1-10.
- Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappaB transcription factor and HIV-1. EMBO J 10: 2247-2258.
- Leff JA, Oppegard MA, Curiel TJ, Brown KS, Schooley RT, et al. (1992) Progressive increases in serum catalase activity in advancing human immunodeficiency virus infection. Free Radic Biol Med 13: 143-149.
- Chiu ST, Hsieh FJ, Chen SW, Chen CL, Shu HF, et al. (2005) Clinicopathologic correlation of up-regulated genes identified using cDNA microarray and real-time reverse transcription-PCR in human colorectal cancer. Cancer Epidemiol Biomarkers Prev 14: 437-443.
- Glorieux C, Auquier J, Dejeans N, Sid B, Demoulin JB, et al. (2014) Catalase expression in MCF-7 breast cancer cells is mainly controlled by PI3K/Akt/mTor signaling pathway. Biochem Pharmacol 89: 217-223.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Citation: Kaur R, KaurR, Sethi RS (2022) Dietary Long-Term Exposures to Fipronil Alter the Expression of Catalase in Lung and Serum. Cell Mol Biol, 68: 245. DOI: 10.4172/1165-158X.1000245
Copyright: © 2022 Kaur R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2725
- [From(publication date): 0-2022 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 2283
- PDF downloads: 442