Review Article Open Access
Diagnostic Challenges in Pediatric Round Blue Cell Tumors of the Bone
Nicoleta C. Arva*Department of Pathology and Laboratory Medicine, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL 60611, USA
- *Corresponding Author:
- Nicoleta C. Arva
Department of Pathology and Laboratory Medicine
Ann & Robert H. Lurie Children's Hospital of Chicago
225 E. Chicago Avenue, Chicago
Illinois 60611, USA
Tel: 312-227-3956
Fax: 312-227-9616
E-mail: NArva@luriechildrens.org
Received date: Feb 29, 2016; Accepted date: Apr 21, 2016; Published date: Apr 25, 2016
Citation: Arva NC (2016) Diagnostic Challenges in Pediatric Round Blue Cell Tumors of the Bone. Diagn Pathol Open 1:110. doi:10.4172/2476-2024.1000110
Copyright: © 2016 Arva NC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Diagnostic Pathology: Open Access
Abstract
Bone lesions, both neoplastic and non-neoplastic, are common in children. The accurate diagnosis is usually established following tissue sampling with histopathologic examination. However, those entities with predominant small round blue cell morphology can be diagnostically challenging. This review highlights distinctive morphologic and molecular features that can help pathologists when encountering these cases.
Introduction
Bone lesions are frequent in the pediatric age group and include both neoplastic and non-neoplastic conditions. In fact, about half of all bone lesions are encountered in the first two decades of life [1]. During childhood, the most common benign entities are represented by osteomyelitis, bone cysts (either aneurysmal or unicameral bone cyst), osteochondroma or non-ossifying fibroma. Malignant lesions are usually represented by osteosarcoma and Ewing sarcoma [2].
In many instances, the diagnosis is established after tissue sampling and pathologic examination. Although some entities, such as conventional-type osteosarcoma, have straightforward histologic features, those cases with predominant small round blue cell morphology can be diagnostically challenging and their correct classification usually requires additional ancillary/molecular studies. This review highlights the distinctive morphological and molecular features of such neoplasms that can help pathologists when encountering these cases.
Ewing sarcoma
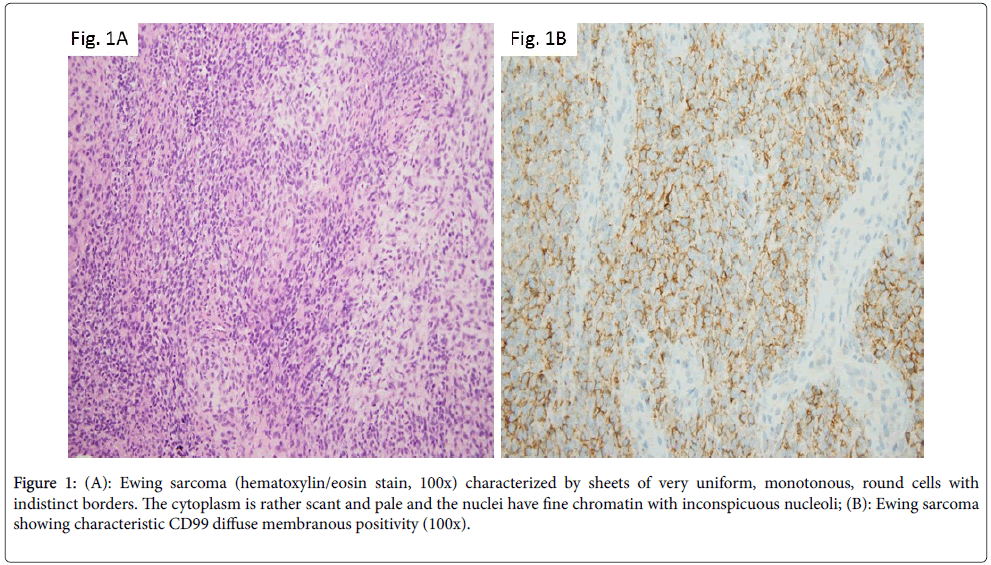
The most common malignant neoplasm of the bone revealing small round blue cell morphology is represented by Ewing sarcoma family of tumors (EFT). This entity accounts for 6% to 8% of primary malignant bone tumors, it is more commonly seen in Caucasians and has a slight male predominance [3]. Classically, the tumor involves the diaphysis or metaphysis of long tubular bones, although it can also arise in the axial bones. On radiology studies, the lesion has aggressive appearance, with multi-layered periosteal reaction creating the characteristic “onionskinning” appearance as well as soft-tissue extension. Morphologically, Ewing sarcoma is characterized by sheets of very uniform, monotonous, round cells with indistinct borders. The cytoplasm is rather scant and pale and the nuclei have fine chromatin with inconspicuous nucleoli [4] (Figure 1A). Ancillary studies reveal cytoplasmic PAS positivity due to high amount of glycogen; there is nuclear FLI1 expression and characteristic CD99 diffuse membranous positivity (Figure 1B). Ewing sarcoma has hallmark chromosomal translocations involving usually a member of the TET family of RNAbinding proteins (most commonly EWSR1, but also FUS) and a member of the ETS (E26 transformation specific) family of transcription factors. In about 95% of instances, these rearrangements result in EWSR1-FLI1 (~85% of cases) or EWSR1-ERG (~10% of cases) fusion transcripts. Reverse transcription PCR is frequently used for their detection. In a small percentage of tumors, EWSR1 gene is replaced by the FUS gene; the FUS-rearranged Ewing sarcomas appear to be morphologically and immunohistochemically similar to EWSR1- positive cases [4]. To make things even more complicated, there are neoplasms within EFT displaying fusion between EWSR1 and genes outside the ETS family members, such as PATZ1, NFATc2 or SP3. The morphology in these instances is atypical, with enlarged cells, more nuclear pleomorphism and prominent nucleoli. FISH analysis using EWSR-1 break-apart probe is useful in diagnosis, demonstrating EWSR1 gene rearrangement. However, it should be kept in mind that EWSR1 also partners with other genes such as WT-1 in desmoplastic small round blue cell tumor or with ATF1 in clear cell sarcoma. However, at morphologic and immunophenotypic level, these entities are distinct from Ewing tumors, suggesting that in fact EWSR1’s partner dictates the morphology [3].
Figure 1: (A): Ewing sarcoma (hematoxylin/eosin stain, 100x) characterized by sheets of very uniform, monotonous, round cells with indistinct borders. The cytoplasm is rather scant and pale and the nuclei have fine chromatin with inconspicuous nucleoli; (B): Ewing sarcoma showing characteristic CD99 diffuse membranous positivity (100x).
Sarcoma with CIC-DUX4 translocation
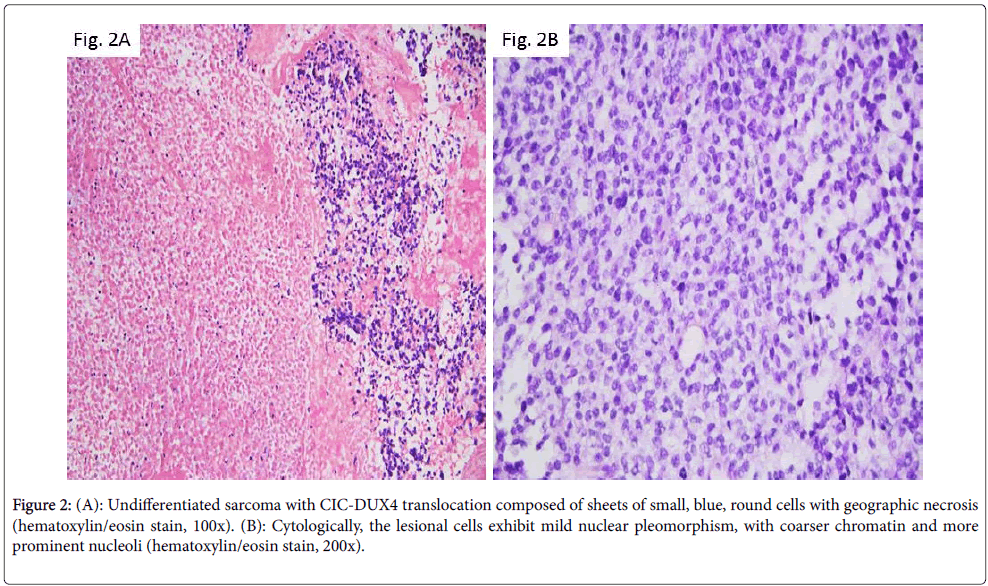
In 2006, Kawamura-Saito et al. described 2 cases of “Ewing-like sarcoma” with a recurrent chromosomal translocation t(4;19) (q35;q13), which resulted in fusion between CIC gene, a human homolog of Drosophila capicua encoding a high mobility group box transcription factor and DUX4, a double homeodomain gene [5]. The resulting fusion product was shown to function as a transcription factor subsequently upregulating members of the ETS family. Following studies demonstrated that this relatively new entity has a wide age distribution ranging from 5 to 62 years, and an equal gender distribution. The tumor, in the vast majority of the patients, is soft tissue based. However, Italiano et al. identified in one of the largest published series two CIC rearranged cases with bone location: one with peritonsillar and mandibular involvement and another centered in the foot soft tissue and bone [6]. Morphologically, these neoplasms are composed of sheets of small, blue, round cells set in a focally extracellular myxoid matrix with variable degree of geographic necrosis. Cytologically, the lesional cells may exhibit mild nuclear pleomorphism, with coarser chromatin and more prominent nucleoli (Figures 2A and 2B). Areas with spindle cells are seen only infrequently. A large number of cases reveal CD99 immunoreactivity, but the degree of staining is variable ranging from focal to diffuse. S-100, desmin, myogenin, cytokeratin, and EMA reactivity was found in rare cases.
Figure 2: (A): Undifferentiated sarcoma with CIC-DUX4 translocation composed of sheets of small, blue, round cells with geographic necrosis (hematoxylin/eosin stain, 100x). (B): Cytologically, the lesional cells exhibit mild nuclear pleomorphism, with coarser chromatin and more prominent nucleoli (hematoxylin/eosin stain, 200x).
Sarcoma with BCOR-CCNB3 translocation
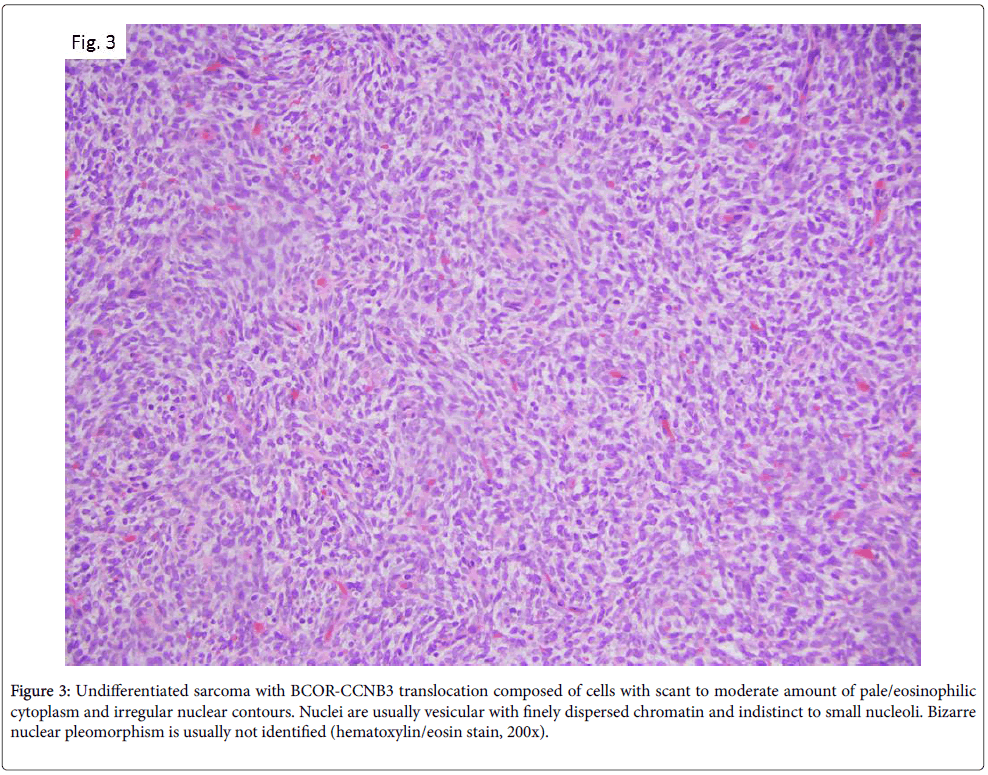
More recently, in 2012, Pierron et al. identified a new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion [7], resulting from a paracentric inversion on chromosome X. Consecutive studies highlighted that this neoplasms is frequently localized to bone, preferentially long bones but also in the axial skeleton, including the spine and pelvis. It usually occurs in adolescents or young adults, ranging from 6 to 26 years with a median age of 13 years and has a male to female ratio of 2:1 [8]. The review by Peters et al. [9] of pretreatment biopsy specimens from six BCOR-CCNB3 positive cases showed tumors of variable cellularity. Cells are often embedded in an edematous and myxoid stroma containing small, compressed thinwalled vessels. Cytologically, lesional cells have scant to moderate amount of pale/eosinophilic cytoplasm, and irregular nuclear contours. Nuclei are usually vesicular with finely dispersed chromatin and indistinct to small nucleoli. Bizarre nuclear pleomorphism is usually not identified (Figure 3). Immunohistochemical studies demonstrate nuclear positivity for CCNB3, whereas CD99 has variable staining pattern [10].
Figure 3: Undifferentiated sarcoma with BCOR-CCNB3 translocation composed of cells with scant to moderate amount of pale/eosinophilic cytoplasm and irregular nuclear contours. Nuclei are usually vesicular with finely dispersed chromatin and indistinct to small nucleoli. Bizarre nuclear pleomorphism is usually not identified (hematoxylin/eosin stain, 200x).
Small cell osteosarcoma
Small cell osteosarcoma also enters the differential diagnosis of pediatric small round blue cell bone tumors. This type of osteosarcoma comprises approximately 1.5% of all osteosarcoma cases [3] and usually involves the metaphysis of the long bones. It is more commonly seen in the second decade of life. Radiologically, it has destructive pattern with extension into the adjacent soft tissue. Many cases demonstrate mineralized matrix, corresponding to the osteoid material seen on histologic sections. Microscopically, the tumor is composed of round/oval cells with hyperchromatic nuclei and scant cytoplasm.
Nucleoli are rarely seen. Presence of osteoid material is required to render this diagnosis. The neoplasm does not have a specific immunophenotype and no recurrent molecular genetic abnormalities have been detected to date.
Mesenchymal chondrosarcoma
Mesenchymal chondrosarcoma should also be considered when dealing with a bone-based small round blue cell neoplasm. This is a rare type of chondrosarcoma showing a peak incidence in the second and third decades of life.
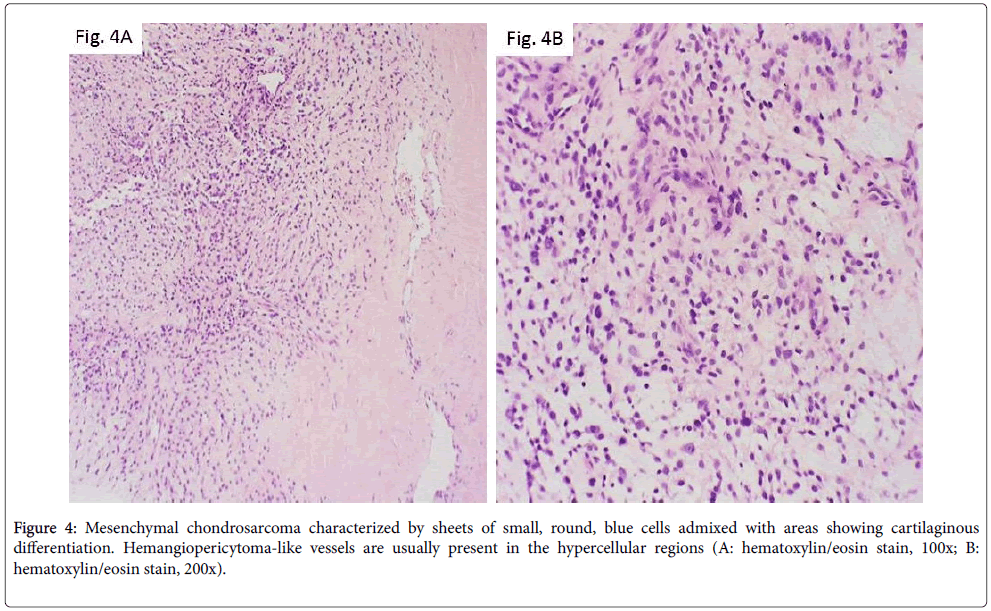
As opposed to other, more common, bone malignancies that usually involve the long bones, mesenchymal chondrosarcoma more commonly arises in the axial skeleton, including craniofacial bones, vertebrae, ribs, pelvis [3]. Radiologically, it appears as an aggressive lesion with ill-defined borders, cortical destruction and soft-tissue mass extension. Histologically, it is characterized by sheets of small, round, blue cells admixed with areas showing cartilaginous differentiation. Hemangiopericytoma-like vessels are usually present in the hypercellular regions (Figures 4A and 4B). The neoplastic cells are variably positive for SOX9 and CD99. A recurrent HEY1-NCOA2 fusion has recently been identified [11].
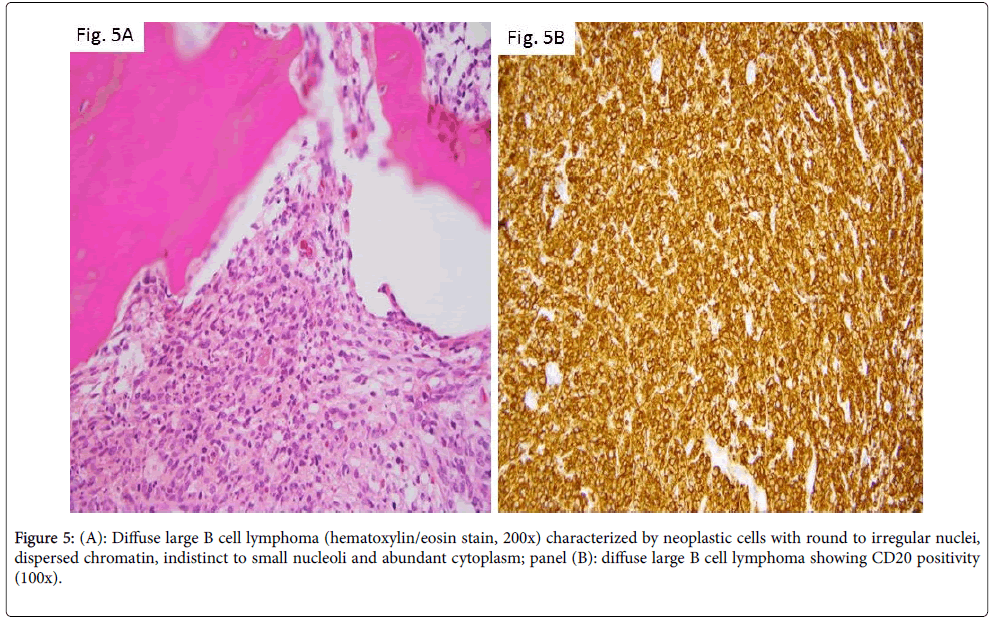
Primary bone lymphoma
Although extranodal sites are a less frequent origin of lymphomas, primary bone lymphomas still account for ~ 7% of malignant bone tumors [12]. The disease commonly affects adults but has been described in pediatric patients as well [13,14]. The majority of cases are diagnosed as diffuse large B cell type lymphomas. In the study by Zhao et al. [13], 10 such pediatric cases were detailed. They involved males in the second decade of life and were often diagnosed after months to years of symptoms (usually bone pain), suggesting an indolent disease. Histologic examination in this disease is characterized by paratrabecular or diffuse location of the neoplastic cells. Cells have round to irregular nuclei, dispersed chromatin, indistinct to small nucleoli, and abundant cytoplasm (Figure 5A). Immunophenotype is that of mature B lymphocytes (Figure 5B, CD20 immunostain).
Metastasis
Secondary bone involvement, in particular in pediatric patients diagnosed with neuroblastoma or rhabdomyosarcoma, also presents histologically as a small round blue cell lesion. A diagnosis of neuroblastoma, in cases that exhibit differentiation, is based on the presence of neuropil or ganglion cells (Figure 6A). Immunohistochemical stains (tyrosine hydroxylase (Figure 6B), PGP9.5, synaptophysin, chromogranin) can aid in diagnosis. Rhabdomyosarcoma is characterized by lesional cells showing rhabdomyoblastic differentiation, with eccentrically placed nuclei, abundant cytoplasm with possible striations (Figure 6C) that are positive for desmin (Figure 6D), myogenin or MyoD1.
Conclusions
Pediatric bone lesions with small round blue cell morphology are often diagnostically challenging. The diagnosis is usually established with the aid of immunohistochemistry and molecular studies. An accurate diagnosis is essential, considering the differences in prognosis between these entities. For instance, sarcoma harboring BCORCCNB3 translocation proved to be very chemosensitive. In the study by Cohen-Gogo et al. [8], ten out of twelve assessed neoplasms displayed less than 10% recognizable and viable tumor cells after neoadjuvant chemotherapy [8]. In addition, Puls et al. [10] found a better overall survival for BCOR-CCNB3 sarcoma patients (75% at 5 year), albeit not statistically different from a large series of classic Ewing sarcoma (54% at 5 year). In contract, patients with CIC-DUX4 sarcomas undergoing surgical resection of the primary neoplasm after preoperative chemotherapy appear to have a poor treatment response, with less than 90% tumor necrosis [15].
References
- Vlychou M, Athanasou NA (2008) Radiological and pathological diagnosis of paediatric bone tumours and tumour-like lesions. Pathology 40: 196-216.
- Wyers MR (2010) Evaluation of pediatric bone lesions. PediatrRadiol 40: 468-473.
- Wei S, Siegal G (2014) Round Cell Tumors of Bone: An Update on Recent Molecular Genetic Advances. Advances in Anatomic Pathology 21: 359-372.
- Antonescu C (2014) Round cell sarcomas beyond Ewing: emerging entities. Histopathology 64: 26-37.
- Kawamura-Saito M, Yamazaki Y, Kaneko K, Kawaguchi N, Kanda H, et al. (2006) Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum Mol Genet 15: 2125-2137.
- Italiano A, Sung YS, Zhang L, Singer S, Maki RG, et al. (2012) High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer 51: 207-218.
- Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, et al. (2012) A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 44: 461-466.
- Cohen-Gogo S, Cellier C, Coindre JM, Mosseri V, Pierron G, et al. (2014) Ewing-like sarcomas with BCOR-CCNB3 fusion transcript: a clinical, radiological and pathological retrospective study from the SociétéFrançaise des Cancers de L'Enfant. Pediatr Blood Cancer 61:2191-2198.
- Peters TL, Kumar V, Polikepahad S, Lin FY, Sarabia SF, et al. (2015) BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of male children. Mod Pathol 28: 575-586.
- Puls F, Niblett A, Marland G, Gaston CL, Douis H, et al. (2014) BCOR-CCNB3 (Ewing-like) sarcoma: a clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J SurgPathol 38:1307-1318.
- Wang L, Motoi T, Khanin R, Olshen A, Mertens F, et al. (2012) Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 51:127-139.
- Bhagavathi S, Fu K (2009) Primary bone lymphoma. Arch Pathol Lab Med 133:1868-1871.
- Zhao XF, Young KH, Frank D, Goradia A, Glotzbecker MP, et al. (2007) Pediatric primary bone lymphomas-diffuse large B-cell lymphoma: morphologic and immunohistochemical characteristics of 10 cases. Am J ClinPathol 127: 47-54.
- Bakshi NA, Ross CW, Finn WG, Valdez R, Ruiz R, et al. (2006) ALK-positive anaplastic large cell lymphoma with primary bone involvement in children. Am J ClinPathol 125:57–63.
- Gambarotti M, Benini S, Gamberi G, Cocchi S, Palmerini E, et al. (2016) CIC-DUX4 Fusion-Positive Round Cell Sarcomas of soft tissue and bone: a single institution morphologic and molecular analysis of 7 cases. Histopathology Apr 15 [Epub ahead of print].
Relevant Topics
- Classic Diagnostic Pathology
- Diagnostic Molecular Pathology
- Diagnostic Pathology
- Diagnostic Pathology of Infectious Diseases
- Diagnostic Pathology Reports
- Diagnostic Surgical Pathology
- Diagnostic Techniques in Pathology
- Forensic Pathologist Communications
- Immunohistochemistry (IHC):
- Immunopathology
- Molecular Pathology
- Pathobiology
- Pathology Diagnostics Market Analysis
- Prognosis-related diagnosis
- Stereology
- Tissue based Diagnosis
- Virtual Microscopy
- Virtual Pathology
Recommended Journals
Article Tools
Article Usage
- Total views: 14939
- [From(publication date):
June-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13834
- PDF downloads : 1105