Diagnostic Approach to Neonatal and Infantile Cholestasis
Received: 04-Jul-2022 / Manuscript No. nnp-22-69976 / Editor assigned: 07-Jul-2022 / PreQC No. nnp-22-69976 / Reviewed: 20-Jul-2022 / QC No. nnp-22-69976 / Revised: 22-Jul-2022 / Manuscript No. nnp-22-69976 / Published Date: 29-Jul-2022 DOI: 10.4172/2572-4983.1000249
Abstract
Neonatal and infantile cholestasis (NIC) can represent the onset of a surgically correctable disease and of a genetic or metabolic disorder worthy of medical treatment. Timely recognition of NIC and identification of the underlying etiology are paramount to improve outcomes.
Upon invitation by the Italian National Institute of Health (ISS), an expert working grouped was formed to formulate evidence-based positions on current knowledge about the diagnosis of NIC.
A systematic literature search was conducted to collect evidence about epidemiology, etiology, clinical aspects and accuracy of available diagnostic tests in NIC. Evidence was scored using the GRADE system. All recommendations were approved by a panel of experts upon agreement of at least 75% of the members. The final document was approved by all the panel components.
This position document summarizes the collected statements and defines the best-evidence diagnostic approach to cholestasis in the first year of life.
Keywords
Alagille syndrome; Biliary atresia; Diagnosis; Genetic liver disease; Inborn errors of metabolism; Jaundice; Monogenic liver disease; Newborn
Introduction
Neonatal/Infantile cholestasis (NIC) is defined as an impairment in bile formation and/or flow presenting by the first year of age, usually in the first three months, and resulting in the retention of bile and biliary substances within the liver that cause liver damage. NIC affects 1 in 2500 children at term and is caused by either surgical or medical disorders, whose prompt recognition may substantially improve the outcome. The identification of the underlying etiology is compelling in order to initiate appropriate surgical or medical treatment. In fact, the most common cause of NIC is biliary atresia (BA), in which an early surgical referral is needed to optimize the success rate. Also in other treatable inherited metabolic conditions, such as galactosemia or the rare inborn errors of bile acids synthesis, a timely diagnosis certainly warrants a prompt specific treatment and a better outcome . The present document aims to provide recommendations to support: a) pediatricians working in primary and secondary care for the initial management of NIC; b) neonatologists and pediatric gastroenterologists working in a tertiary referral center for the extensive diagnostic work-up. To the purposes of this document, NIC is defined in presence of: i) serum conjugated bilirubin > 1 mg/dl, when total bilirubin is < 5 mg/dl or ii) a conjugated component > 20% of the total, when total bilirubin is > 5 mg/dl, in a child aging less than 1 year.
Methods
The project started in October 2019, under the auspices of the SIGENP (Italian Society for Pediatric Gastroenterology, Hepatology and Nutrition), in response to the invitation by the ISS (Italian National Institute of Health) to draw specific recommendations on diagnosis and management of NIC. A working group consisting of 39 members (26 pediatric hepatologists, 6 pediatric surgeons, 2 radiologists, 2 neonatologists, 1 metabolic diseases specialist, 1 pathologist, 1 geneticist) was formed.A core panel identified the relevant questions to be addressed, with subgroups of experts being assigned to specific sections, and providing positions based on evidence resulting from a selection of key publications. Every subgroup was responsible for identifying relevant evidence, which included existing guidelines,literature reviews and/or systematic literature search from 1980 to 2020, using accessible relevant databases (PubMed, MEDLINE). For this search, English language restriction was applied while geographical restrictions were not [1].
Two grades of recommendation were considered (1 = strong and 2 = weak); quality of evidence was scored according to the GRADE system as
A = High (further research is unlikely to change our confidence in the estimate of effect)
B = Medium (further research is likely to have impact on our confidence in the estimate of effect)
C = Low (any estimate of effect is very uncertain).
This position paper does not analyze aspects regarding management and treatment of NIC, that are object of another document.
Consensus and voting
Panel meetings took place in May 2019 and December 2019 to agree upon document specific issues and the graded positions. In September 2020, due to the COVID-19-related distancing measures, two meetings involving the whole working group took place online to vote each recommendation using the nominal voting technique as follows: agree/ disagree. The agreement reached for each recommendation is listed as percentage.
An agreement of at least 75% of the panel members was required to accept the recommendations [2].
The final document was drafted in January 2021 and was reviewed and approved by all members.
Causes of neonatal/infantile cholestasis
Hyperbilirubinemia occurs in around 50% of newborns - although not always clinically evident - as an expression of physiological jaundice in the vast majority of cases . Prolonged jaundice (defined as jaundice lasting for longer than 2 weeks), observed in up to 15% of all infants, must evoke the suspicion of a cholestasis promoting further investigations and in particular the determination of serum conjugated bilirubin. Elevated serum levels of conjugated bilirubin define cholestasis. Once cholestasis has been documented in an infant, achieving a rapid etiological definition is of primary importance. The wide spectrum of causes potentially underlying NIC entails a complex diagnostic approach that depends on the accompanying clinical picture. For practical purposes, causes of cholestasis are divided in surgical and medical conditions [3].
Surgical causes of cholestasis
Biliary atresia (BA)
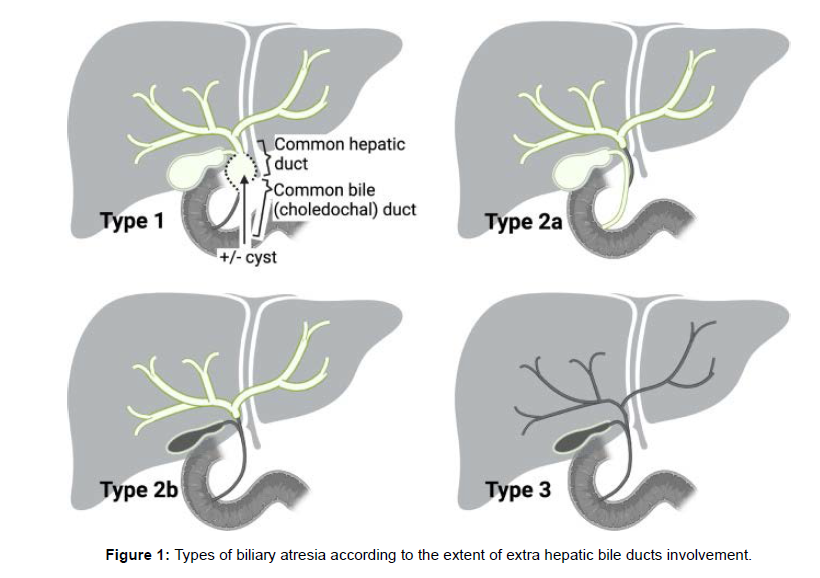
BA is the most common identifiable cause of NIC, with an incidence ranging from 0.55 to 1.3 /10,000 live births, the highest incidence being observed in Asia. In addition, it represents the most frequent reason for liver transplantation in childhood . BA is the result of a rapidly progressive inflammatory and fibrotic process causing the partial or complete obliteration of the extrahepatic and often the intrahepatic bile ducts. Genetic, epigenetic, malformative, vascular, infective, toxic or autoimmune factors have been investigated in the attempt of clarifying BA pathogenesis that is believed to be likely multifactorial . BA is classified into three types according to morphology In the rare types 1 and 2 ( ≈20%), the disease is limited to the distal extrahepatic bile ducts, i.e., the choledocal duct (type 1) and the common hepatic duct (type 2). In these types – more often associated with a better outcome after surgical correction – part of the extrahepatic biliary system can be spared from the inflammatory destruction and thus remains patent and usable for the intervention. In Type 2A, the lower part of the bile ducts (gallbladder and choledocus) can be found at the cholangiography. An extrahepatic biliary cyst at “porta hepatis” is rare (but allowing prenatal suspicion) and is mostly associated with type 1 (once called “correctable forms” of BA, as the atretic process may be, in these cases, limited to only the lower part of the bile ducts) (Figure 1).
In the most common type 3, a deep fibrous plate involves the extrahepatic bile ducts, associated with a fibrous transformation of the gallbladder in most cases, and transformation in mucocele in few ones. In the alternatively adopted French classification, types 2A, 2B, and 3 of the Japanese nomenclature are referred to as types 3, 2, and 4, respectively .Apart from the isolated form, BA can occur in the context of other clinical scenarios. In 20% of cases, BA is associated with extrahepatic malformations, mainly splenic (BA with splenic malformations, BASM). BASM is characterized by asplenia or polysplenia, laterality defects (intestinal malrotation, situs inversus) and cardiovascular malformations (dextrocardia, preduodenal portal vein, agenesis of retro-hepatic inferior vena cava, anomalies superior vena cava) [4].
Choledochal malformation (CM)
CM (or congenital biliary dilatation, CBD) is a pancreaticobiliary anomaly characterized by dilatation of the biliary tract and, in the vast majority of cases, a pancreatico-biliary maljunction (PBM)
The diagnosis is based on the abdominal imaging, essentially with ultrasound, but it needs to be confirmed by cholangio-MRI. Regardless of the symptoms or age at presentation, the surgical correction (generally, excision of the extrahepatic bile ducts followed by a bilio-digestive reconstruction using a jejunal Roux-en-Y loop) is mandatory. Timely management is important to prevent chronic damage to the liver, biliary obstruction with jaundice, cholangitis and pancreatitis, growth retardation, increased hepatic fibrosis and even cirrhosis. Late malignant transformation is described and can occur in any portion of the biliary system that will remain in the patient. There is however no need for urgent surgery in most cases, and many authors have recommended to delay surgery when possible beyond 6 months and/or a weight > 5 kg, to reduce the risk of complications .
The differential diagnosis involves liver cyst, duodenal duplication and more importantly cystic biliary atresia.
Medical causes of neonatal/infantile cholestasis
Beyond the extrahepatic (surgical) causes, the knowledge about the underlying etiology of the medical (non-surgical, so called “intrahepatic”) cholestasis has been rapidly expanding in the last decades. These conditions can be mainly grouped into geneticmetabolic, endocrine, infectious, and related to hematologic conditions. The extraordinary advance in sequencing techniques has demonstrated that the majority of these conditions are monogenic liver disorders . Genetic causes include defects in canalicular bile acid transport (e.g. progressive familial intrahepatic cholestasis, PFIC), tight junction defects, inborn errors of bile acid metabolism, inborn errors of metabolism (IEM) not related to bile acid synthesis, and other pictures in the context of syndromic/systemic conditions. The main clinical and laboratory findings are listed in Table 1. Although these diseases are generally poorly identifiable on the clinical grounds, definite diagnosis is important to identify treatable conditions. Congenital endocrine disorders also may present with cholestatic jaundice, while in presence of an acutely/critically ill infant with severe liver function impairment, a severe systemic infection, or a decompensated metabolic disorder should be suspected [5] (Table 1).
| Disease | Laboratory hallmarks and biomarkers | Gene |
|---|---|---|
| EXTRA-HEPATIC CAUSES | ||
| Biliary atresia | High platelets count; high GGT, sBA. | NA |
| Choledocal cyst | - | NA |
| Cholelithiasis | - | NA |
| Inspissated bile/mucous plug | - | NA |
| Congenital perforation of common bile duct | - | NA |
| INTRAHEPATIC CHOLESTASIS | ||
| Cystic fibrosis | Elevated sweat chloride | CFTR |
| * | ||
| α−1-antitrypsin deficiency | Low serum A1AT | SERPINA1 |
| Defects of the biliary canalicular transport | ||
| PFIC1 | High sBA; low GGT; elevated sweat chloride | ATP8B1 |
| PFIC2 | High sBA; low GGT; high AFP | ABCB11 |
| PFIC3 | High sBA; high GGT | ABCB4 |
| FXR deficiency (PFIC5) | High sBA; low GGT; high AFP | NR1H4 |
| MYO5B cholestasis | High sBA; low GGT | MYO5B |
| Tight junction defects | ||
| TJP2 deficiency (PFIC4) | High sBA; low GGT | TJP2 |
| USP53 deficiency | High sBA; low GGT | USP53 |
| NISCH syndrome | High sBA; high GGT | CLDN1 |
| Bile acid synthesis and conjugation disorders | ||
| 3-β-HSD-oxidoreductase deficiency | Low sBA, GGT; bile acid mass spectrometry | HSD3B7 |
| D4–3-oxosteroid 5 β-reductase deficiency | High GGT; low sBA; biliary acid mass spectrometry | AKR1D1 |
| Cerebrotendinous xanthomatosis | Low sBA; high cholesterol and cholestanol | CYP27A1 |
| BACL deficiency | Low GGT, sBA; biliary acid mass spectrometry | SLC27A5 |
| BAAT deficiency | Low GGT, sBA; biliary acid mass spectrometry | BAAT |
| 2-methylacil-CoA racemase deficiency | Low GGT, sBA; biliary acid mass spectrometry | AMACR |
| Oxisterol-7α-hydroxylase deficiency | Low GGT, sBA; biliary acid mass spectrometry | CYP7B1 |
| Biliary development defects | ||
| Alagille syndrome | High GGT, sBA, cholesterol, triglycerides | JAG1, NOTCH 2 |
| Neonatal sclerosing cholangitis | High GGT, sBA | DCDC2; CLDN1 |
| ARC syndrome | High sBA; low or high GGT; metabolic acidosis, hypophosphatemia | VPS33B, VIPAS39 |
| Caroli disease | High GGT, sBA | PKHD1 |
| Ciliopathies | High GGT, sBA | different genes |
| Lysosomal storage disease | ||
| Niemann-Pick disease type C | Markedly elevated oxysterols and lysosphingolipids in plasma; accumulation of intracytoplasmic unesterified cholesterol in skin fibroblasts (filipin staining); elevated chitotriosidase; sea blue histiocytes in bone marrow | NPC1; NPC2 |
| * | ||
| Acid sphyngomyelinase deficiency (Niermann-Pick disease type A and B) | Markedly elevated oxysterols and lysosphingolipids in plasma; reduced acid sphingomyelinase activity (dried blood spot, leukocytes, fibroblasts); | SMPD1 |
| * | ||
| Lysosomal acid lipase deficiency (Wolman disease) | Reduced acid lipase activity (dried blood spot, leukocytes, fibroblasts); abnormal lipid profile; vacuolated lymphocytes; macrophage activation | LIPA |
| * | ||
| Gaucher disease (neurologic) | Reduced glucocerebrosidase activity (dried blood spot, leukocytes, fibroblasts); foamy cells in bone marrow; high angiotensin-converting enzyme, tartrate-resistant acid phosphatase, chitotriosidase; thrombocytopenia | GBA |
| * | ||
| Mitochondrial disorders | ||
| Mitochondrial DNA depletion syndrome | Hypoglycemia; lactic acidosis; high plasma alpha-fetoprotein; hyperferritinemia iron overload; coagulopathy; abnormal urine organic acid | POLG, DGUOK, MPV17 |
| SUCLG1, C10ORF2, elongation factor G1,TRMU related and BCS1L deficiency | Hypoglycemia; lactic acidosis; abnormal urine organic acid | SUCLG1, C10ORF2, EGF1, TRMU, BCS1L |
| Mitochondrial Fatty Acid Oxidation defects | ||
| LCHAD/MTP deficiency | Hypoketotic hypoglycemia; high levels of CPK; abnormal blood acylcarnitine and urine organic acids profiles | HADHA, HADHB |
| * | ||
| Peroxisomal disorders | ||
| Zellweger spectrum disorders | Elevated very long-chain fatty (VLCFA), phytanic and pristanic acids in plasma; reduced plasmalogenes in erythrocytes and fibroblasts; reduced catalase activity in fibroblasts | PEX genes |
| Aminoacidopaties | ||
| Tyrosinemia type 1 | Elevated succinylacetone (dry blood spot, plasma, urine); elevated tyrosine and methinine (dry blood spot, plasma); elevatedAFP; coagulopathy; hypophosphatemia; hypoglycemia; hyperaminoaciduria; elevated delta-aminolevulinic acid urine | FAH |
| * | ||
| Adenosine kinase deficiency | Hypoglycemia (hyperinsulinemic); elevation (intermittent) of methionine in plasma; coagulopathy; elevated adenosine in dry blood spot and urine (transient) | ADK |
| * | ||
| S-adenosylhomocysteine hydrolase deficiency | Elevated methionine, S-adenosyl-homocysteine, S-adenosyl-methionine and homocysteine in plasma; high levels of CPK | AHCY |
| * | ||
| Inborn error of polyols and pentose metabolism | ||
| Transaldolase deficiency | Coagulopathy; anemia and thrombocytopenia; hypothiroidism; renal tubulopathy; abnormal profile of urinary polyols | TALDO1 |
| Carbohydrate metabolism defects | ||
| Classic galactosemia | Elevated galactose (dry blood spot, erythrocytes); reduced GALT activity in erythrocytes; coagulopathy; hypoglycemia; renal tubulopathy; neonatal E. Coli sepsis; positive urinary reducing substances | GALT |
| * | ||
| Glycogen storage disease type IV | Fasting hypoglycemia; high levels of CPK; PAS positive inclusions at liver and muscle histology; reduced enzyme activity (liver tissue, muscle, leukocytes, fibroblasts) | GBE1 |
| Congenital disorders of glycosylation | Low coagulation factors (VII, IX, X, XI, AT-III, protein C and S); abnormal serum transferrin isoforms | different genes |
| Urea cycle defects | ||
| Urea cycle defects | Hyperammonemia; abnormal aminoacid profile in plasma; elevated orotic acid in urine | OTC, ASS, ASL, ARG |
| * | ||
| Citrin deficiency (NICCD) | Elevated citrulline (dry blood spot; plasma); elevated galactose and AFP; hypoglycemia; hyperammonemia | SLC25A13 |
| Cholesterol metabolism disorders | ||
| Smith-Lemli-Opitz syndrome | Elevated 7-dehydrocholesterol and 8-dehydrocholesterol in plasma | DHCR7 |
| Mevalonic aciduria | Anemia and thrombocytopenia; hyper-Ig D; abnormal organic urine acids | MVK |
| Cellular trafficking abnormalities | ||
| NBAS deficiency | Hypoglycemia; lactic acidosis; coagulopathy, abnormal urine organic acid | NBAS |
| CALFAN syndrome | Low GGT | SCYL1 |
| Metal metabolism disorder | ||
| MEDNIK syndrome | Low serum copper and ceruloplasmin; high urinary copper; mild elevation of plasma VLCFA | AP1S1 |
| Syndromic cholestasis (most relevant) | ||
| Down and Edwards Syndrome | Abnormal kariotype | Trisomy 21, 18 |
| Kabuki syndrome | Low or high GGT | KMT2D, KDM6A, MLL2 |
| Noonan syndrome | High GGT | PTPN11, SOS1, RAF1 and KRAS |
| Aagenaes syndrome | High GGT | LSC1, CCBE1 |
| ENDOCRINOLOGIC DISEASES | ||
| Thryroid disorders | TSH and FT4 values | different genes. in congenital hypotiroidism (e.g., FOXE1, NKX2–1/5, PAX8, SLC26A4, TSHR) |
| Panhypopituitarism | TSH and FT4, ACTH, cortisol, GH, IGF1, PRL, LH, FSH, stimulating test, brain MRI | different genes in genetic forms (e.g., HESX1, PROP1, POUF1, LHX3, LHX4, GLI2, SOX3) |
| Adrenal insufficiency | ACTH, cortisol stimulating test | monogenic forms (e.g., POR, MC2R, MRAP, StAR, AYP11A1, NNT, TRXR2) syndromic forms (eg.CDKN1C, MCM4, SAMD9, SGPL1) |
| INFECTIOUS DISORDERS | ||
| Cytomegalovirus, Herpes virus type 1–2–6; toxoplasma; rubella; parvovirus B19; enterovirus (including coxsackievirus, echovirus), adenovirus, syphilis, HIV, listeria monocitogenes, congenital tubercuolosis | Low or high GGT, serology in the proband and mother, specific direct nucleic acid testing via PCR | NA |
| HEMATOLOGIC AND IMMUNE-MEDIATED DISORDERS | ||
| Hemophagocitic lymphoistiocytosis | HLH clinical and testing criteria (cytopenia, hypertriglyceridemia or hypofibrinogenemia, hemophagocytosis in biopsy samples, low or absent NK activity, high serum ferritin, elevated CD25 levels | different genes (e.g., PRF1, UNC13D, STX11, STXBP2, RAB 27, XLP) |
| Neonatal hemocromatosis (GALD and non-GALD) | Hypoglycemia, coagulopathy, high ferritin, high alpha-fetoprotein, low trasferrin and high iron saturations | non-GALD (e.g., DGUOK, SRD5B1, BCS1L) |
| Congenital lupus | ANA, positive Coombs-test | NA |
| Post-hemolytic cholestasis | Hemolytic disease of the newborn due to Rh or ABO alloimmunization | NA |
| TOXIC AND SECONDARY CHOLESTASIS | ||
| Parenteral nutrition associated cholestasis (PNALD); drugs; intestinal obstruction; cardiovascular disorders, neoplastic disorders; perinatal asphyxia | Low or High GGT | NA |
Table 1: Intra- and extra-hepatic causes of neonatal and infantile cholestasis and their biochemical features.
* detectable by extended newborn screening, where available. A1AT, α−1-antitrypsin; PFIC, progressive familial intrahepatic cholestasis; sBA, serum bile acids; GGT, γ-glutamyl-transpeptidase; AFP, α-fetoprotein; ARC, Arthrogryposis renal dysfunction cholestasis; CDG, congenital disorders of glycosylation; CPK, creatinephosphokinase; GALT, Galactose-1-Phosphate Uridyltransferase; PAS, Periodic acid–Schiff; ATIII, Antithrombin; NICCD, Neonatal intrahepatic cholestasis caused by citrin; NBAS, neuroblastoma amplified sequence; CALFAN, low γ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration; MEDNIK, Mental retardation, Enteropathy, Deafness, Neuropathy, Ichthyosis, Keratodermia; LCHAD/MTP, long-chain 3-hydroxyacyl-CoA dehydrogenase/ mitochondrial trifunctional protein; VLCFA, verylong- chain fatty acid; TSH, thryroid-stimulating hormone; FT4, free tethraiodotyroxine; ACTH, adrenocorticotropic hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1; PRL, prolactin; FSH, follicle-stimulating hormone; LH, luteinizing hormone; MRI, magnetic resonance imaging; PCR, Polymerase chain reaction; GALD, Gestational alloimmune liver disease; ANA, anti-nucleus antibodies; PNALD, Parenteral nutrition associated cholestasis.
Diagnostic work-up of biliary atresia
Children with BA are candidate for Kasai hepatoportoenterostomy (HPE) with the aim of restoring bile flow, clearing the jaundice, and preserving liver function . The importance of an early diagnosis and/or referral is justified by the fact that the patient should be proposed for surgical exploration early in life, as the success rate of surgery decreases with the age of the child at the operation. Early suspicion of BA is necessary to allow a timely diagnostic work-up aiming to exclude other causes of neonatal cholestasis and to collect clinical, laboratory and non-invasive imaging elements in favor of the diagnosis of BA. Since there is no a single test or procedure to establish a positive diagnosis, a number of concordant elements contribute to establish a wellfounded suspicion ultimately setting the indication for performing a cholangiography . An effective work-up protocol should select patients for cholangiography within one week with the minimum number of invasive procedures.Clinical examination and laboratory results can suggest the diagnosis; in the last decade the diagnostic potential of fasting abdominal ultrasonography (US) was extensively investigated and a combination of US signs can offer an extremely high positive predictive power. Liver biopsy can be of great value in ruling in or out BA; however, the only procedure to definitely establish the diagnosis is the cholangiography and/or surgical exploration (usually performed together), which represents the gold standard for the diagnosis of BA [6]. The time to referral to an expert team for the assessment is a cornerstone problem, as the mean age at referral in most countries has remained as high as 6 weeks and a minority of children with BA undergoes HPE under 60 days of age.Thus, timing and accuracy of the diagnostic work-up are essential, as the surgical exploration should be ideally proposed only to patient with a high index of suspicion – thereby avoiding unnecessary laparotomy in those who are not BA.Infrequently, the clinical presentation may be atypical exacerbating the challenge of a positive diagnosis in an adequate time. Some children may present with hypocholic stools, a complex neonatal clinical history and not specific findings at laboratory tests or even at liver biopsy, and are not immediately proposed for cholangiography. In the case of persisting cholestasis, the pediatrician should not hesitate to reassess a child shortly later if the first work-up did achieved a certain diagnosis, as the clinical picture may evolve rapidly in case of BA .As a future direction, the serum matrix metalloproteinase-7 (MMP-7) has recently emerged as a promising standalone non-invasive marker of BA, bearing a sensitivity of 95–98% and a specificity of 83–95% .The lack of standardized collection and dilution protocols, and – subsequently – of clear cut-offs, have limited so far its large-scale validation and clinical use.
Children with initially cholic stools, and evolution towards acholic stools between the age of 2 to 4 months have been described . To what extent these cases represent a misclassification of the newborn’s stool color or they are late forms of BA, is controversial. However, the diagnosis of BA should not be definitely excluded on the basis of the presence of normal stools in the early neonatal period [7] .
Initial evaluation
Medical history
Familial and personal medical details should be collected in children with prolonged jaundice and especially in those in which NIC has been already demonstrated . Details in the family history including previous and current pregnancy and information on miscarriages, pruritus or liver disease, hemolytic diseases and/or cardiac anomalies in maternal history or other family members should be noted. Detailed medical history can highlight some clues important to the final diagnosis. Gestational and perinatal history are crucial in defining factors associated with some forms of multifactorial, transient cholestasis, such as low birth weight and prematurity, a low Apgar score, sepsis, asphyxia, total parenteral nutrition, history of organ failure. Geneticmetabolic causes or congenital infection should be considered in the differential diagnosis in presence of intrauterine growth retardation, fetal hydrops with or without malformations [8]
Red flags suggesting IEM as the cause of the liver disease include suggestive family history, early-onset (or triggered by intercurrent illnesses) episodes of acute metabolic decompensation (e.g. hypoglycemia, hyperammonemia, acidosis, acute liver failure/ involvement), recurrent/chronic vomiting, selective food aversion, unusual odor of body fluids, associated neurological signs or multiorgan involvement .Careful information about the onset of jaundice, changes in stool pigmentation and urine color must be collected
Recommendation In jaundiced infants with suspected or confirmed cholestasis a detailed pre-, peri‑, and postnatal medical and family history should be collected (GRADE 1B; 100% agree).
Physical examination
Typically, the infant with BA is born at term and has been feeding nicely since birth until the jaundice is recognized as a pathologic condition. Less frequently, the suspicion of BA may be evoked in a premature baby because of a rising level of direct bilirubin in presence of acholic stools; in these small babies, jaundice can be multifactorial and the diagnostic work-up may even be more difficult than usual. The most important element in physical examination is the direct observation of stools color and a careful abdominal inspection looking at hepatomegaly and/or splenomegaly. Acholic stools, totally decolored with clay-like aspect (https://www.atresiabiliare.org/guidafotografica- sulla-cacca-del-bambino/) are the strongest argument to suspect a biliary obstructive disease but can be observed in only a few other diseases, notably Alagille syndrome (AGS), alpha-1-antitrypsin deficiency (A1ATD), and cystic fibrosis. Hypocholic (light yellowish) stools are not specific of any disease and can be found in late onset BA cases as a transition period towards acholic stools (during the second month of life). However, either parents nor youth healthcare doctors nor general practitioners seems to reliably recognize discolored stool .Although the association of acholic stools and dark urine is very typical of BA, the observation of dark urine is per se not specific, as it is present in any cholestatic disease [9]
The clinical observation of a firm hepatomegaly with or without splenomegaly in a jaundiced infant is the other most relevant clinical finding in BA infants. However, because splenomegaly in BA is secondary to the development of portal hypertension, it may be less pronounced in very young infants or in late onset BA cases; massive splenomegaly is rather suggestive of lysosomal storage diseases or hematological conditions.
An exhaustive physical examination in any infant with cholestasis has great importance because it may reveal hallmarks of other diseases - as for example, a systolic murmur audible from the back in Alagille syndrome (AGS) (peripheral pulmonary artery stenosis). Clinical findings suggestive of specific causes of NIC are listed in (Table 2).
| Assessment of general health | Ill appearance may indicate infection or metabolic disease; infants with biliary atresia typically appear in good general and nutritional conditions; severe pruritus (biliary canalicular transport, development and tight junction defects) |
| Stool and urine observation | Acholic stools, totally decolored with clay-like aspect, are the strongest argument to suspect a biliary obstructive disease such as BA; colored urine are strongest suggestive of cholestasis |
| General appearance | Dysmorphic features: e.g., Alagille syndrome (rarely exhibits characteristic facial anomalies before age 6 months), Zellweger spectrum disorders, Smith-Lemli-Opitz, ARC syndrome, syndromic cholestasis |
| Vision/slit lamp examination | Posterior embryotoxon (Alagille syndrome), cataract (galactosemia, Zellweger spectrum disorders, lysosomal storage disorders, congenital infections), cherry red spot (lysosomal storage disorders); rotatory eye movement (DGUOK deficiency) |
| Hearing | Hear loss (e.g., congenital infections, Zellweger spectrum disorders, other IEM, CDG) |
| Cardiac examination | Murmur/congenital cardiac malformations (Alagille Syndrome, biliary atresia with splenic malformation syndrome); situs inversus (biliary atresia) cardiomyopathy (mitochondrial disorders, fatty acid oxidation defects, glycogen storage diseases) |
| Abdominal examination | Presence of ascites, abdominal wall visible veins, liver size and consistency, spleen size (splenomegaly suggests Niemann-Pick and Gaucher diseases), abdominal mass, umbilical hernia, cystic kidneys (ciliopathies, Zellweger spectrum disorders) |
| Neurologic evaluation | Note overall vigor and tone (severe hypotonia suggests peroxisomal disorders or mitochondrial cytopathies), lethargy, poor feeding (several IEM, CDG) |
| Skin evaluation | Ichtyosis/collodion baby (ARC syndrome, MEDNIK, Gaucher, congenital disorders of glycosylation), laxity (CDG, transaldolase deficiency), rash (mevalonic aciduria) |
| Genitalia | Micropenis (hypofisarian dysfuntion), ambigous genitalia (e.g., Smith-Lemli-Opitz) |
Table 2 : Clinical findings in the physical examination of a newborn or infant with cholestatic jaundice.
BA, serum bile acids; ARC, Arthrogryposis, renal dysfunction, cholestasis; CDG, Congenital disorders of glycosylation; IEM, inborn errors of metabolism; MEDNIK: Mental retardation, Enteropathy, Deafness, Neuropathy, Ichthyosis, Keratodermia.
Recommendations Infants with jaundice should receive a complete physical examination in particular searching for the presence of hepatomegaly and/or splenomegaly, dysmorphic features, growth and nutritional status, skin lesions, cardiac murmurs and neurologic examination (GRADE 1A, 100% agree).Direct visualization of stools color and its monitoring should be part of the clinical evaluation of any infant with jaundice (GRADE 1A, 100% agree).
Laboratory investigations
Laboratory is essential in confirming the suspicion of a cholestatic jaundice, estimating the presence and the proportion of conjugated bilirubin. Specific measurements of conjugated bilirubin are optimal if available. However, the most commonly used laboratory determination, the diazo or van den Bergh method, does not specifically measure conjugated bilirubin but reports direct reacting bilirubin (entailing free and albumin-bound conjugated bilirubin). The measurement of direct bilirubin with standard methods is accurate in identifying children with BA also when performed shortly after birth, and recent studies demonstrated its feasibility and cost-effectiveness as a newborn screening
Recommendations Infants with jaundice persisting after 2 weeks of age should be evaluated for cholestasis by measurements of serum total and direct-reacting (conjugated) bilirubin (GRADE 1A, 97% agree) Conjugated bilirubin >1 mg/dL (17 mmol/L) if total bilirubin is <5 mg/ dl, or >20% if total bilirubin is >5 mg/dL, is considered diagnostic of cholestasis and warrants further evaluation (GRADE 1A, 100% agree).
Further investigations
Table 3 displays a targeted investigation protocol for infantile cholestasis. First-level laboratory tests represent the initial work-up to evaluate type and severity of liver involvement. In some cases, these tests point to a specific diagnosis or rule out confounding clinical pictures, such as alpha-1-antitrypsin deficiency that can present with neonatal hepatitis syndrome mimicking BA . Also, some conditions such as Alagille syndrome and cystic fibrosis are reported causes of false positive intraoperative cholangiogram, and should be excluded before surgical exploration, as a mistakenly performed HPE may worsen their condition . After exclusion of BA, second-line investigations aim at identifying other disorders responsible for non-obstructive (intrahepatic) causes of cholestasis (Table 3).
| TIER 1: Investigations to evaluale the severity of the liver involvement and first line work-up | Comment |
|---|---|
| Total and conjugated bilirubin, ALT and AST, alkaline phosphatase, GGT, total protein and albumin, INR/PT and aPTT | To define the severity of liver involvement |
| WBC + differential | To suspect the presence of infection and metabolic disease |
| Serum bile acids | Usually elevate in cholestasis, low levels suggestive of BASD (according to upper limit of normal value for age) |
| TSH and T4 values, ACTH, cortisol | Screen for congenital hypothyroidism, adrenal insufficiency and hypopituitarism |
| Urine: urine analysis, culture | To rule out infection |
| Glucose, electrolytes, blood gas analysis with evaluation of bicarbonate and lactate, ammonium | First level screening for metabolic diseases |
| CPK, BUN, creatine, Ca/P, AFP*, lipid profile (C, C-LDL, C HDL, triglycerides) | To analyze systemic involvement and suspect the presence of a metabolic conditions; high levels of C in Alagille syndrome |
| Sweat chloride analysis (trypsinogen level and CFTR genetic testing if appropriate) | To diagnose CF where newborn screening is not available |
| α−1-antitrypsin level, α − 1-antitrypsin phenotype (where available) | To screen for α−1-antitrypsin deficiency |
| Serum iron, ferritin and transferrin | If high, suspicion of GALD and HLH |
| Bacterial cultures of blood and other fluids if clinically ill infant | To rule out infections |
| Serology (IgG and IgM) for infections in the propositus and mother | To diagnose antenatal/perinatal infections |
| Abdominal ultrasound | To detect presence of ascites, to explore extrahepatic biliary tree, adrenal calcification (Wolman), renal cysts (ciliopathies, Zellweger), hepato-splenomegaly (lysosomal storage disorders), liver echogenicity |
| Echocardiogram | to find congenital heart defects or cardiomyopathy (mitochondrial disorders, fatty acid oxidation defects) |
| Eye visit, FO and slit lamp examination | To detect ocular involvement (hallmarks of perinatal infections, genetic and metabolic disorders) |
| TIER 2: Second-line work-up | Comment |
| GH, IGF1, PRL, LH, FSH | To rule out panhypopituitarism |
| Vitamin A, D, E | To detect fat-soluble vitamins depletion |
| Infective work-up (specific direct nucleic acid testing via PCR) | As suggested by serology and clinical findings |
| Selected metabolic work-up (driven by first line clinical and laboratory evaluation) | Amino acids (P), acylcarnitine (DBS, P), organic acids (U), orotic acid (U), succinylacetone (DBS, P,U), galactose (DBS), VLCFA (P), sialotransferrins (S), oxysterols (P), 7-dehydrocholesterol (P), lysoshingolipids (P), bile acids (DBS/S/U), cholestanol (P), polyols (U), lysosomal enzymes (DBS/WBC/F), GALT activity (DBS/RBC), mithocondrial enzymes (M/F) |
| α−1-antitrypsin genotype | To diagnose α−1-antitrypsin deficiency |
| Genetic testing | Karyotype, array-CGH, targeted gene panels, WES, WGS |
| Skeletal X-ray | Spinal abnormalities (such as butterfly vertebrae Alagille), bone dysplasia (lysosomal storage disorders), punctate calcification (Zellweger and peroxisomal disorders), signs of congenital infections, arthrogryposis (ARC syndrome) |
| Bone marrow aspiration | Storage cells suspecting a lysosomal storage disease; hematological/infiltrative conditions (e.g., HLH) |
| EEG, cerebral ultrasound, brain MRI, evoked potentials | Neurological impairment |
| Liver Biopsy | See paragraph |
| MRI cholangiogram/scintigraphy/ERCP | See paragraph |
Table 3: First- and second-tier investigations in newborn and infants with cholestasis.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl-transpeptidase; INR, international normalized ratio; PT, prothrombin time; aPTT, activated partial thromboplastin time; WBC, white blood cells; TSH, thyroid-stimulating hormone; FT4, thyroxine; CPK, creatine-phosphokinase; BUN, Blood Urea Nitrogen; BASD, Bile acid synthesis defects; CF, Cystic Fibrosis; FO, fundus oculi; ACTH, adrenocorticotropic hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1; PRL, prolactin; FSH, folliclestimulating hormone; LH, luteinizing hormone; PCR, Polymerase chain reaction; WES, whole-exome sequencing; WGS, whole-genome sequencing; ARC, Arthrogryposis renal dysfunction cholestasis; P, plasma; S, serum; DBS, dried blood spot; U, urine; RBC, red blood cells; F, fibroblasts; M, muscle; GALD, gestational alloimmune liver disease; EEG, Electroencephalogram; MRI, Magnetic resonance imaging;ERCP, Endoscopic Retrograde Cholangiopancreatography; HLH,hemophagocytic lymphohistiocytosis. A biochemical crossroad in the evaluation of NIC is the serum gamma-glutamyl transpeptidase (GGT) concentration. GGT is typically markedly increased in BA, in fact high levels (> 200 IU/L) in presence of acholic stools strongly suggest BA, although this finding is not specific to this condition. Notably, in a large retrospective cohort, GGT values higher than 500 IU/L were found only in BA patients Conversely, a normal GGT level is may suggest a poor prognosis as in progressive intrahepatic familial cholestasis type 1 and 2 and other inborn defects of canalicular transport or synthesis of bile acids .Elevated (above 7 μmol/L) total serum bile acids can be considered a very sensitive biomarker of cholestasis. However, slight elevations may reflect physiologic bilirubin metabolism immaturity in infants under 6 months of age, while their concentration may be low/ normal in bile acid synthesis defects
Recommendation In cholestatic infants, alpha1-antitrypsin deficiency, Alagille syndrome, and cystic fibrosis must be ruled out by specific tests before any invasive diagnostic procedure, since these diseases may mimic biliary atresia (GRADE 1 B, 100% agree).
Metabolic workup for inborn errors of metabolism presenting with NIC
IEM causing infantile cholestasis include a wide and heterogeneous group of diseases. In some disorders the liver is the only affected organ, while others present NIC in the context of a multisystem disease. The orientation of second level laboratory tests requires a careful evaluation of the clinical picture and of first-level laboratory analyses. Recently, expanded newborn screening (NBS) may identify some treatable IEM causing NIC. Depending on the different screening panels, the following disorders can be detected at birth: tyrosinemia type 1, galactosemia, urea cycle disorders [e.g., citrullinemia type I and II (citrin deficiency), argininosuccinate lyase and arginase deficiencies], long-chain fatty acid oxidation disorders (e.g., CPT I, LCHAD/MTP, MAD deficiencies). All disorders identified by NBS should be confirmed by biochemical and molecular analysis . However, confounding NBS results may occur in preterm infants and in those on prolonged parenteralutrition. The red flags suggesting an IEM as the underlying cause of NIC are listed in Table 4. Second-level laboratory testing and/or genetic testing should be then performed based on the diagnostic suspicion. (Table 4)
| Red flags | Disorders |
|---|---|
| Hyperammonemia, letargy/coma, feeding difficulties | Urea cycle disorders |
| Cataract, hypoglycemia, coagulopathy, E.Coli sepsis | Galactosemia |
| Hypoglycemia, hepatomegaly | Disorders of carboydrate metabolism, mitochondrial disorders, adenosine kinase deficiency |
| Early nodular liver lesions, high alfafetoprotein, coagulopathy, renal tubular dysfunction | Tyrosinemia type 1 |
| Hepato-splenomegaly, neurologic involvement, cherry red spot, non-immune fetal hydrops | Lysosomal storage disorders |
| Selective coagulation factor deficiency (factor VII, IX, X, XI, AT3, protein C/S), neurological and systemic signs | Congenital disorders of glycosylation (CDG) |
| Lactic acidosis, neurological signs, cardiomiopathy | Mitochondrial disorder |
| Rotatory eye movements, hypoglycemia, coagulopathy, elevated blood lactate, hyperferritinemia | Mitochondrial DNA depletion syndrome (DGUOK) |
| Adrenal calcification, hepatosplenomegaly, failure to thrive, macrophage activation syndrome | Wolman disease (LAL deficiency) |
| Hypotonia, wide anterior fontanel, cataract, renal cysts | Zellweger spectrum disorders |
| Chronic diarrhea | Bile acid synthesis and conjunction disorders, PFIC 1 |
| Hypoketotic hypoglycemia, cardiomyopathy, maternal HELPP, rhabdomyolysis | Fatty acid oxidation defects (e.g., LCHAD/MTP) |
| Renal tubular dysfunction | Tyrosinemia, mitochondrial diosorders, transaldolase deficiency, galactosemia, ARC syndrome |
| Polydactily/Syndactily, ambigous genitalia, micrognatia | Smith-Lemli-Opitz syndrome |
| Non immune fetal hydrops | Lysosomal storage disorders, Smith-Lemli-Opitz, mevalonic aciduria, congenital disorders of glycosylation (CDG), peroxisomial disorders, mitochondrial disorders |
| Acute liver failure | Tyrosinemia type 1, galactosemia, mitochondrial disorders, urea cycle disorders, CDG, NBAS deficiency, CALFAN syndrome, adenosine kinase deficiency, Transaldolase deficiency, fatty acid oxidation defects, mevalonic aciduria, Wolman disease |
Table 4 : “Red flags” for inborn error of metabolism in cholestatic infant.
AT3, Antithrombin; LAL, Lysosomal acid lipase; PFIC, progressive familial intrahepatic cholestasis; HELPP, hemolysis, elevated liver enzyme levels, and low platelet levels; LCHAD/MTP, long-chain 3-hydroxyacyl-CoA dehydrogenase/ mitochondrial trifunctional protein; ARC, Arthrogryposis renal dysfunction cholestasis; CDG, congenital disorders of glycosylation; CDG, congenital disorders of glycosylation; NBAS, neuroblastoma amplified sequence; CALFAN, low γ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration. The diagnosis of an IEM causing NIC may allow the identification of treatable disorders, provides the clues for genetic counselling and should alert clinicians on potentially associated risk of liver failure, acute metabolic events and of a multiorgan/multisystem involvement.
Recommendation In infants with cholestasis, inborn errors of metabolism (IEM) should always be considered in the differential diagnosis (GRADE 1A, 100% agree). Clinicians should be aware of which IEM causing cholestasis is included in the expanded newborn screening of their own region/country (GRADE 1A, 100% agree). A careful clinical evaluation, combined with first-level laboratory testing, may arise the suspicion of specific IEM, indicating second level laboratory analyses for confirmatory diagnosis (GRADE 1 C, 100% agree).
Imaging
Liver ultrasound
Abdominal ultrasound is helpful in evaluating cholestatic infant,particularly to look for signs of BA and other biliary obstructive diseases.A 4-hour fasting abdominal ultrasound is the first-line diagnostic imaging technique to assess several hepatic sonographic parameters because it is noninvasive, easily available, painless, safe and without need for sedation. The main goal of the ultrasound in cholestatic infants is to exclude other anatomic causes of cholestasis, such as a choledochal cyst or complicated gallstone disease. The triangular cord sign, abnormal gallbladder length and contractility index, gallbladder ghost triad, nonvisualization of the common bile duct, hepatic artery diameter, hepatic artery to portal vein diameter ratio, hepatic subcapsular blood flow, and splenic, portal vein and/or inferior vena cava abnormalities have been considered ultrasound findings suggestive of BA. However, none by itself can confirm the diagnosis, and their detection and interpretation imply substantial radiological expertise . In particular, findings such as laterality anomalies, midline liver, polysplenia, asplenia, absence of inferior vena cava and preduodenal portal vein raise the suspicion of BASM .A recent meta-analysis showed ultrasound pooled sensitivity of 77% and specificity of 93% for the diagnosis of BA, with a positive predictive value of 88.6% and a negative predictive value of 85.3% .Another metanalysis showed that a combination of the triangular cord sign and an abnormal gallbladder yielded a sensitivity of 95% and specificity of 89%; therefore, these are considered the two most accurate ultrasound findings used for the diagnosis or exclusion of BA. However, clinicians should remember that a normal ultrasonography, does not rule out non-syndromic BA. Shear Wave Elastography and transient elastography appear to help discriminate BA from other causes of NIC, but they still are not routinely performed
Recommendation Infants with cholestasis should undergo liver ultrasound to exclude biliary obstructive conditions other than biliary atresia, such as choledochal malformations or gallstone disease (GRADE 1A, 100% agree). Ancillary findings such as the absence of the gallbladder or its dysmorphic aspect, along with other minor features suggestive of biliary atresia, should always be searched at liver ultrasound (GRADE 1 A, 100% agree).
Other imaging
Hepatobiliary scintigraphy
Used in the past to assess biliary tract patency, the hepatobiliary scintigraphy (HBS) is not anymore part of standard work-up, because of its low specificity and positive predictive value . Falsely positive results are seen in non-obstructive bile flow alteration (non-BA severe cholestasis). On the contrary, a normal bile passage in the intestine has nearly 100% negative predictive value for BA, but since these children generally also have colored stools, HBS seems not to add much to a thorough clinical examination . In no case the HBS should delay the referral of a child with suspect BA to a center with experience in pediatric hepatology.
Cholangiogram
The lack of visualization of the bile ductal system is the cornerstone in the diagnosis of BA. Direct or indirect biliary tract imaging essentially distinguishes between BA from all other non-BA cholestasis. However, in low-weight infants, a normal biliary system is not visible on standard imaging, thus making definite identification of BA very challenging. All the cholangiographic techniques may give false positive results (e.g., in AGS or in cystic fibrosis) due to marked ductal hypoplasia.
Direct cholangiography is obtained through different approaches:
Surgical direct cholangiography (intraoperative cholangiography, IOC): IOC allows a direct opacification of the biliary tree, and is performed as the second step of a laparoscopic or open surgical assessment (after abdominal and liver macroscopic evaluation). IOC is currently the gold standard to diagnose BA, and it allows to perform a HPE at the same time Endoscopic Retrograde Cholangio- Pancreatography (ERCP) has been shown to be a possible alternative to intra-operative cholangiogram, with similar diagnostic accuracy. However, the limited availability on the market of pediatric ERCP equipment and the lack of experienced endoscopists in this field limit the utility of this approach to selected centers Percutaneous cholangiography (PC): direct cholangiography by percutaneous transhepatic puncture of an intrahepatic bile duct is only feasible in patients with dilated intrahepatic bile ducts and it is rarely proposed in NIC. Alternatively, a PC can be carried out in some patients through gallbladder puncture (cholecystography). This is the case of suspected BA with patent gallbladder, such as type 1. Overall, that approach can be used in selected jaundiced babies with cholestasis and absence of dilatation of the biliary system but a normal gallbladder.
Magnetic Resonance Cholangio-Pancreatography (MRCP) is not reliable as the normal biliary tract is not consistently visualized in children under 3 months of age; false negative results are thus common.
Recommendations Surgical exploration and direct evaluation of biliary tract by cholangiogram is considered the gold standard to positively and definitively establish the diagnosis of biliary atresia and neonatal sclerosing cholangitis (GRADE 1A, 82% agree). Hepatobiliary scintigraphy and magnetic resonance cholangio-pancreatography should not be used as a single test in the diagnosis of BA due to their scarce specificity (GRADE 1B, 100% agree).
Histology
Liver biopsy often remains the cornerstone of the diagnostic work-up of infants with cholestatic jaundice as interpretation by an experienced pathologist will provide the correct diagnosis in 90% to 95% of cases, avoiding unnecessary surgery in patients with intrahepatic disease . In addition to its diagnostic role, the liver biopsy may also reveal histologic features of significant prognostic value, such as the estimated degree of fibrosis, which may help predict outcome following HPE and the decision to proceed with HPE. Accuracy depends on several factors, especially on the adequacy of the specimen and on the patient’s age. Paraffin-embedded liver biopsies are routinely stained with hematoxylin and eosin, periodic acid-Schiff (PAS), PAS after diastase and Masson trichrome stain. Adequacy in size and number of portal tracts, as well as size and shape of portal bile ductules should be reported. The main role of histology in the investigation of NIC is to distinguish between intrahepatic and extrahepatic (biliary obstructive, essentially BA) causes, ultimately allowing timely surgery for infants with BA. Intrahepatic cholestasis could be further distinguished between cholestatic conditions caused by a disturbance located at the level of the liver cell or canaliculus (intralobular cholestasis) and pathological states where the primary lesion is thought to operate the intrahepatic biliary passage (extralobular cholestasis).
Ductal/ductular bile plugs, generalized moderate to marked ductular reaction and bile duct proliferation, portal stromal edema, higher stages of portal fibrosis, prominent pseudorosette formation, moderate to marked peribiliary neutrophilic infiltrates, and interlobular bile duct injury are acknowledged markers of biliary obstructive cholestasis, such as BA. Conversely, bile duct paucity, predominant giant cell transformation, macrovesicular steatosis, and extramedullary hematopoiesis are commonly associated with non-obstructive causes.Parenteral nutrition-associated cholestasis, cystic fibrosis and alpha-1-antitrypsin deficiency may show variable ductular reaction and may be impossible to distinguish from BA without clinical data.
However, early histological changes of BA may be relatively nonspecific, and biopsies performed too early in life may result in a falsely negative diagnosis . Moreover, although ultrasound-guided percutaneous core liver biopsy is generally considered to be a safe and effective procedure, complications are reported in 1.7 to 4.6% of infants . For the above-mentioned reasons, the decision as to whether and when a liver biopsy should be performed has to be taken on a caseby- case basis by experienced pediatric hepatologists, also considering the local pathology expertise.
Recommendations Liver biopsy is useful to discriminate between extrahepatic and intrahepatic causes of NIC and may provide important clues for specific underlying etiologies, when performed at the appropriate timing and evaluated by an experienced pathologist (GRADE 2C; 100% agree). Percutaneous liver biopsy should not be performed when histology is not expected to add substantial information on the etiology of NIC, the diagnosis of biliary atresia is very likely on clinical and ultrasound findings, and surgical exploration and treatment could be delayed (GRADE 2C; 100% agree).
Genetic testing
In a child with NIC, once surgical (BA, CM), infectious and secondary causes are ruled out, the picture is most likely related to a monogenic liver disease. Over the last decade, next-generation sequencing (NGS)-based testing strategies have been refined, attaining high-throughput massively parallel sequencing platforms, thus providing simultaneous analysis of a large number of genes . NGS is increasingly used in clinical setting with different approaches varying in wideness and analytic complexity, from selected targeted gene panels (TGP) to whole exome sequencing (WES) and whole genome sequencing (WGS) Their role has been moving upwards in the diagnostic algorithm of NIC, from that of retrieving a genetic diagnosis among patients with indefinite conditions, to a second-tier screening for genetic causes just after having ruled out the “extrahepatic” ones
The overall accuracy of NGS testing for neonatal/infantile cholestasis is poorly known. Considering as positive results the detection of pathogenic or likely pathogenic variants in accordance with Mendelian inheritance, sensitivity and specificity are close to 100% in different reports However, the real-life diagnostic yield largely varies according to study population, design and setting: TGP identified a genetic etiology in 20% to 61% of children with persistent cholestasis while a tiered clinically-steered approach with TGP and WES was able to accomplish a genetic diagnosis in 60% of the children
Selection bias accounts for the variability in the diagnostic capacity of such approaches
Taken together, these results show that NGS is a promising tool to differentiate within different causes of intrahepatic cholestasis, and could be adopted as a second-tier evaluation after the exclusion of etiologies susceptible of rapid surgical or medical treatment, provided that a reasonable turnaround time and sufficient expertise in results’ interpretation are warranted, and that quality parameters are met. Interpretation of genetic results and variant classification should comply with the American College of Medical Genetics and Genomics guidelines and confirmation should be obtained by a different method (e.g., Sanger sequencing) using a second independent DNA sample of the parent-proband trio. The wideness of the genetic testing should be adapted to the index of suspicion as defined per clinical, biochemical, instrumental and histologic features. If NGS sequencing is not diagnostic for an etiology highly suspected on the phenotype, further testing is recommended to detect large deletions, duplications or inversions that can be missed at NGS. In this setting, WGS has proven superior to WES to detect all major classes of variants.
Recommendations NGS-based genetic testing has high diagnostic accuracy in identifying monogenic liver disorders causing NIC (GRADE 1C, 100% agree). NGS-based genetic testing should be considered early after exclusion of BA, in parallel with a metabolic work-up, and based on local availability (GRADE 2C, 100% agree).
Non-invasive diagnostic scores
The clinical differential diagnosis of BA cases from any other causes of neonatal cholestasis can be challenging. Early studies focused on laboratory data , in recent years all described clinical, radiological and laboratory signs were combined to obtain a scoring system with enough sensitivity and specificity to allow a positive diagnosis of BA. These efforts led in recent years to propose several “diagnostic scoring systems” for BA, including histological findings in some.In the United States a huge multicentric prospective study conducted among the Childhood Liver Disease Research Network (ChiLDReN - formerly BARC) failed in this effort of generating an algorithm that could discriminate BA and Non-BA cases, without the need for including the results of invasive procedures Overall, there are no currently available algorithm that has shown enough accuracy and/or that has been validated in independent pediatric cohorts to avoid a standard work-up.
Recommendation A positive diagnosis of BA is the result of a combination of clinical elements and different diagnostic tools. Clinical scoring systems for biliary atresia should not replace standard work-up due to the lack of validation in clinical setting (GRADE 1B, 100% agree).
Cholestasis in neonatal intensive care unit
Neonatal intensive care unit Beside prematurity, other common risk factors are long-term parenteral nutrition, sepsis, delayed enteral intakes, intrauterine growth retardation, asphyxia, surgery, hemodynamic instability, male sex. These conditions, often associated with higher oxidative stress, represent risk factors for developing transient neonatal cholestasis. Preventative measures should include the use of adequate caloric intakes and appropriate proportion of fish oil-based lipid emulsions in children requiring parenteral support, the early introduction of enteral feeding, as well as prompt recognition and treatment of infectious comorbidities .Although generally selflimiting, in a child with multifactorial transient neonatal cholestasis, other causes should be investigated if direct bilirubin levels > 2 mg/ dL persists after 2 weeks to avoid the risk of delaying the diagnosis of an underlying primary liver disease. Time and diagnostic effort should be tailored on the clinical appearance and on the course of cholestasis. Persistent cholestasis in any infant should be considered pathologic and detectable causes of cholestasis, including biliary atresia should be excluded in a timely and appropriate manner. Incidence of biliary atresia or genetic forms of cholestasis is the same in premature as in term infants
Conclusion
NIC poses a diagnostic challenge for the pediatrician. A delay in referral to the specialist can have a detrimental impact on outcomes.
A stepwise approach, aiming at ruling out first diseases with available treatments, is proposed.The recommendations reported in the present document assist the primary care pediatrician and the neonatologist in recognizing the newborns and infants with cholestasis among those with prolonged jaundice. Secondarily, they orient the pediatric gastroenterologist to the rationale use of the available diagnostic repertoire. SIGENP is not responsible for the practices of physicians and provides guidelines and Position Papers as indicators of best practice only. Diagnosis and treatment are at the discretion of the health care provider.
References
- Jakobsen LP, Knudsen MA, Lespinasse J, Ayuso CG, Ramos C, et al. (2006)The genetic basis of the Pierre Robin Sequence. Cleft Palate Craniofac J 43: 155-159.
- Dobby N, Black A, Ong KB (2012) Airtraq vs Glidescope airway management of a pediatric population with a documented difficult airway; Cormack and Lehane Grade III or IV. Pediatr Anesth 22: 921.
- Asai T, Nagata A, Shingu K (2008)Awake tracheal intubation through the laryngeal mask in neonates with upper airway obstruction. Paediatr Anaesth 18: 77-80.
- Asai T, Shingu K (2004)Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: incidence, causes and solutions. Br J Anaesth 92: 870-881.
- Parotto M, Cooper RM, Behringer EC (2020) Extubation of the Challenging or Difficult Airway. Curr Anesthesiol Rep 4: 1-7.
- Patel MR, Piazza CC, Martinez CJ, Volkert VM, Christine MS (2002)An evaluation of two differential reinforcement procedures with escape extinction to treat food refusal.J Appl Behav Anal 35: 363-374.
- Bernard-Bonnin AC (2006)Feeding problems of infants and toddlers. Can Fam Physician 52: 1247-1251.
- Davies WH, Satter E, Berlin KS (2006)Reconceptualizing feeding and feeding disorders in interpersonal context: the case for a relational disorder.J Fam Psychol 20: 409-417.
- Poppert KM, Patton SR, Borner KB (2015)Systematic review: mealtime behavior measures used in pediatric chronic illness populations.J Pediatr Psychol 40: 475-486.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Dianes AG (2022) Diagnostic Approach to Neonatal and Infantile Cholestasis. Neonat Pediatr Med 8: 249. DOI: 10.4172/2572-4983.1000249
Copyright: © 2022 Dianes AG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2335
- [From(publication date): 0-2022 - Nov 23, 2025]
- Breakdown by view type
- HTML page views: 1833
- PDF downloads: 502