Review Article Open Access
Diagnosing Progressive Supranuclear Palsy: Role of Biological and Neuroimaging Markers
Borroni B1*, Benussi A1, Pilotto A1, Gazzina S1, Turrone R1, Gardoni F2, Di Luca M2 and Padovani A1
1Centre for Neurodegenerative Disorders, Neurology Unit, University of Brescia, Brescia, Italy
2Centre of Excellence in Neurodegenerative Disorders, University of Milan, Milan, Italy
- Corresponding Author:
- Barbara Borroni
Clinica Neurologica
Università degli Studi di Brescia Pza Spedali Civili, 1 - 25100 Brescia, Italy
Tel: +39-0303995632;
Email: bborroni@inwind.it
Received date: August 04, 2014; Accepted date: November 05, 2014; Published date: November 12, 2014
Citation: Borroni B, Benussi A, Pilotto A, Gazzina S, Turrone R et al. (2014) Diagnosing Progressive Supranuclear Palsy: Role of Biological and Neuroimaging Markers. J Alzheimers Dis Parkinsonism 4:168. doi: 10.4172/2161-0460.1000168
Copyright: 2014 Borroni B et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Progressive Supranuclear Palsy (PSP) is a neurodegenerative disorders characterised by a kinetic-rigid syndrome with ocular motor dysfunction, postural instability, and frontal lobe and bulbar dysfunction. In most of the cases, especially at early disease stages, diagnosis is still challenging. PSP signs and symptoms may indeed overlap with both dementing neurodegenerative syndromes and movement disorders. In the last few years, a better definition of clinical picture along with the identification of biological and neuroimaging markers have increased diagnostic accuracy. In the present work, we reviewed the current literature on PSP diagnosis and the usefulness of potential diagnostic markers.
Keywords
Progressive supranuclear palsy; Biological markers; Neuroimaging
Introduction
Progressive Supranuclear Palsy (PSP) is an adult-onset progressive neurodegenerative disorder leading to an akinetic-rigid syndrome with ocular motor dysfunction, postural instability, and frontal lobe and bulbar dysfunction [1]. PSP usually occurs in the sixth decade, with clinical features that are often subtle and difficult to distinguish from other neurodegenerative disorders such as Parkinson's Disease (PD), Dementia with Lewy Bodies (DLB), Multiple System Atrophy (MSA) and Cortico Basal Syndrome (CBS) [2]. Early diagnosis can hence be challenging and the definite diagnosis is frequently deferred for many months. Neuropathologically, PSP is characterized by accumulation of Tau protein, neurofibrillary tangles and tufted astrocytes [3] in subcortical areas with a variable involvement of brain cortex.
The aetiology still remains elusive, but genetic background has a key-role in disease pathogenesis. Although the majority of PSP cases are sporadic, recent studies have reported high familial aggregation in PSP patients, and it has been widely demonstrated that Microtuble Associated Protein Tau (MAPT) gene mutations are causative of rare monogenic autosomal dominant PSP [4,5]. In sporadic cases, genetic advances have further confirmed the role of MAPT in increasing disease risk, and the H1 MAPT haplotype has been consistently associated with PSP, while the H2 haplotype seems protective [6]. Conversely, no major environmental risk factors have been reported so far [7]. A proper evaluation of known susceptibility factors related to PSP pathogenesis may help in defining neuroprotective therapeutic approaches [8].
In most cases PSP presents clinically as Richardson's syndrome (RS), or Steele-Richardson-Olszewski syndrome, with prominent postural instability, supranuclear vertical gaze palsy and frontal dysfunction [9,10].The classical description of RS, although, did not include all neuropathologically confirmed cases of PSP. To justify this heterogeneity, PSP was recently divided clinically and pathologically into two main phenotypes: classical RS and PSP-parkinsonism (PSP-P), the latter characterised by bradykinesia, rigidity and sometimes tremor, which tends to be asymmetric and modestly responsive to levodopa therapy [11-13].
Clinical heterogeneity is furthermore augmented by PSP presenting as CBS (PSP-CBS), as pure akinesia with gait freezing (PSP-PAGF), or, rarely, as PSP associated to bvFTD (PSP-FTD) or progressive non-fluent aphasia (PSP-PNFA)[14]. These subtypes are not included in the currently used diagnostic criteria [9,15].
The National Institute of Neurological Disorders and Stroke and the Society for PSP (NINDS-SPSP) clinical diagnostic criteria were compiled to reliably identify patients who had underlying PSP-tau pathology [9,15]. Briefly, the NINDS-SPSP criteria depend on the identification of a progressive disorder with onset after age 40 combining the two cardinal features (i.e. postural instability with falls during the first year of the disease and slow vertical saccades or supranuclear gaze palsy). A list of exclusion criteria aims to sort out look-alike disorders [1,16]. The sensitivity, specificity, and positive predictive value of the NINDS-SPSP criteria have been evaluated retrospectively in a pathologically confirmed series of patients, showing specificity and sensitivity of clinician diagnosis, which can be as high as 90% [17]. Because the specificity and PPV of the probable NINDS-SPSP clinical criteria have been found to be near perfect, and the specificity and PPV of the possible criteria to be high, a redefinition of this set of criteria has been proposed [15].
Recently, the Neuroprotective and Natural History in Parkinson Plus Syndromes (NNIPPS) consortium has proposed modified clinical diagnostic criteria, aiming to facilitate the clinical diagnosis of PSP. Briefly, the NNIPPS criteria allow an age of onset after 30 and postural instability or falls within 3 years from disease onset [16,18].
A recent study has evaluated the efficacy of both criteria for the diagnosis of PSP. The authors conclude that the total NINDS-SPSP criteria, accepting both possible and probable for diagnosis, yielded the highest sensitivity and might thus be most useful for routine clinical care. For scientific clinical trials, the NINDS-SPSP probable criteria might be preferred, possessing the highest positive predictive value and allowing, at present, the earliest, most specific diagnosis, thus allowing to include correct positives early on and to reliably exclude false positives [16].
Diagnosis according to clinical criteria is still hindered by low levels of specificity and sensitivity, because of clinical overlap with other neurodegenerative disorders characterised by Parkinsonism and dementia. These results should encourage the development of reliable biomarkers to increase diagnostic accuracy and improve early detection of disease, when neuropathologic burden of disease is not so severe as to prevent the action of upcoming disease-modifying therapies.
Genetics in the Diagnosis of PSP
Both environmental and inherited factors contribute to the risk of developing PSP [19,20]. Large clinical series have observed a positive family history for either parkinsonism or dementia in about a third of PSP patients[21,22], about 10% of which had an autosomal dominant inheritance pattern, thus genetic screening need to be considered in selected cases. Mutations within MAPT gene have been associated with PSP and other phenotypes within the FTLD spectrum, such as frontotemporal dementia, PPA or CBS [4,5]. Tau is involved in microtubule assembly and stabilization, which is altered in the presence of known mutations. Tau protein is translated from a single gene named microtubule associated protein tau (MAPT) located on chromosome 17 q21.31. Its expression is regulated by an alternative splicing mechanism resulting in six isoforms in the human brain [23]. Disease-specific deposition of Tau isoforms has been identified, with either 3-repeat (3R) or 4-repeat (4R) Tau inclusions on the basis of exon 10 skipping [24-26].
Of the 44 pathogenic mutations in MAPT currently described (http://www.molgen.ua.ac.be/FTDMutations/) only 9 have been associated with a PSP phenotype [27-36] (see Table 1). Intriguingly, all MAPT mutations associated with PSP affect exon 10, increase the ratio of 4R Tau isoforms (Table 1) [4,5,25,31].
| MAPT region | Mutation | Clinical features | Origin | Age at onset | Age at death | References |
|---|---|---|---|---|---|---|
| Exon 1 | R5L | Falls, dysarthria, micrographia | U.S.A | 62 | 67 | [30] |
| Exon 10 | N279K | *1 bradyphrenia, parkinsonism, apathy | France | 40 | 47 | [28] |
| *2 apathy, attentive deficits | 41 | - | ||||
| *3 parkinsonism with tremor, personality change, ocular apraxia, postural instability | Japan | 42 | 54 | [91] | ||
| *4 parkinsonism, gaze palsy, postural instability | 44 | 56 | ||||
| *5 Parkinsonism, gaze palsy, falls | 46 | - | ||||
| *6 Cognitive impairment, gaze palsy, postural instability, pyramidal signs | 41 | 51 | ||||
| *7 Parkinsonism, gaze palsy, falls | 42 | 54 | ||||
| *8 Parkinsonism, gaze palsy, falls, pyramidal signs | 43 | 51 | ||||
| ** 4 families with a common founder, 39 affected members; parkinsonism or dementia associated with behavioural changes | U.S.A (Caucasian) | 32-58 | 6-9 y of duration | [36] | ||
| ** 1 family, 3 affected members; parkinsonism or dementia associated with behavioural changes | France | 38-45 | 6-8 y of duration | |||
| L284R | *1 multiple falls with progressive dementia | UK (South England) | early-40s | 48 | [31] | |
| *2 personality change, falls, progressive aphasia | mid-40s | 52 | ||||
| *3 personality change, apathy, falls, dysarthria | 43 | 47 | ||||
| S285R | *1 Sporadic, supranuclear gaze palsy, bradykinesia, falls | Japan | 46 | 49 | [91] | |
| �??N296 | *1 gaze palsy, cognitive deficits, emotional lability | Spain | 38 | - | [92] | |
| *2 personality change, parkinsonism | 39 | - | ||||
| *3 Personality change, cognitive impairment, gaze palsy, falls | Japan | 44 | - | [91] | ||
| *4 Dysarthria, anterocollis, falls, parkinsonism | Italy | 39 | - | [33] | ||
| P301L | *1 Parkinsonism not further characterized | Nederland | - | - | [22] | |
| G303V | *1 Parkinsonism, falls, gaze palsy, dysarthria | Spain | 37 | 45 | [32] | |
| *2 Not well characterized parkinsonism | 41 | 44 | ||||
| *3 Not well characterized parkinsonism | late-30s | - | ||||
| *4 Parkinsonism with tremor and rigidity | France | 41 | 45 | [27] | ||
| *5 Parkinsonism, gaze palsy, eye apraxia | 37 | 40 | ||||
| *6 Parkinsonism with dystonia, eye apraxia | 51 | 57 | ||||
| *7 Parkinsonism with dystonia, gaze palsy | 39 | 44 | ||||
| *8 Parkinsonism, gaze palsy, dysphagia | 42 | 46 | ||||
| *9 Parkinsonism with dystonia, gaze palsy | 32 | 36 | ||||
| S305S | *1 Apathy/cognitive deficits | Australia (Caucasian) | 55 | - | [35] | |
| *2 Dementia, apathy, aphasia | 48 | 51 | ||||
| *3 Clumsiness, dysarthria, rigidity | 49 | 56 | ||||
| Intron 10 | 10+3 | *1 “atypical PSP-presentation” not further characterized | U.S.A. | 47 | 48 | [34] |
| *2 “atypical PSP-presentation” not further characterized | 52 | 58 | ||||
| 10+16 | *1 Sporadic parkinsonism with fatigue, apathy, micrographia, falls | UK | 40 | 45 | [29] |
Table 1: MAPT mutation associated with clinical diagnosis of PSP. *: studies with single patients descriptions; **studies with families descriptions.
Despite the fact that gene mutations are absent in most patients, since 1997 a large number of studies showed a significant association of PSP with the MAPT locus [6,37-40]. The MAPT locus exists as two major haplotype groups termed as “H1†and “H2†in European populations, defined by at least 100 SNPs that are inherited in strong linkage disequilibrium with each other. Inheritance of two copies of the H1 haplotype (H1/H1) has been associated with a major genetic risk factor for PSP [6]. A large collection of pathologically confirmed PSP samples have also been used to fine map PSP risk on H1 chromosomes [40,41]. The most likely explanation of the association with the MAPT H1 haplotype and PSP is that variants in the H1/H2 haplotypes confer risk/protection against disease by altering expression at the locus, with the H1 haplotypes expressing higher levels of MAPT [40,42].
Projections of population-attributable risk suggest that only about 60% of the risk of developing PSP can be accounted for by the MAPT H1 haplotype, suggesting there may be additional risk genes involved in PSP. In 2007 the first GWAS analysis on PSP patients identified a new locus on chromosome 11, starting from a small cohort of pathologically proven PSP patients [39]. This locus contains the DNA damage-binding protein 2 (DDB2) and lysosomal acid phosphatase 2 (ACP2) genes. Since DNA damage and lysosomal dysfunction have been implicated in ageing and neurodegenerative processes, both genes were viable candidates for conferring disease risk. Searching for new candidates genes, Höglinger et al. performed a world-wide GWAS analysis in 2011 considering two different PSP groups of more than 1000 individuals each with pathologically and clinically-diagnosed PSP patients [43]. After confirming the MAPT locus, the study identified previously unidentified signals associated with PSP risk at STX6, EIF2AK3 and MOBP. These genes encode proteins for vesicle-membrane fusion at the Golgi-endosomal interface, for the endoplasmic reticulum unfolded protein response and for a myelin structural component, generating testable translational hypothesis, further extending the complex pathophysiology of PSP [43].
Biological Markers in the Diagnosis of PSP
The CSF analyses represent the most direct and convenient means to study biochemical changes occurring in the central nervous system as they are directly related to specific pathogenetic mechanisms of neurodegeneration [44]. In Alzheimer’s disease, CSF analyses show a typical decrease in amyloid ß42 (Aß42), and an increase in tau (t-tau) and phospho-tau (p-tau) levels compared with controls and other neurodegenerative diseases [45-47]. Despite the fact that PSP is a primary tauopathy, most studies evaluating t-tau and p-tau CSF levels did not show any significant difference in PSP compared to controls or other parkinsonisms [47,48]. CSF Abeta amyloid levels are also comparable between PSP and healthy controls in most but not all studies [47,49-52].
In addition, other CSF biological markers may be of help to exclude other neurodegenerative disorders. In PD and DLB, there is a consistent decrease in total alpha-synuclein levels and an increased in oligomeric or phosphorylated form [53,54], and the level of neurofilament light chain alone may differentiate between PD and other parkinsonisms [48], but cannot selectively recognise PSP[55,56]. Taken together, these results indicate that classical CSF levels have limited utility in distinguishing PSP from controls and other parkinsonian disorders [47,50,56-58].
To date, the most sensitive biomarker to detect PSP cases seems to be the evaluation of CSF full-length (55 kDa) and truncated (33 kDa) tau forms [59,60]. Using western blot and immuno-precipitation assays it has been demonstrated a reduction in 33 kDa/55 kDa in PSP, differentiating the condition from healthy controls, Alzheimer’s disease and Parkinson’s disease [59]. Interestingly, for the first time, the assay distinguished also PSP from bvFTD and Cortico-basal degeneration, part of the complex FTLD spectrum. These findings were reproduced by the same group in another larger cohort of patients [60].More recently, by developing ELISA kit on tau fragments, Wagshal et al. [52]observed lower CSF N-terminal and C-terminal tau concentrations in PSP compared to controls and AD patients[61], thus confirming tau fragment abnormalities in PSP. Finally, Luk et al. showed a decrease in 4R-tau isoform in PSP and AD compared with CBS, PDD and healthy controls using an adapted immune-PCR procedure [62].
In the near future, providing a more reliable assay for tau fragments could be pivotal in order to extend these observations in larger clinical and pathological PSP sample.
Neuroimaging in the Diagnosis of PSP
According to clinical research criteria, Magnetic Resonance Imaging (MRI) utility in PSP pathology is limited to the exclusion of relevant structural abnormalities, such as basal ganglia-brainstem infarcts or lobar atrophy [9]. However, different structural and functional techniques have been shown to be useful in PSP in vivo diagnosis, and could represent a first-phase approach when pathology exhibits an atypical presentation or in the first phases when differentiation from others movement disorders can be challenging.
Structural Imaging
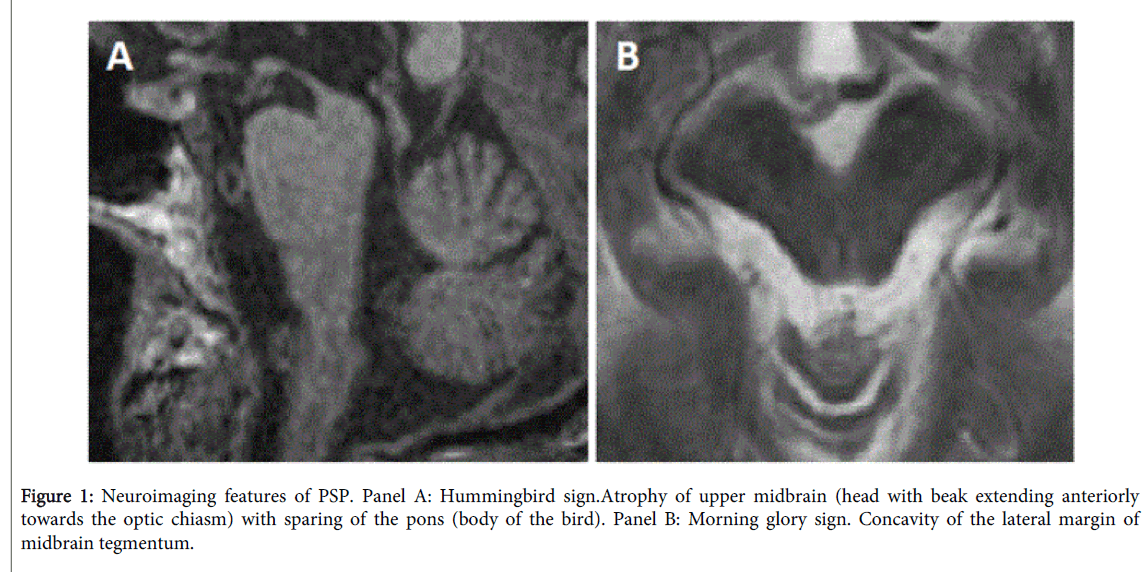
Atrophy of the upper brainstem, of the superior cerebellar peduncles and dilatation of third ventricle are the radiological and pathological features of PSP [10,63,64].
Two useful “easy to assess†structural signs typical of PSP pathology have been described, namely the hummingbird [65] or giant penguin sign [66] and the morning glory sign [67]. The hummingbird sign is the expression of atrophy of the rostral tegmentum on mid-sagittal view, with sparing of the pons (Figure 1, panel A). Morning glory sign can be seen on axial images and is due to midbrain atrophy with concavity of the lateral margin of the midbrain tegmentum (Figure 1, panel B). The latter sign has been related to supranuclear gaze palsy and is more frequently observed in the RS. The sensitivity, specificity, and accuracy of radiological diagnosis of PSP are 72.7%, 94.3%, and 86.0%, respectively [68], thus being of help in clinical practice.
Beside conventional MRI, different quantitative measures of atrophy, to assess an early diagnosis in patients with atypical presentation, have been studied. The area of the midbrain in patients with PSP is about half of the area of the midbrain in patients with PD, MSA-P and age-matched controls. However, patients with MSA-P showed some overlap of individual areas with values from patients with PSP [66]. On the other hand, pons area is smaller in MSA-P, and a reduced midbrain/pons ratio has been proposed as a more sensitive quantitative marker of PSP [66], even in combination with CSF markers [69].
Despite that, other studies failed in replicating the stability of midbrain/pons ratio [70,71],and suggested other quantitative approaches, such as the MR parkinsonism index (MRPI, calculated by multiplying the pons area-midbrain area ratio by the middle cerebellar peduncle width-superior cerebellar peduncle width ratio, sensitivity and specificity 100%) [71]. Interestingly, it has been reported that an abnormal MRPI (> 13.55) could also predict evolution of clinically unclassifiable parkinsonisms. Morelli found that 78.5% of patients with an abnormal MRPI developed PSP after 2 year-follow-up [72].
Other work evaluated white matter damage in PSP, as neuropathological studies indicated this as hallmark of the disorder. Both fractional anisotropy and mean diffusivity reveal a diffuse cerebellar, brainstem, cerebral, thalamic involvement [73-75], with a more frontal spatial distribution in the RS [76]. In particular, it has been reported that corpus callosum and superior cerebellar peduncle abnormalities can discriminate PSP from PD patients with high accuracy [77].Studies with diffusion tensor imaging have identified extensive white matter lesions in corticobasal degeneration and progressive supranuclear palsy, showing strong diagnostic marker potential for these diseases [73-75].
Functional Imaging
Functional imaging techniques employ tracers or blood-oxygen contrasts to measure changes in brain metabolism. In clinical practice, post-synapticdopamine tracers or dopamine transporter (DaT SCAN) may assist in differentiating PD or neurodegenerative parkinsonisms from other neurodegenerative disorders, but this is not useful in the differential diagnosis among parkinsonisms [78-80].
Brain 18F fluorodeoxy glucose (FDG)-PET allows the characterization of PSP hypometabolism [81], with brainstem, medial thalamus, caudate nuclei and the medial frontal cortex involvement [82,83]. Moreover, in PSP patients, it may be of help in discriminating RS from PSP-P: when comparing RS to PSP-P, a reduced uptake in frontal and thalamic regions in the former group, and putaminal in the latter, was found. These patterns are in line with clinical presentation, with frequent cognitive decline in RS and predominant motor symptoms in PSP-P [84]. Only few studies explored functional connectivity in PSP pathology. This technique is promising as a very early marker of pathology but it cannot be still applied in single subject analysis [85,86].
An interesting and modern approach is represented by support vector machine analysis. This approach is based on algorithms able to automatically extract multiple information from image sets without requiring an a priori hypothesis of where this information may be coded. By combining different techniques, the automated algorithm allowed to discriminate correctly between PSP and PD with 100% accuracy, 90% sensitivity and 96% specificity. These results could be encouraging for computer-based diagnosis in the near future [87,88].
Combined Markers in PSP
Despite the efforts to establish reliable clinical criteria, PSP diagnosis still represents a clinical challenge, because of clinical overlap with either tau or synuclein-based neurodegenerative disorders that lead to parkinsonism and dementia [89]. Several studies have attempted to identify reliable techniques for improving PSP diagnosis, especially in early disease stages. As a result, different biological markers, neuroimaging techniques, and sonography studies have been proposed.
Research on Alzheimer's disease has long been taking advantage of different biomarkers and using them in combination to obtain higher levels of diagnostic accuracy. Conversely, in PSP, combined biomarkers are currently lacking. A recent study by Borroni et al. has sought to improve diagnostic accuracy by combined evaluation of biological and neuroimaging biomarkers. The authors, employing the CSF tau 33 kDa/55 kDa ratio [60] and MRI midbrain to pons measure, obtain significantly improved sensitivity and specificity for PSP detection, achieving diagnostic accuracy ranging from 86.3% to 92.5% in different comparison groups [90]. The use of combined biomarkers seems to be a rational and promising approach for enhancing diagnostic accuracy in identifying PSP, especially in the early stages of disease when clinical diagnosis is still challenging.
Conclusions
In spite of the recent advances in the comprehension of the underlying pathophysiology of PSP, combined with an improvement of neuroimaging techniques and genetic analyses, the diagnostic accuracy of PSP remains low in some clinical settings. Structural, functional and metabolic imaging techniques show great potential in refining the diagnostic accuracy in atypical cases. On the other hand, as sustained by the great body of literature, CSF biomarkers will be chief in the differential diagnosis of neurodegenerative diseases. However, most of promising PSP CSF biomarkers have been evaluated by western- and immune-blot and a validation of reliable clinical assays (e.g. ELISA) in larger cohort will be pivotal in order to further understand their clinical relevance [52, 56]. Furthermore, there is a crucial need to diagnose neurodegenerative diseases early in their progression, when therapeutic approaches are likely to be most effective, to monitor disease progression and response to future treatments. The precise recognition of a specific molecular pathology in the preclinical or prodromal stages of disease will provide a proper diagnostic and therapeutic approach in early phases of disease where neuronal loss is not so severe as to be irreversible.
A limitation of many studies is that they are cross-sectional and retrospective, frequently lacking post-mortem neuropathological assessment. Hence, there is a necessity for prospective longitudinal studies with neuropathological confirmation to establish a better estimate of the accuracy of clinical diagnosis, and to determine whether additional investigations can improve diagnostic reliability, both individually and in combination.
Acknowledgements
This work was supported by grant from Ministero della Salute RF-FSL-2008-1281075 to A.P. and M.D.L.
References
- Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, et al. (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46: 922-930.
- Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54 Suppl 5: S5-19.
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, et al. (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44: 2015-2019.
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, et al. (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 95: 7737-7741.
- Van Swieten J, Spillantini MG (2007) Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol 17: 63-73.
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, et al. (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8: 711-715.
- Vidal JS, Vidailhet M, Derkinderen P, de Gaillarbois TD, Tzourio C, et al. (2009) Risk factors for progressive supranuclear palsy: a case-control study in France. J Neurol Neurosurg Psychiatry 80: 1271-1274.
- Borroni B, Agosti C, Magnani E, Di Luca M, Padovani A (2011) Genetic bases of Progressive Supranuclear Palsy: the MAPT tau disease. Curr Med Chem 18: 2655-2660.
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, et al. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47: 1-9.
- Steele jc, richardson jc, olszewski j (1964) progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10: 333-359.
- Liscic RM, Srulijes K, Gröger A, Maetzler W, Berg D (2013) Differentiation of progressive supranuclear palsy: clinical, imaging and laboratory tools. Acta Neurol Scand 127: 362-370.
- Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, et al. (2005) Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain 128: 1247-1258.
- Williams DR, Lees AJ (2010) What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord 25: 357-362.
- Williams DR, Holton JL, Strand C, Pittman A, de Silva R, et al. (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson's syndrome. Brain 130: 1566-1576.
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, et al. (2003) Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18: 467-486.
- Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, et al. (2013) Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 28: 504-509.
- Lopez OL, Litvan I, Catt KE, Stowe R, Klunk W, et al. (1999) Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology 53: 1292-1299.
- Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, et al. (2009) Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 132: 156-171.
- Golbe LI, Rubin RS, Cody RP, Belsh JM, Duvoisin RC, et al. (1996) Follow-up study of risk factors in progressive supranuclear palsy. Neurology 47: 148-154.
- Stamelou M, de Silva R, Arias-Carrión O, Boura E, Höllerhage M, et al. (2010) Rational therapeutic approaches to progressive supranuclear palsy. Brain 133: 1578-1590.
- Borroni B, Goldwurm S, Cerini C, Cosseddu M, Meucci N, et al. (2010) Familial aggregation in Progressive Supranuclear Palsy and Corticobasal Syndrome. Eur J Neurol .
- Donker Kaat L, Boon AJ, Azmani A, Kamphorst W, Breteler MM, et al. (2009) Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology 73: 98-105.
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3: 519-526.
- Delacourte A, Robitaille Y, Sergeant N, Buée L, Hof PR, et al. (1996) Specific pathological Tau protein variants characterize Pick's disease. J Neuropathol Exp Neurol 55: 159-168.
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, et al. (1998) Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702-705.
- Lieberman AP, Trojanowski JQ, Lee VM, Balin BJ, Ding XS, et al. (1998) Cognitive, neuroimaging, and pathological studies in a patient with Pick's disease. Ann Neurol 43: 259-265.
- Choumert A, Poisson A, Honnorat J, Le Ber I, Camuzat A, et al. (2012) G303V tau mutation presenting with progressive supranuclear palsy-like features. Mov Disord 27: 581-583.
- Delisle MB, Murrell JR, Richardson R, Trofatter JA, Rascol O, et al. (1999) A mutation at codon 279 (N279K) in exon 10 of the Tau gene causes a tauopathy with dementia and supranuclear palsy. Acta Neuropathol 98: 62-77.
- Morris HR, Osaki Y, Holton J, Lees AJ, Wood NW, et al. (2003) Tau exon 10 +16 mutation FTDP-17 presenting clinically as sporadic young onset PSP. Neurology 61: 102-104.
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, et al. (2002) An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol 52: 511-516.
- Rohrer JD, Paviour D, Vandrovcova J, Hodges J, de Silva R, et al. (2011) Novel L284R MAPT mutation in a family with an autosomal dominant progressive supranuclear palsy syndrome. Neurodegener Dis 8: 149-152.
- Ros R, Thobois S, Streichenberger N, Kopp N, Sánchez MP, et al. (2005) A new mutation of the tau gene, G303V, in early-onset familial progressive supranuclear palsy. Arch Neurol 62: 1444-1450.
- Rossi G, Gasparoli E, Pasquali C, Di Fede G, Testa D, et al. (2004) Progressive supranuclear palsy and Parkinson's disease in a family with a new mutation in the tau gene. Ann Neurol 55: 448.
- Spina S, Farlow MR, Unverzagt FW, Kareken DA, Murrell JR, et al. (2008) The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain 131: 72-89.
- Stanford PM, Halliday GM, Brooks WS, Kwok JB, Storey CE, et al. (2000) Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain 123 : 880-893.
- Tsuboi Y, Uitti RJ, Delisle MB, Ferreira JJ, Brefel-Courbon C, et al. (2002) Clinical features and disease haplotypes of individuals with the N279K tau gene mutation: a comparison of the pallidopontonigral degeneration kindred and a French family. Arch Neurol 59: 943-950.
- De Silva R, Hope A, Pittman A, Weale ME, Morris HR, et al. (2003) Strong association of the Saitohin gene Q7 variant with progressive supranuclear palsy. Neurology 61: 407-409.
- Higgins JJ, Golbe LI, De Biase A, Jankovic J, Factor SA, et al. (2000) An extended 5'-tau susceptibility haplotype in progressive supranuclear palsy. Neurology 55: 1364-1367.
- Melquist S, Craig DW, Huentelman MJ, Crook R, Pearson JV, et al. (2007) Identification of a novel risk locus for progressive supranuclear palsy by a pooled genomewide scan of 500,288 single-nucleotide polymorphisms. Am J Hum Genet 80: 769-778.
- Rademakers R, Melquist S, Cruts M, Theuns J, Del-Favero J, et al. (2005) High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet 14: 3281-3292.
- Pittman AM, Myers AJ, Abou-Sleiman P, Fung HC, Kaleem M, et al. (2005) Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet 42: 837-846.
- Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R (2006) Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 15: 3529-3537.
- Höglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, et al. (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43: 699-705.
- Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, et al. (2010) Biomarker discovery for Alzheimer's disease, frontotemporal lobar degeneration, and Parkinson's disease. Acta Neuropathol 120: 385-399.
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, et al. (2012) Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 367: 795-804.
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, et al. (2010) Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 9: 1118-1127.
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, et al. (2012) Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 78: 47-54.
- Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, et al. (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69: 1445-1452.
- Bech S, Hjermind LE, Salvesen L, Nielsen JE, Heegaard NH, et al. (2012) Amyloid-related biomarkers and axonal damage proteins in parkinsonian syndromes. Parkinsonism Relat Disord 18: 69-72.
- Süssmuth SD, Uttner I, Landwehrmeyer B, Pinkhardt EH, Brettschneider J, et al. (2010) Differential pattern of brain-specific CSF proteins tau and amyloid-β in Parkinsonian syndromes. Mov Disord 25: 1284-1288.
- Noguchi M, Yoshita M, Matsumoto Y, Ono K, Iwasa K, et al. (2005) Decreased beta-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci 237: 61-65.
- Wagshal D, Sankaranarayanan S2, Guss V2, Hall T2, Berisha F3, et al. (2014) Divergent CSF Ä alterations in two common tauopathies: Alzheimer's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry .
- Parnetti L, Castrioto A, Chiasserini D, Persichetti E, Tambasco N, et al. (2013) Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol 9: 131-140.
- Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, et al. (2012) Phosphorylated α-synuclein in Parkinson's disease. Sci Transl Med 4: 121ra20.
- Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B (2010) Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord 16: 142-145.
- Magdalinou N, Lees AJ, Zetterberg H2 (2014) Cerebrospinal fluid biomarkers in parkinsonian conditions: an update and future directions. J Neurol Neurosurg Psychiatry 85: 1065-1075.
- Eller M, Williams DR (2009) Biological fluid biomarkers in neurodegenerative parkinsonism. Nat Rev Neurol 5: 561-570.
- Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, et al. (2011) α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 10: 230-240.
- Borroni B, Gardoni F, Parnetti L, Magno L, Malinverno M, et al. (2009) Pattern of Tau forms in CSF is altered in progressive supranuclear palsy. Neurobiol Aging 30: 34-40.
- Borroni B, Malinverno M, Gardoni F, Alberici A, Parnetti L, et al. (2008) Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology 71: 1796-1803.
- Meredith JE JR, Sankaranarayanan S, Guss V, Lanzetti AJ, Berisha F, et al. (2013) Characterization of novel CSF Tau and ptau biomarkers for Alzheimer's disease. PLoS One 8: e76523.
- Luk C, Compta Y, Magdalinou N, Martà MJ, Hondhamuni G, et al. (2012) Development and assessment of sensitive immuno-PCR assays for the quantification of cerebrospinal fluid three- and four-repeat tau isoforms in tauopathies. J Neurochem 123: 396-405.
- Aiba I, Hashizume Y, Yoshida M, Okuda S, Murakami N, et al. (1997) Relationship between brainstem MRI and pathological findings in progressive supranuclear palsy--study in autopsy cases. J Neurol Sci 152: 210-217.
- Warmuth-Metz M, Naumann M, Csoti I, Solymosi L (2001) Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Arch Neurol 58: 1076-1079.
- Kato N, Arai K, Hattori T (2003) Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 210: 57-60.
- Oba H, Yagishita A, Terada H, Barkovich AJ, Kutomi K, et al. (2005) New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 64: 2050-2055.
- Adachi M, Kawanami T, Ohshima H, Sugai Y, Hosoya T (2004) Morning glory sign: a particular MR finding in progressive supranuclear palsy. Magn Reson Med Sci 3: 125-132.
- Massey LA, Micallef C, Paviour DC, O'Sullivan SS, Ling H, et al. (2012) Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord 27: 1754-1762.
- Borroni B, Del Bo R, Goldwurm S, Archetti S, Bonvicini C, et al. (2010) VEGF haplotypes are associated with increased risk to progressive supranuclear palsy and corticobasal syndrome. J Alzheimers Dis 21: 87-94.
- Gröschel K, Kastrup A, Litvan I, Schulz JB (2006) Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology 66: 949-950.
- Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, et al. (2008) MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 246: 214-221.
- Morelli M, Arabia G, Novellino F, Salsone M, Giofrè L, et al. (2011) MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study. Neurology 77: 1042-1047.
- Knake S, Belke M, Menzler K, Pilatus U, Eggert KM, et al. (2010) In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: a DTI study using TBSS. Mov Disord 25: 1232-1238.
- Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, et al. (2006) Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 77: 457-463.
- Sajjadi SA, Acosta-Cabronero J, Patterson K, Diaz-de-Grenu LZ, Williams GB, et al. (2013) Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 136: 2253-2261.
- Saini J, Bagepally BS, Sandhya M, Pasha SA, Yadav R, et al. (2012) In vivo evaluation of white matter pathology in patients of progressive supranuclear palsy using TBSS. Neuroradiology 54: 771-780.
- Agosta F, Galantucci S, Svetel M, Lukić MJ, Copetti M, et al. (2014) Clinical, cognitive, and behavioural correlates of white matter damage in progressive supranuclear palsy. J Neurol 261: 913-924.
- Brooks DJ (2010) Imaging approaches to Parkinson disease. J Nucl Med 51: 596-609.
- Südmeyer M, Antke C, Zizek T, Beu M, Nikolaus S, et al. (2011) Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med 52: 733-740.
- Schwarz J, Tatsch K, Gasser T, Arnold G, Pogarell O, et al. (1998) 123I-IBZM binding compared with long-term clinical follow up in patients with de novo parkinsonism. Mov Disord 13: 16-19.
- Eidelberg D, Takikawa S, Moeller JR, Dhawan V, Redington K, et al. (1993) Striatal hypometabolism distinguishes striatonigral degeneration from Parkinson's disease. Ann Neurol 33: 518-527.
- Eckert T, Tang C, Ma Y, Brown N, Lin T, et al. (2008) Abnormal metabolic networks in atypical parkinsonism. Mov Disord 23: 727-733.
- Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, et al. (2014) FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol 261: 710-716.
- Srulijes K, Reimold M, Liscic RM, Bauer S, Dietzel E, et al. (2012) Fluorodeoxyglucose positron emission tomography in Richardson’s syndrome and progressive supranuclear palsy-parkinsonism. Mov Disord 27: 151-155.
- Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, et al. (2013) Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 73: 603-616.
- Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, et al. (2011) Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord 17: 599-605.
- Salvatore C, Cerasa A2, Castiglioni I3, Gallivanone F4, Augimeri A5, et al. (2014) Machine learning on brain MRI data for differential diagnosis of Parkinson's disease and Progressive Supranuclear Palsy. J Neurosci Methods 222: 230-237.
- Cherubini A, Morelli M, Nisticó R, Salsone M, Arabia G, et al. (2014) Magnetic resonance support vector machine discriminates between Parkinson disease and progressive supranuclear palsy. Mov Disord 29: 266-269.
- Osaki Y, Ben-Shlomo Y, Lees AJ, Daniel SE, Colosimo C, et al. (2004) Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord 19: 181-189.
- Borroni B, Malinverno M, Gardoni F, Grassi M, Parnetti L, et al. (2010) A combination of CSF tau ratio and midsaggital midbrain-to-pons atrophy for the early diagnosis of progressive supranuclear palsy. J Alzheimers Dis 22: 195-203.
- Ogaki K, Li Y, Takanashi M, Ishikawa K, Kobayashi T, et al. (2013) Analyses of the MAPT, PGRN, and C9orf72 mutations in Japanese patients with FTLD, PSP, and CBS. Parkinsonism Relat Disord 19: 15-20.
- Pastor P, Muñoz E, Ezquerra M, Obach V, Martà MJ, et al. (2001) Analysis of the coding and the 5' flanking regions of the alpha-synuclein gene in patients with Parkinson's disease. Mov Disord 16: 1115-1119.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 16555
- [From(publication date):
December-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11856
- PDF downloads : 4699