Review Article Open Access
Diabetes Effects in Alzheimer Disease: The Interactive Role of Insulin and Aβ Peptide
Maria Elisa de Oliveira Lanna1,2*, Maria Lucia Vellutini Pimentel2 and Sergio Augusto Pereira Novis2
1Department of Cognitive Disorders - 24a e 25a Infirmaries– Neurology Service - Professor Sergio Augusto Pereira Novis - Hospital Geral Santa Casa da Misericórdia do Rio de Janeiro (HSCMRJ), RJ, Brazil
2Neurology Service - 24a e 25a Infirmaries– HSCMRJ, RJ, Brazil
- Corresponding Author:
- Maria Elisa de Oliveira Lanna
- Enfermarias 24 e 25 Serviço de Neurologia
do Professor Sergio Augusto Pereira
Novis – HSCMRJ. Rua Santa Luzia
206 – Centro/Castelo. Cep: 20020-022
Rio de Janeiro, RJ, Brazil
Tel: 5 (21) 2540-0659
Fax: 55 (21) 2524-4424
E-mail: deo_lanna@terra.com.br
Received date: February 25, 2014; Accepted date: April 20, 2014; Published date: May 20, 2014
Citation: de Oliveira Lanna ME, Pimentel MLV, Novis SAP (2014) Diabetes Effects in Alzheimer Disease: The Interactive Role of Insulin and Aβ Peptide. J Alzheimers Dis Parkinsonism 4:151. doi: 10.4172/2161-0460.1000151
Copyright: © 2014 de Oliveira Lanna ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Insulin resistance, hyper-insulinemia and products associated to insulin metabolism can affect the amyloid cascade and promote the onset of Alzheimer`s disease or aggravate the condition, in early or old age regardless of the development of type 2 diabetes. The changes described in pathological studies and molecular research, classify two types of mechanism involved with cognitive impairment in these cases: one related to cerebrovascular events due the action of vascular risk factors, and the other more controversial, non-cerebrovascular mechanism involving the interaction of insulin with Aβ in the entorhinal cortex and hippocampus, as well as its synaptogenesis action that involves signaling of intracellular molecular paths in the modulating of neurotransmitters such as acetylcholine, norepinephrine and glutamate receptors. Based on a literature review, the role of insulin in the Central Nervous System is examined along with its participation in the amyloidogenesis process in progression to Alzheimer Disease. This review also addresses the consequence of chronic peripheral hyperinsulinemia, leading to down-regulation of insulin receptors in the blood-brain barrier and decreased insulin up-take, causing a state of central hypoinsulinism. This state interferes mainly in the process of Aβ degradation, emphasizing the role of the catalytic enzymes in Aβ clearance, particularly of the insulinase. Among others, increasing synaptic toxicity by disrupting PI3K/Akt inhibition of the GSK3 intracellular molecular pathway increasing tau phosphorylation, as well as PKC synaptogenesis signaling, causing clinical and anatomic changes that favor Alzheimer Disease.
Keywords
Insulin resistance; Diabetes; Aβ clearance; Alzheimer disease
Introduction
Type II diabetes mellitus (T2DM) affects an estimated 250 million people in the world and epidemic projections predict a rise to 300 million cases by 2030 [1]. The relationship between diabetes and lateonset Alzheimer Disease (AD) has been extensively discussed and, a growing incidence of this association is reported in clinical practice. Studies have evolved to recognize this condition. The main mechanism of convergence between the two diseases is insulin resistance, a stage that precedes the onset of T2DM [2].
Insulin resistance is defined as the resistance of tissues to the insulin action. In the periphery it is attributed to resistance to the uptake of glucose used by the muscles, liver, kidney and other organs, while in the brain it impairs insulin signaling. Insulin resistance involves the insulin receptors and is influenced by genetic predisposition as well as environmental factors such as feeding and obesity [3,4].
Insulin resistance with hyperinsulinemia can precede the onset T2DM by several years. This pre-diabetic stage is responsible for vascular changes that may or may not be associated with other vascular risk factors (hypertension, dyslipidemia and obesity), which together comprise the metabolic syndrome [5]. It may also lead to the more discussed non-vascular changes of the hippocampus and other regions related to the cognitive circuit, similar to AD, a combination explored in numerous studies [6].
Among the changes observed, focus has centered on the role of hyperinsulinemia in promoting deposition of the peptide amyloid β, contributing to the accumulation of amyloid plaques in the brain, an important hallmark of AD [7,8].
The process of amyloid plaque deposition entails the stages of formation, degradation and transport of the peptide out off the brain (clearance). Specific enzymes are involved in each step. In the mechanism of forming the peptide that involves the precursor protein cleavage is emphasized the role of β secretase (BACE1), a disintegrin enzyme responsible for the cleavage that forms the amyloid peptides in familial early onset AD, that is related with the genes of the amyloid precursor protein (APP), presenilin 1 and presenilin2 [8,9].
In sporadic forms, insufficient degradation of the amyloid protein [5] with the accumulation and deposition of the peptide, and the more known genetic linkage with the APOE allele 4, appear to predominate. The enzymes physiologically involved in the process include neprilysin, endothelin-converting enzyme, the uPA/tPA/ plasminogen system, angiotensin-converting enzyme and insulin degrading enzyme (IDE or insulinase) [10]. The activity of the latter playsa central role in the process associated with hyperinsulinemia, with or without T2DM, and in the absence of the APOE allele 4 [2,11].
Type II diabetes and AD are characterized by insoluble protein aggregates: amylin in pancreatic cells (T2DM) versus Aβ and tau tangles (AD). Amylin is a pancreatic protein analogous to amyloid peptide dependent on insulinase for degradation. Deposition of amylin is observed in the pancreas of diabetic patients, similar to that which occurs in the brain with the amyloid peptide in cases of T2DM with AD [12].
Various substrates compete for the insulin degrading enzyme, including the β amyloid protein, but the most competitive substrate is insulin. Experimental mechanisms described in both animals and humans suggest that in the presence of hyperinsulinemia or of genetic mutation in the locus of IDE expression, the relationship between the substrates becomes favorable to the deposition of amyloid peptide, in view of the decreased degradation of the substance [13].
The competitive mechanism of substrates may be observed in the early stages of hyperinsulinemia [13]. However, controversies exist over the hippocampus alterations observed with prolonged and chronic hyperinsulinemia [14] that reduce insulin uptake in the brain by saturable transport across the blood brain barrier (BBB), decreasing insulin levels in the central nervous system (CNS) with the development of brain insulin resistance and AD [11].
This review aims using synoptic approach to explore the manner in which peripheral hyperinsulinism with brain insulin resistance affect this cascade, given that some mechanisms explaining this relationship are known while a clear understanding on others remain elusive. It encourages the continuity of studies in this area in the future with new therapeutic strategies.
Mechanism of Insulin Action and Brain Insulin Resistance
The main role of insulin in the CNS is not related to glucose uptake into tissue, there is a class of peptide isoform – glucose transporter isoforms (GLUTs 1-8) to play this role, which are modulated by the action of insulin in some important regions of cognitive circuits [2,15].
The insulin action in the brain, is related to cognitive functions in specific regions such as the hippocampus, and influences the process of forming short-term memory and learning with improved performance. In addition, it plays part in long-term memory potentiation with the modulation of the expression of neurotransmitter receptors involved in the task of synapses plasticity such as acetylcholine, glutamate and dopamine [11]. A action is also exerted on hypothalamus-pituitary axis, regulating the mechanism of feeding and body energy balance [3,4].
These actions in the CNS are carried out through specific insulin receptors that are structurally and functionally different from the IR present in peripheral tissue, and are distributed in large numbers in the entorhinal cortex, hippocampus, frontal cortex and hypothalamus regions, as well as in the BBB where insulin is actively transported into the brain. There is little local production of insulin within the brain itself, most of which is transported from the periphery across the BBB where it reaches the cerebral spinal fluid (CSF) and binds to its specific receptors in the CNS [16].
brief summary now ensues outlining some of the main events related to the mechanism of insulin action reviewed by Kahn and Suzuki [16]. The insulin receptors (IR) belong to the tyrosine kinase family, a classical allosteric enzyme. This has a mechanism of action that starts by inducing conformational changes in the receptor by binding to it, a critical step for activating insulin signaling and triggering phosphorylation of the receptor in 12 substrates of intracellular protein. The main four first identified in the brain (IRS1 – IRS4) [2,16], and in which insulin action activates the paths of the intracellular molecular cascade responsible for performing the insulin function in neurons and astrocyte cells.
After phosphorylation [2,16], these substrates function by intermediating signal transduction through interaction with other intracellular molecules. The main molecules with the clearest mechanisms include: Phosphatidylinositol-3-kinase (PI3K/Akt), Protein Kinase C (PKC), Glycogen Synthetase Kinase 3(GSK3) [17].
Regulatory actions include the activation and inhibition of signaling pathways. The activated PI3K path is the most important link of signaling for insulin metabolic effects and via Akt activity is an important signaling pathway for IDE effects while also inhibits the GSK3 path which has deleterious cellular action. Akt, a Protein Kinase B – PKB, acts as a multifaceted intermediary between signals arising from IR activation and signals to be transmitted downstream for biological effects. PKC constitutes another path of neuronal activation and via IR and IGF-R activity produce the signaling for synaptogenesis and neuronal survival effects [2,9,16].
The GSK3 pathway, when activated by insulin resistance facilitates the binding of Aβ oligomers linked to tau toxicity action. There are many mechanisms related to insulin resistance signaling that may originate at the BBB, in T2DM model [2,5,16].
T2DM and the role of amyloid β
In type 2 diabetes, peripheral hyperinsulinemia is a consequence of the insulin resistance that follows the glucose intolerance stage until the onset of diabetes. In the brain, insulin resistance with impairment of insulin signaling is a consequence of peripheral hyperinsulinemia, and can precede by many years the development of the diabetes with pancreas born out [12].
In an early stage of the process, central hyperinsulinism appears to occur concomitantly with peripheral hyperinsulinemia, because the insulin crosses the BBB at a proportional concentration. With onset of chronic hyperinsulinemia, saturation of the carrier receptors of insulin at the BBB takes place, with down-regulation of the receptors (internalization with degradation of the receptor) and decreased insulin uptake into the brain, producing a stage with central hypoinsulinism. One of the processes of insulin resistance in the CNS that begins at the BBB, is a mechanism extensively explored in the studies of Suzanne Craft [11,18,19].
According to descriptions by Kahn and Suzuki [16], mutations in the insulin receptor (IR) gene have been reported in a substantial number of patients with severe insulin resistance, and there are many mechanisms involved in the acquired alterations of IR signaling at different stages of the disease. The most common down-regulation occurs in variable degrees with the hyperinsulinemia.
The brain depends on adequate levels of insulin to keep its signaling healthy. The consequent reduction of insulin levels in the CNS promotes a decrease in components dependent on insulin such as insulin growth factor (IGF), IDE and other IR in the brain tissue [2]. This contributes by aggravating the process of resistance to insulin signaling, at several stages of the intracellular molecular pathways, leading to the histopathological changes found in AD. These changes include deposition of amyloid plaques of insoluble oligomers (Aβ42) in the extracellular space, intracellular tau phosphorylation with neurofibrillary tangles and amyloidal angiopathy, resulting in synapse dysfunction and neuronal death [2,8,9,20].
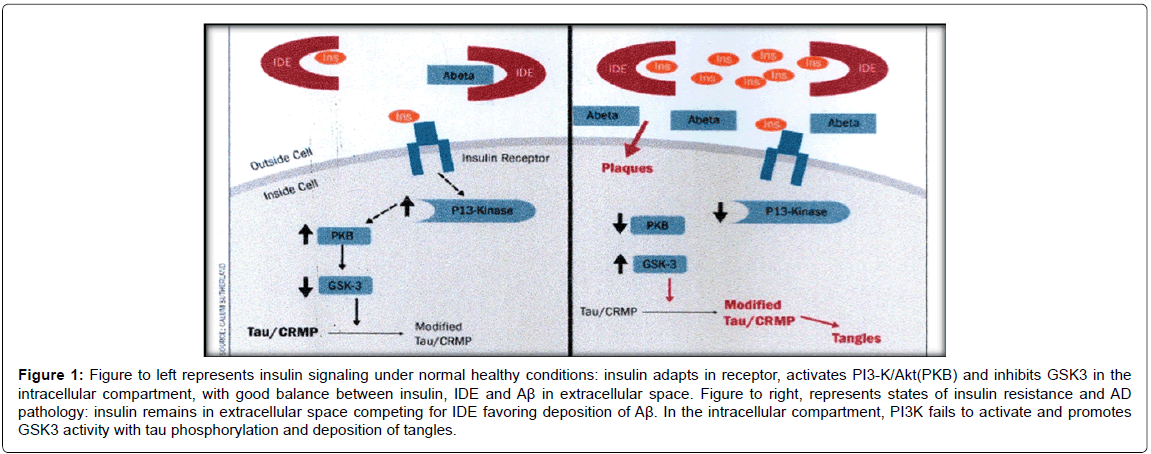
Low levels of insulin and IR are unable to activate and phosphorylate the receptor to start activation of the intracellular molecular events, necessary to allows insulin to fulfil its metabolic functions. Under these conditions, PI3K is not activated, leading to the cytotoxic action of the GSK3 path (Figure 1) [2,5,10,17].
Figure 1: Figure to left represents insulin signaling under normal healthy conditions: insulin adapts in receptor, activates PI3-K/Akt(PKB) and inhibits GSK3 in the intracellular compartment, with good balance between insulin, IDE and Aß in extracellular space. Figure to right, represents states of insulin resistance and AD pathology: insulin remains in extracellular space competing for IDE favoring deposition of Aß. In the intracellular compartment, PI3K fails to activate and promotes GSK3 activity with tau phosphorylation and deposition of tangles.
In the presence of the hypoinsulinismstate, insulin cannot adapt to IR and remains in the extracellular space, competing for IDE substrate and decreasing Aβ degradation (Figure 1). Insulin modulates Aβ clearance to regulate IDE expression and activate Akt required for upregulation. Low levels of insulin decrease IDE expression, aggravating the situation cyclically.IDE (110 kd zinc metalloprotease that catabolize insulin) deficit expressions are sufficient to cause both T2DM and amyloidogenesis [5,10].
Several studies have explored the role of IDE dysfunction in the process of amyloidogenesis in the states of chronic hyperinsulinemia and T2DM with progression to late-onset AD. Compared to the well established genetic influence of APOE in late-onset AD, which contributes to insufficient degradation and deposition of amyloid plaques, the pathologic hallmark of AD [2,7,11,13,14].
However, the crucial issue surrounding IDE performance can be identified in the absence of the APOE genetic factor. Suzane and Craft [11,18] reported that 50% of late-onset AD cases are unrelated to APOE and have no identified genetic inheritance. This 50% encompasses cases of T2DM, pre-T2DM stages and metabolic syndrome (obesity, hypertension and dyslipidemia) that is associated with changes resulting from the mechanisms of insulin resistance strongly predictive of AD. These studies emphasize the relationship of insufficient action of IDE with Aβ accumulation, and the strong contribution of inflammation in the process with the participation of tumoral necrosis factor-α (TNFα) and interleukins from the microglia.
The several cross-sectional and longitudinal studies that investigated the influence of hyperinsulinemia, metabolic syndrome elements and T2DM to evaluate the contribution of these risk factors in late-onset AD, found disparate results among histopathological analyses [5]. Most of the studies, described AD pathological changes in association with the APOE allele 4.
Other studies however, described pathological alterations associated with hyperinsulinemia as the longitudinal study in elderly black, Caribbean Hispanic, and Non-Hispanic whites in New York City–Luchsinger et al. [5]. One study conducted in Israel found a progressive relationship between T2DM in midlife and risk of dementia in elderly subjects [5].
By contrast, some studies failed to find a relationship of elevated Aβ peptide load deposited in pathological material of individuals with T2DM and AD, findings emphasized in the study of Alafuzoff et al. [14]. These divergent pathological results point to the need for further studies on the role of insulin and its influence in cognition and cases of sporadic AD.
One of the limitations of the study methods concerns the facts that the findings in animal models cannot be replicated in human beings [21,22]. Type 3 diabetes mellitus (T3DM), a recent well established reverse condition, associates peripheral insulin resistance with T2DM during the course of sporadic AD. This model relates the diabetogenic effect of AD [23,24].
Although the metabolic disorder can be primary or secondary in origin, in fact, not all T2DM cases progress to late-onset AD, while not all cases of late-onset AD evolve with T3DM [25,26]. Moreover, the studies are pre-dominants in an age group in which metabolic disorder and AD can occur in both directions. This issue could be clarified by conducting further studies involving young adults with hyperinsulinemia/T2DM [27].
However, among the numerous factors that can influence the amyloid cascade and contribute to the precipitation of dementia, the therapeutic strategy aiming at potentiating insulinase action in the degradation and clearance of Aβ peptide, in an attempt to attenuate the deleterious effects of Aβ deposition, is gaining attention [2,13,28].
In addition to the reduction of activity in hypoinsulinism states by the mechanism of counter-regulation with insulin levels, there are also cases with genetic mutation in the locus of expression of this enzyme, a factor that can cause 15% to 30% reduction in this enzyme`s activity, apparently a sufficient drop to cause T2DM and impair Aβ degradation [10], even with the co-participation of other catalytic enzymes also involved in Aβ degradation, as mentioned in the introduction.
This genetic resistance is encoded in chromosome 10, found in T2DM and late-onset AD. It alters IDE expression in the brain and in the periphery. In the reviews of Kahn CR and Suzuki [16], a schematic action of IDE was described and demonstrated in an IR internalization model.IDE with normal action degrades the ligand insulin at the endosome, and the free receptor is transferred to the membrane and recycled. In the mutations, IDE does not recycle, impairing the number of insulin receptors and consequently the levels of insulin and the counter-regulation mechanism with IDE, aggravating IDE insufficiency and insulin resistance as a deleterious cycle. This mechanism can occur in selective regions of the brain and in the peripheral organs with insulin action.
Hyperinsulinemia also reduces Aβ clearance in the periphery, IDE-mediated, precipitating the process, which is aggravated by the imbalance of the drainage brain-periphery, and periphery-brain [5,13]. The mechanisms linking insulin in the periphery with brain Aβ clearance are multiple and complex, and warrant future investigation.
Another interaction of insulin and Aβ is related to modulation of synaptotoxicity in AD. Soluble oligomers of Aβ are synaptotoxic. Oligomers have an affinity to the insulin receptor (and other receptors) and disrupt PI3K and PKC signaling with activation of GSK3 path, disrupting the LTP synaptogenesis signaling process. Insulin prevents binding of Aβ to synapses, preserving synaptic integrity [10,20].
Evidence shows that besides amyloid peptide deposition induced by insulin resistance in CNS, activation of the GSK3path linked to tau also occurs. This action promotes phosphorylation of this axonallylocated protein and consequent intracellular toxicity, culminating in the formation of tangles, another pathological hallmark of AD [8,20,29]. Simon Lovestone [20] describes with details the mechanisms underlying genotypes, phenotypes and insulin signaling in tau phosphorylation, dependent on the GSK3 pathway in AD and other related tauopathies. The author suggest that maintaining GKS3 signaling inhibited, even in the presence of stimulation from oligomers, induces no tau phosphorylation, representing one of the strategies for attenuating the deleterious effects of soluble oligomers and of insulin resistance state.
In addition to the effects in Aβ regulation and neuromodulatory action of neurotransmitters, there are long descriptions in the literature exploring the inflammatory effects of insulin resistance in AD pathology. These mechanisms of oxidative stress involving numerous inflammatory products, aggravate the process and impact Aβ clearance [18,19,30].
Conclusion
Insulin action may not be as important in AD as it is in T2DM, but, is emerging as a promising strategy for improving cognitive decline in AD.
The actions of insulin resistance in T2DM with AD progression include: reduced IDE-mediated Aβ degradation and Aβ clearance with lower expression of IDE due to decreased levels of insulin in the brain and anti-synaptogenesis effects via GSK3.
The recent studies focusing on multiple choice of therapeutic agents able to mediate the molecular and inflammatory signaling pathways of interaction of the insulin with the amyloid cascade: strategies of IDE to improve Aβ degradation (clearance), of synaptogenesis to potentiate PKC signaling (LTP) and inhibition of GSK3 signaling to prevent synaptic toxicity and tau phosphorylation among others [23-27].
References
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053.
- Bosco D, Fava A, Plastino M, Montalcini T, Pujia A (2011) Possible implications of insulin resistance and glucose metabolism in Alzheimer's disease pathogenesis. J Cell Mol Med 15: 1807-1821.
- Lebovitz HE (2001) Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 109 Suppl 2: S135-148.
- Cheung WW, Mao P (2012) Recent advances in obesity: genetics and beyond. ISRN Endocrinol 2012: 536905.
- Luchsinger JA (2010) The relationship between the continuum of elevated adiposity, hyperinsulinemia, and type 2 diabetes and late-onset alzheimer`s disease: an epidemiological perspective. In: CRAFT, S. (Ed.).Diabetes, Insulin and Alzheimer`s Disease. Berlin: Springer-VerlagBerlin Heidelberg 89-107.
- Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, et al. (2004) Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53: 474-481.
- Biessels GJ, Kappelle LJ; Utrecht Diabetic Encephalopathy Study Group (2005) Increased risk of Alzheimer's disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem Soc Trans 33: 1041-1044.
- Mao P, Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: implications for early intervention and therapeutics. Biochim Biophys Acta 1812: 1359-1370.
- Cole SL, Vassar R (2008) The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J Biol Chem 283: 29621-29625.
- Sun MK, Nelson TJ, Alkon DL (2010) PKC and insulin pathways in memory storage: targets for synaptogenesis, anti-apoptosis, and the treatment of AD. In: CRAFT, S. (Ed.). Diabetes, Insulin and Alzheimer`s Disease. Berlin: Springer-Verlag Berlin Heidelberg 153-182.
- Craft S (2007) Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res 4: 147-152.
- Götz J, Lim YA, Eckert A (2013) Lessons from two prevalent amyloidoses-what amylin and Aβ have in common. Front Aging Neurosci 5: 38.
- Qiu WQ, Folstein MF (2006) Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging 27: 190-198.
- Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H (2009) Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol 35: 60-68.
- S Roriz-Filho J, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, et al. (2009) (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta 1792: 432-443.
- Kahn CR, Suzuki R (2010) Insulin Action in the brain and the pathogenesis of Alzheimer`s disease. In: CRAFT, S. (Ed.). Diabetes, Insulin and Alzheimer`s Disease. Berlin: Springer-VerlagBerlin Heidelberg 1-20.
- Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol 490: 115-125.
- Craft S (2006) Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord 20: 298-301.
- Craft S (2010) The role of insulin dysregulation in aging and Alzheimer`s Disease. In: CRAFT, S. (Ed.). Diabetes, Insulin and Alzheimer`s Disease.Berlin: Springer-VerlagBerlin Heidelberg 109-127.
- Lovestone S, Killick R (2010) Is Alzheimer`s a disorder of ageing and why don`t mice get it? The centrality of insulin signaling to Alzheimer`s disease pathology. In: CRAFT, S. (Ed.). Diabetes, Insulin and Alzheimer`s Disease.Berlin: Springer-VerlagBerlin Heidelberg 129-152.
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, et al. (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A 107: 7036-7041.
- Park SA (2011) A common pathogenic mechanism linking type-2 diabetes and Alzheimer's disease: evidence from animal models. J Clin Neurol 7: 10-18.
- Tang J, Pei Y, Zhou G (2013) When aging-onset diabetes is coming across with Alzheimer disease: comparable pathogenesis and therapy. Exp Gerontol 48: 744-750.
- de la Monte SM, Wands JR (2008) Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol 2: 1101-1113.
- de la Monte SM, Tong M2 (2014) Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol 88: 548-559.
- Formiga F, Reñe R, Pérez-Maraver M (2014) Dementia and diabetes: Casual or causal relationship? Med Clin 142: [Epub ahead of print].
- Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, et al. (2011) Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br J Clin Pharmacol 71: 365-376.
- Ralat LA, Kalas V, Zheng Z, Goldman RD, Sosnick TR, et al. (2011) Ubiquitin is a novel substrate for human insulin-degrading enzyme. J Mol Biol 406: 454-466.
- Diniz BS, Pinto Júnior JA, Forlenza OV (2008) Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer's disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 9: 172-182.
- Sato M, Murakami K, Uno M, Nakagawa Y, Katayama S, et al. (2013) Site-specific inhibitory mechanism for amyloid β 42 aggregation by catechol-type flavonoids targeting the Lys residues. J Biol Chem 288: 23212-23224.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14846
- [From(publication date):
August-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10252
- PDF downloads : 4594