Development of Solid Acid Catalyst from Biomass Obtained from Cake of Vitellaria paradoxa and Its Application in Biodiesel Production Via a TwoStep Reaction System
Received: 19-Nov-2018 / Accepted Date: 01-Jan-2019 / Published Date: 05-Jan-2019 DOI: 10.4172/2469-9764.1000129
Abstract
An integrated approach is needed to make biodiesel production cost-effective and a suitable choice of fuel. This study was therefore designed to utilize the seed cake of Vitellaria paradoxa obtained during oil extraction stage for the synthesis of a solid acid catalyst capable of hydrolysis and/or esterification reaction. The seed cake was pyrolyzed in an inert tube furnace at 420°C for 5 h, and then sulfonated in fuming sulphuric acid (15 wt.%) at 150°C for 10 h to obtain a biomass-derived catalyst. The catalyst was characterized by elemental analysis, FT-IR spectroscopy, X-ray diffraction (XRD) spectroscopy and total acid density. The hydrolytic activity of the catalyst was performed using vegetable oil obtained from V. paradoxa biomass and the reaction was optimized using a randomized central composite rotatable design (CCRD). The optimization results showed that catalyst derived from seed cake of V. paradoxa was active in hydrolysis reaction and a conversion of 34.3% was obtained and it was also observed that reaction time and weight of catalyst have significant effect on the reaction. When the catalyst was further employed for esterification of free fatty acids in V. paradoxa oil, higher conversion (70%) was achieved at a prescribed reaction condition. The approach of utilizing the waste generated during oil extraction from plants could make the process much greener when viewed from the context of resource efficiency and has the potential to kick start the commercialization of biodiesel production especially in developing countries.
Keywords: Vitellaria paradoxa; Solid acid catalyst; Hydrolysis; Esterification; Biodiesel; Optimization
Introduction
Biodiesel, mainly derived from oil-bearing plants, has the potential to replace petro-diesel in parts or whole in the energy sectors of both developed and developing countries. In the automobile industries, biodiesel can be used with little or no modification of engines with improved combustion because of its oxygen content and can improve engine performance due to its higher lubricity [1]. In addition, biodiesel can be used in engines in its pure form (B100) or may be blended with petroleum diesel in different ratios [2].
There are many potential feedstocks available for use in biodiesel, which include vegetable oils as well as animal fats. Common raw materials for biodiesel production include soybean oil, sunflower, corn and olive oils, rapeseed oil, castor and lesqurella oils, milkweed seed oil, Jatropha curcas oil, Calophyllum inophyllum oil [3-9]. Algae and waste vegetable oils are also being considered as suitable feedstock for biodiesel production [10,11].
Transesterification reactions are commonly employed to convert vegetable oils to biodiesel in the presence of either alkali or acid catalysts. Qiu et al. [12] utilized mixed soybean and rapeseed oil with sodium hydroxide as catalyst and 94% yield of biodiesel was reported at the optimized conditions. Dawodu et al. [13] also used Sesamum indicum L. as feedstock to produce biodiesel in the presence of sodium methoxide (NaOCH3) catalyst and a yield of 87.8% was reported at the optimum reaction condition. Alkali-catalyzed reactions are only possible with feedstock having low free fatty acids, thus edible vegetable oils are preferred for biodiesel production since alkalicatalyzed reaction is much faster than acid-catalyzed reaction [14]. However, acid-catalyzed reaction requires high reaction temperature, retention time, molar ratio of vegetable oil to methanol/ethanol and catalyst weight to achieve high conversion of vegetable oils to biodiesel [9,15,16].
Therefore, new methods are required to convert feedstock with high free fatty acids to biodiesel and the methods have to be energy efficient in terms of recovery, recycling and re-use. A two-step solid acid/akalicatalyzed reaction is now preferred due to easy catalyst separation and purity of the product [15]. Cavalcanti-Oliveira et al. [17] proposed a hydro esterification route to convert triglycerides in soybean oil to free fatty acids (FFAs) via hydrolysis reaction, which was followed by esterification of FFAs with alcohol to biodiesel in the presence of niobic acid in pellets.
Vitellaria paradoxa , commonly known as shea tree is a tree of the Sapotaceae family. It is the only species in genus Vitellaria and is indigenous to Afirca. V. paradoxa fruit consists of a thin, tart, nutritious pulp that surrounds a relatively large, oil-rich seed from which shea butter is extracted and the oil is of interest in biodiesel production. Two-stage process involving hydrolysis and esterification reactions is rarely used to produce biodiesel and even when used, edible vegetable oils were the preferred feedstock. Therefore, this study adopts two-step process to catalyze V. paradoxa oil to biodiesel. The catalyst was developed through pyrolysis and sulfonation of V. paradoxa seed cake to obtain a solid acid catalyst. The catalyst was utilized in the hydrolysis of V. paradoxa oil to FFAs and the reaction was optimized using a randomized central composite rotatable design (CCRD). The product from hydrolysis experiment was further converted to FAME (biodiesel) using sulfonated V. paradoxa biochar catalyst.
Experimental
Materials
Dried Shea butter (Vitellaria paradoxa ) kernels were collected from Fufu village in kwara state, the Southern part of Nigeria. The seeds were manually removed from the kernels. The seeds and the shaft were dried to acceptable moisture content, pulverized to increase the surface area and then stored in airtight bags prior to further use.
Oil extraction and characterization
The dried seeds of V. paradoxa were extracted by means of soxhlet apparatus using n-hexane (99.0%). After the extraction, the set up was dismantled and the mixture of oil and n-hexane was distilled to obtain the V. paradoxa oil and waste product (seed cake) while the recovered solvent was used for subsequent extraction. Fatty acids profile of V. paradoxa oil was determined using GC-FID equipped with capillary fused silica column SPTM 2380 (30 m × 0.25 mm × 0.2 mm film thickness). Fatty acids identification was done using standards of palmitic (99.0%), stearic (99.0%), oleic (99.0%), linoleic (99.0%) and arachidic (99.0%) fatty acids. Saponification value, iodine value, acid value, free fatty acid, viscosity, ash, moisture content was determined by standard methods described by the Association of Official Analytical Chemist [18].
Catalyst synthesis
The pulverized V. paradoxa seed cake was sieved to 0.6 m mesh size and oven dried at 105°C for 10 h. Thereafter, the sample was carbonized at 420°C in a tube furnace under nitrogen for 5 h. The process is a form of destructive distillation in which a carbonaceous residue of highly porous structure is obtained.
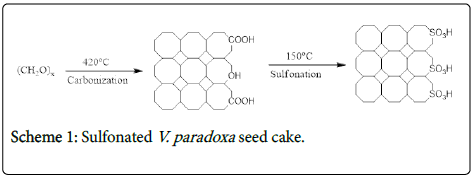
The obtained carbon residue (10 g) was immersed in 100 ml of fuming sulphuric acid for 10 h at 150°C. The mass ratio of acid to shaft was 18.4:1; the resultant sample was washed with distilled water until a neutral pH was achieved (litmus paper test). The sulfonated V. paradoxa seed cake was then dried at 110°C for 24 h. The scheme 1 is shown below.
Catalyst characterization
The carbon, hydrogen, nitrogen, sulfur and oxygen contents of the sulfonated V. paradoxa catalyst were determined by Elemental Analyzer (Vario cube). The Fourier transform infrared (FT-IR) spectroscopy was obtained using Perkin Elmer FTIR instrument. Spectra were taken in the range of 350 to 4000 cm using KBr pellet method (solid sample) or Nujol method (liquid sample). X-ray diffractometer (XRD) pattern was obtained using Rigaku D/Max-IIIC X-ray diffractometer operated at 40 kV and 20 mA using Cu Kα radiation source. The sample was scanned in the range of 2θ of 5-80°C at a scanning speed of 2°/min. The surface morphology of the catalyst was done using JOEL JSM-6400 microscope. The total acid density of the catalyst was determined using the standard acid-base back titration method. The catalyst samples were pre-dried in the oven at 110°C for 2 h, and then ~ 0.10 g of catalyst was added into 60 ml of 0.0080 mol/L NaOH and stirred for 30 minutes before back-titration with 0.02 mol/L HCl. For the sulfonic group density determination, it is assumed that all the sulfur content of the catalyst is in the form of -SO3H and thus sulfonic group density was calculated based on the weight percentage of S content obtained from elemental analysis.
Catalyst activity: Hydrolysis and esterification of Vitellaria paradoxa oil
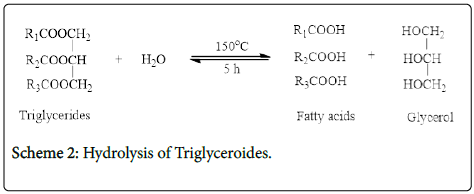
Hydrolysis reaction was carried out in a quick fit round bottom flask of 250 ml equipped with a hot plate and magnetic stirrer. 5 g of V. paradoxa oil, 50 g of distilled water, and catalyst of 0.1 g were charged into the reactor for 5 h at 140°C. At the end of the reaction, the reactor was cooled, and catalyst was separated by filtration. The reaction product consisted of two layers, an upper oily layer of fatty acids, catalyst and unreacted triglycerides and a lower, aqueous layer of water and glycerol which was separated by funnel.
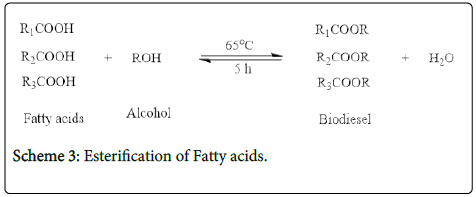
Esterification of fatty acids with alcohols in the presence of sulfonated V. paradoxa catalyst was carried out. 4 g of hydrolyzed oil, 40 g of methanol and 0.2 g of catalyst were charged inside a 250 ml reactor which was then placed in an oil-bath at 65°C, magnetic stirring (600 rpm) for 5 h under a reflux condition. The reaction was terminated by placing the reactor in a cool water bath, excess methanol recovered, and the catalyst was phase separated by decantation. The reaction scheme is shown below.
The percentage conversions for both hydrolysis and esterification reactions were calculated by titrimetry method. Titration was carried out using 0.25 N NaOH solution and phenolphthalein as indicator. The percentage conversion of triglyceride was calculated using Equation 1.
FFA conversion=at−ai × 100…………eqn(1)
Where ai is the initial acid value of the V. paradoxa oil and at is the final acid value at the end of the reaction.
Optimization study
Central Composite Rotatable Design technique (CCRD) was used for optimization of the hydrolysis reaction. The experimental design employed was a full 32 factorial design (2 factors each at 3 levels) with 4 centre points making a total of 12 runs (Table 1). Experiments were randomly carried out in order to minimize errors from systematic trends in the variables. Optimization conditions chosen were: molar ratio of oil/water: 1:10; catalyst concentration 1-3 wt.% based on the weight of oil; reaction temperature of 150°C. The reaction was timed between 4-6 h soon as the catalyst was added. After a specific time, interval, the reaction was quenched in a cold-water bath. The percentage conversion of triglyceride was determined by titration and percentage converted was calculated using Equation 1.
| Levels | |||||
|---|---|---|---|---|---|
| Variables | 0.414 | 0 | 1 | 1.414 | |
| Wt% catalyst (A) | 0.59 | 1 | 2 | 3 | 3.41 |
| Time (B) | 3.59 | 4 | 5 | 6 | 6.41 |
Table 1: Coded levels and real values of tested variables in the CCRD.
The Design Expert 6.0 software was therefore used for regression, analysis of variance (ANOVA) and graphical analysis of the data obtained. A quadratic polynomial was developed to predict the response as a function of independent variables and their interactions and a second-order polynomial equation was used [19,20].
Results and Discussion
Properties of Vitellaria paradoxa oil
Vegetable oils generally used for biodiesel should have low moisture content since alkali catalyzed transesterification reactions are susceptible to water which usually lead to soap formation [21]. The properties of V. paradoxa oil are shown in Table 2. The oil yield is considerably high thus making the oil feedstock economically viable for biodiesel production. Also, low contents of ash and moisture made the oil a suitable feedstock for biodiesel production. In addition, the fatty acids profile of V. paradoxa oil are palmitic (6.5%), stearic (28.7%), oleic (55.5%), linoleic (6.2%) and arachidic (0.7%) making the oil a suitable candidate for transesterification reaction.
| Properties | Values |
|---|---|
| Moisture (%) | 1.2 ± 0.4 |
| Ash (%) | 0.2 |
| Colour | Light yellow |
| Oil yield (%) | 53.7 ± 1.2 |
| State of the oil at room temperature | Solid |
| Saponification value (mg KOH/g) | 160.2 ± 0.9 |
| Relative density (g/cm3) | 0.9 |
| Iodine value (I2/100 g) | 42 |
| Dynamic viscosity (cP) | 2.4 |
| Free fatty acid (%) | 4.7 ± 0.1 |
| Acid value (mg KOH/g) | 9.4 |
Table 2: Proximate and physico-chemical properties of Vitellaria paradoxa oil.
The saponification value of the oil was 160.2 ± 0.9 mg KOH/g and the value is within the same range of some edible oils reported by Eromosele et al. [22]. The oil has an iodine value of 42.0 I2/ 100 g thus classified as a non-drying. The oil has free fatty acid (FFA) of 4.7% while the acid value is 9.4 mg KOH/g, thus rendering the oil feedstock unsuitable for alkali-catalyzed transesterification because of soap formation. An alternative conversion route is then required to convert the oil to biodiesel. The oil has a relative density of 0.9 g/cm3 which showed that it is less dense than water and a kinematic viscosity of 9.2 centipoises which showed that is not as thick as most of drying oils.
Catalyst characterization
X-ray diffraction (XRD) pattern of sulfonated V. paradoxa catalyst (Figure S1) exhibited diffraction peaks around the 2θ=15-30° region which is due to the presence of an amorphous carbon structure with randomly oriented aromatic carbon sheets [23-25]. The XRD pattern obtained was similar to that obtained from seed cake of Calophyllum inophyllum [26].
The functional groups present on V. paradoxa biochar and sulfonated V. paradoxa catalyst contained aromatic and aliphatic hydrocarbons, as well as some functional oxygen-containing groups, including -OH and C=O [27]. The peaks at around 3440 cm are more intense in V. paradoxa biochar than in sulfonated V. paradoxa catalyst (Figure S2). Therefore, V. paradoxa biochar contains larger phenolic OH groups. Both V. paradoxa biochar and sulfonated V. paradoxa catalyst spectra contain peaks attributable to aromatic ring modes, at 1463 and 1467 cm respectively [28]. After sulfonation of the carbonized materials prepared at 400°C, the vibration bands observed at 1079 cm (SO3 stretch) and 1216 cm (S=O asymmetric vibration in SO3H) confirmed that the catalyst possess SO3H groups [29]. The sulfonated V. paradoxa catalyst also exhibited peaks at 1702 cm and 1608 cm which can be attributed to C=O of a carboxylic acid and the stretching vibration of a polycarboxylic aromatic compound containing carboxylic groups [15]. However, the peaks attributed to carboxylic groups at (1700 cm) and polycarboxylic aromatic compounds (1608 cm) were absent in the spectra of V. paradoxa biochar. These peaks could have been overlapped by the peak at 1631 cm. The band at 2300-2700 cm was as a rusult of an overtone due to – OH-O= (bend) and this is linked by a strong hydrogen bond, which suggests that some SO3H groups are within a short distance to each other [9,29].
The morphological analysis of the sulfonated V. paradoxa biochar catalyst by SEM revealed that the material has a loose irregular network structure (Figure S3). The main determinant of the structural properties of a carbon-based material is the nature of the starting material. A char obtained by slow pyrolysis retains the fibrous structure while char produced by rapid pyrolysis has a porous structure. Removal of cell contents and consequently, the opening-up of cellular structures give rise to a porous network of distinctive features, which depends on the heating rate and the raw material [30].
Elemental analysis on V. paradoxa seed cake, V. paradoxa biochar and sulfonated V. paradoxa catalyst are presented in Table 3. It can be seen from Table 3 that carbonization of biomass results in an increase in the H/C ratio of the material. This is due to the removal of oxygen from the biomass in the form of carbon dioxide (and carbon monoxide) during the carbonization process and successful incorporation of SO3H group was also confirmed. In addition, sulfonated V. paradoxa catalyst SO3H density as determined from the EA was 3.35 mmol/g and this was absent from V. paradoxa seed cake and V. paradoxa biochar (Table 4). However, the total acid density for the catalyst was higher than biomass-derived solid acid catalysts obtained in our previous studies [15,26].
| Material | Elemental Analysis Results (%) | Composition | H/C | ||||
|---|---|---|---|---|---|---|---|
| C | H | O | N | S | |||
| V. paradoxa seed cake | 52.1 | 0.72 | 41.68 | 5.5 | - | CH0.17O0.06N0.09 | 0.014 |
| V. paradoxa biochar | 61.8 | 2.56 | 30.14 | 5.5 | - | CH0.50O0.37N0.08 | 0.041 |
| Sulfonated V. paradoxa | 62.08 | 4.12 | 17.58 | 5.5 | 10.72 | CH0.80O0.21S0.07N0.08 | 0.067 |
Table 3: Elemental composition of catalyst materials.
Catalytic activity of sulfonated Vitellaria paradoxa catalyst
Hydrolysis of Vitellaria paradoxa oil: The activity of the catalyst was tested under pre-determined reaction conditions. The yield obtained was compared to those obtained without the catalyst and the V. paradoxa biochar. The results of hydrolysis of V. paradoxa oil with or without catalyst are presented in Table 4. Blank experiment in the absence of catalyst, at the reaction condition (Temperature: 150°C; Oil/ molar: 1:10; Catalyst: 2 wt.% of oil; Stirring speed: 600 rpm; Time: 5 h), showed that the hydrolysis reaction takes place, to a certain extent. This may be due to the presence of free fatty acids in the oil which acts as weak acid catalyst for the reaction [31]. The result also showed that a higher conversion was obtained with V. paradoxa biochar. The catalytic activity of the non-sulfonated carbon material is due to presence of - COOH and phenolic -OH groups which confer acidic properties on the material. Table 4 also shows the total acid density on the carbon material to be 0.28 mmol/g, which is a combination of the -COOH and phenolic -OH group acidic group. The presence of FFAs in the oil acting as weak acid catalyst also complement the activity of the acidic group in the non-sulphonated carbon material in driving the hydrolytic reaction forward. Under the same reaction conditions, sulfonated V. paradoxa catalyst gave 34.32% conversion of triglycerides to FFAs. Despite high acid density of the catalyst, which should have translated to a higher catalytic activity of the catalyst, the obtained conversion was relatively low. Comparing the conversion rate with similar work, Satyrarthi et al. [32] were able to achieve a triglyceride conversion of up to 93.9% employing a solid Fe-Zn double-metal cyanide (DMC) complex catalyst (acid density of 0.84 mmol/g) in a Teflon-lined, stainless steel autoclave at a temperature of 190°C and autogeneous pressure. Since the rate of the forward reaction of an endothermic reaction is favoured by an increase in temperature, the reaction yield is significantly high. The pressure generated within the system is also of greater magnitude compared to that obtained under the reflux condition under which this study was done.
| S | Acid Density (mmol/g) | Conversion (wt.%)c | Acid value (mg KOH/g) |
||
|---|---|---|---|---|---|
| (wt.%) | SO3Ha | Totalb | |||
| No catalyst | - | - | - | 12.84 | 10.67 |
| V. paradoxa biochar | - | - | 0.28 | 21.26 | 11.46 |
| Sulfonated V. paradoxa | 10.72 | 3.35 | 4.45 | 34.32 | 12.7 |
aCalculated based on the sulphur content from elemental analysis. bObtained by titration.
cReaction Conditions: Oil/water: 1:10, Catalyst: 2 wt.% of oil, temperature: 150°C, Stirring speed: 600 rpm; Time: 5hrs.
Table 4: Properties and activities of derived catalyst.
Optimization study
An optimization study of the hydrolysis reaction was carried out in order to study the effect of temperature and amount of catalyst on the reaction yield, while keeping other factors affecting the reaction constant. This optimization was carried out using a Randomized Central Composite Rotatable Design (CCRD). The design matrix and the results obtained are shown in Table 5. The best conversion of FFAs was obtained in Run 1, with a catalyst ratio of 3% and a reaction time of 6 h. From the model, variables with P-values lower than 0.05 were considered statistically significant (Table 6). Based on the statistical analysis of the results, an empirical model was constructed to describe the variation in the free fatty acid concentration as a function of catalyst ratio (A) and reaction time (B) as shown in equation (2). Only terms A and B were significant. A Fisher F-test with a P-value lower the 0.05 indicates a high significance for the model.
%Conversion=33.80+5.65A+4.34B+1.00AB +1.79A2-0.83B2…….eqn(2)
| Standard order | Run Order | %wt of catalyst | Time (Hrs) | % Conversion |
|---|---|---|---|---|
| 4 | 1 | 3 | 6 | 45.4 |
| 3 | 2 | 1 | 6 | 30.9 |
| 5 | 3 | 2 | 5 | 34.5 |
| 1 | 4 | 1 | 4 | 28 |
| 2 | 5 | 3 | 4 | 38.5 |
| 9 | 6 | 2 | 6.41 | 40 |
| 10 | 7 | 2 | 5 | 36.4 |
| 7 | 8 | 3.41 | 5 | 43.6 |
| 8 | 9 | 2 | 3.59 | 22.4 |
| 6 | 10 | 0.59 | 5 | 29.3 |
| 11 | 11 | 2 | 5 | 33.8 |
| 12 | 12 | 2 | 5 | 30.5 |
Table 5: Design matrix and table of result for optimization experiments.
| Source | Sum of Squares | Df | Mean Square | F value | P-value |
|---|---|---|---|---|---|
| Model | 440.1 | 5 | 88.02 | 9.35 | 0.0085 s |
| A | 255.64 | 1 | 255.64 | 27.15 | 0.002 |
| B | 150.43 | 1 | 150.43 | 15.98 | 0.0071 |
| AB | 4 | 1 | 4 | 0.42 | 0.5387 |
| A2 | 20.59 | 1 | 20.59 | 2.19 | 0.1896 |
| B2 | 4.42 | 1 | 4.42 | 0.47 | 0.5187 |
| Residual | 56.49 | 6 | 9.41 | ||
| Lack of Fit | 38.35 | 6 | 12.78 | 2.11 | 0.2772 ss |
| Pure Error | 18.14 | 6 | 6.05 | ||
| Cor Total | 496.59 | 11 |
Table 6: ANOVA for response surface quadratic model.
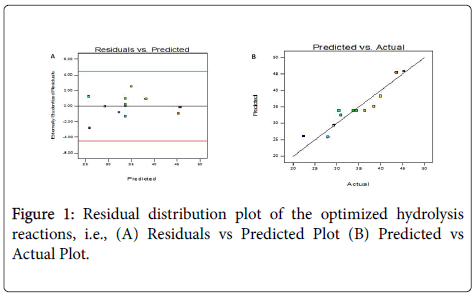
The model predicts that percent yield of 33.8% could be obtained under the optimum operating conditions. To test the fit of the model, residual distribution graph was plotted, and it is observed that the residual distribution does not follow a particular trend with respect to the predicted values (Figure 1A). This test the assumption of constant variance and should be random scatter (constant range of residuals across the graph). This shows the relationships between the experimental response values and predicted response values. This graph helps to detect a value, or group of values, that are not easily predicted by the model. To test the fit of the model, the data points should be split evenly by the 45-degree line. The graph agrees with this assumption and the fit of the model was further confirmed by normal plot of residuals (Figure 1B).
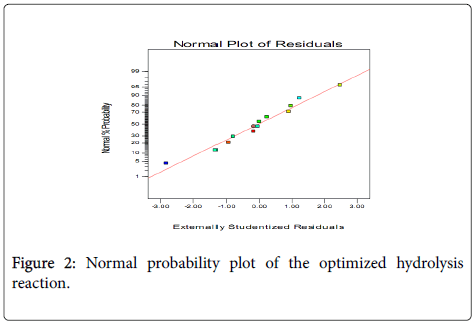
The normal probability plot indicates whether the residuals follow a normal distribution, in which case the points will follow a straight line (Figure 2). This indicates that the errors are normally distributed for all the responses. It can thus be concluded that the proposed empirical model adequately correlates the relationship between the reaction variables and the conversion of triglycerides to FFAs.
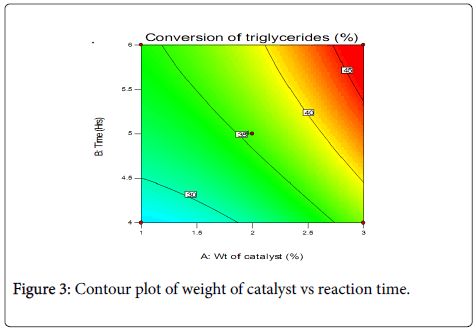
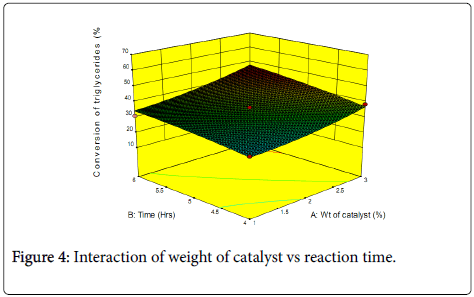
Contour plots and three-dimensional plots for the interaction between the variables and percentage conversion of triglycerides are shown in Figures 3 and 4.
Test from the analysis of variance, showed the predicted model has R-square value of 0.8862. This shows that the sample variation of 88.62% for triglyceride conversion is attributed to the independent variables selected A and B and 11.38% of the total variations are not explained by the model. Therefore, other variables within the process may have influenced the triglyceride conversion.
Statistical analysis shows that catalyst concentration is the most important factor in the response. Catalysts alter the rate of a chemical reaction by providing alternative pathway with lower activation energy for the reaction. It is therefore expected that the reaction yield should increase with increase in the amount of catalyst used. The reaction time also exerts a positive influence on the response, though lower than that of the catalyst. Higher product yield can be directly related to long reaction time which is due to higher effective collision between the reacting molecules. The interaction between weight of catalyst and reaction time is also positive, though it is slightly not significant. The interaction between the variables and the response can be further understood from the three-dimensional surface response plots (Figure 4). To evaluate the reliability of the developed empirical model, a test reaction was carried out setting a target of 2 wt.% catalyst and a reaction time of 4 h. A conversion of 27% was obtained, with the theoretical value at 30%.
Esterification of FFAs to biodiesel
The hydrolyzed V. paradoxa oil was subjected to esterification reaction with methanol in the presence of sulfonated V. paradoxa catalyst. The conversion of FFAs to biodiesel was calculated by comparing the acid value of the oil before and after esterification. A conversion of 70% was obtained at the reaction condition. The resultant biodiesel was confirmed based on the functional groups of the product (Figure S4). The two most polar bonds in esters (containing the -CO-O-C-unit) are the C=O and C-O bonds and these bonds produce the strongest bands in the spectrum of any ester. Table 7 shows the summary of the characteristic IR bands of biodiesel and the biodiesel produced in the present work. Aliphatic esters produce C=O and C-O bands at 1750730 cm and 1300100 cm, respectively, which is an indicator of conversion of FFAs to FAME.
| Wave number (cm) | Present work | Group Assignment | Vibration type |
|---|---|---|---|
| 720 | 722.08 | 0 | Plane rocking |
| 1112070 | 1097.96 | -C-O | Stretching |
| 1300100 | 1117.76 | -C-O | Stretching |
| 1275100 | 1241.88 | -CH | In-plane bending |
| 1475350 | 1377.72 | -CH2, -CH3 | Bending |
| 1472427 | 1465.21 | 0 | Asymmetric bending |
| 1750730 | 1747.4 | -C=O | Stretching |
| 3000-2800 | 2853.27 | 0 | Symmetric stretching |
| 3000-2800 | 2925.3 | 0 | Asymmetric stretching |
| 3050-3000 | 3004.46 | 0 | Stretching |
Table 7: Characteristic infrared bands of biodiesel.
Conclusion
A promising heterogeneous catalyst for hydrolysis of triglycerides has been prepared through the sulfonation of Vitellaria paradoxa biochar with fuming sulphuric acid. Structural study through SEM, elemental analysis and FT-IR spectroscopy suggests that biochar-based catalyst consists of polycyclic aromatic carbon sheets bearing three different acidic groups of phenolic, carboxylic, and sulphonic. The catalytic activity of the developed biochar-based catalyst was evaluated in a two-step reaction involving hydrolysis of V. paradoxa oil and subsequent esterification of FFAs to FAME (biodiesel). The hydrolysis reaction showed that the catalyst increased the conversion of V. paradoxa oil to FFAs when compared to the reaction carried out without the catalyst. A percent conversion of 34.32% was obtained with the catalyst compared to the 12.84% obtained without the catalyst. Optimization study of the hydrolysis reaction using a randomized central composite rotatable design showed that a percent yield of 33.80% was obtainable at optimum reaction condition of the study. Evaluation of the esterification activity of the catalyst showed a FFAs conversion of 70%.
Conflict of Interest
The authors declare no conflict of interest.
References
- Dora E, James G, David A, Edgar L (2005) Transesterification of triacetin with methanol on solid acid and base catalysts. Appl Catal A: General 295: 97-105.
- Lebedevas S, Vaicekauskas A (2006) Research into the application of biodiesel in the transport sector of Lithuania. Transport 2: 80-87.
- Valente O, da-Silva M, Pasa V, Belchior C, Sodre J (2010) Fuel consumption and emissions from a diesel power generator fuelled with castor oil and soybean biodiesel. Fuel 89: 3637-3642.
- Anastopoulo G, Lois E, Karonis D, Kalligeros S, Zannikos F (2005) Impact of oxygen and nitrogen compounds on the lubrication properties of low sulfur diesel fuels. Energy 30: 415-426.
- Mamat R, Abdullah N, Hongming X, Wyszynski M, Tsolakis A (2009) Effect of fuel temperature on performance and emissions of a common rail diesel engine operating                with rapeseed methyl ester (RME).
- Goodrum J, Geller D (2005) Infuence of fatty acid methyl esters from hydroxylated vegetable oils on diesel fuel lubricity. Bioresour Technol 96: 851-855.
- Holser R, Harry O (2006) Transesterified milkweed (Asclepias) seed oil as a biodiesel fuel. Fuel 85: 2106-2110.
- Ilham Z, Saka S (2010) Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour Technol 101: 2735-2740.
- Ayodele O, Dawodu FA (2014a) Conversion of Calophyllum inophyllum oil with high free fatty acid content to biodiesel over starch-derived catalyst. Energ Technol 2: 912-920.
- Krohn BJ, McNeff CV, Yan B, Nowlan D (2011) Production of algae-based biodiesel using the continuous catalytic Mcgyan process. Bioresour Technol 102: 94-100.
- Guzatto R, Defferrari D, Reiznautt QB, Cadore ÃR, Samios D (2012) Transesterification double step process modification for ethyl ester biodiesel production from vegetable                and waste oils. Fuel 92: 197-203.
- Qiu F, Li Y, Yang D, Li X, Sun P (2011) Biodiesel production from mixed soybean oil and rapeseed oil. Appl Energ 88: 2050-2055.
- Dawodu FA, Ayodele OO, Bolanle-Ojo T (2014) Biodiesel production from Sesamum indicum L. seed oil: An optimization study. Egy J Petrol 23: 19199.
- Endalew AK, Kiros Y, Zanzi R (2011) Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenerg 35: 3787-3809.
- Dawodu FA, Ayodele O, Xin J, Zhang S, Yan D (2014) Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl Energ 114: 819-826.
- Ayodele O, Dawodu FA (2014) Production of biodiesel from Calophyllum inophyllum oil using a biomass-derived catalyst. Biomass Bioenerg 70: 239-248.
- Cavalcanti-Oliveira EA, da Silva PR, Ramos AP, Aranda DAG, Freire DMG (2011) Study of soybean oil hydrolysis catalyzed by Thermomyces lanuginosus lipase and its application to biodiesel production via hydroesterification. Enzyme Res, pp: 1-8.
- AOAC (1984) Official methods of analysis. 14th edn. Arlington, VA, 67: 503-515.
- Montgomery DC (2001) Design and analysis of experiments. John Wiley and Sons, New York, USA.
- Balusu R, Paduru RR, Kuravi SK, Seenayya G, Reddy G (2005) Optimization of critical medium components using response surface methodology for ethanol production from cellulosic biomass by Clostridium thermocellum SS19. Process Biochem 40: 3025-3030.
- Chen C, Huang C, Tran D, Chang J (2012) Biodiesel synthesis via heterogeneous catalysis using modified strontium oxides as the catalysts. Bioresour Technol 113: 8-13.
- Eromosele IC, Eromosele CO, Akitoye AO, Komolafe TO (1994) Characterization of oils and chemical analysis of wild plants. Plant Foods Hum Nutr 46: 361-365.  Â
- Okamura M, Atsushi T, Masakazu T, Junko N, Kazunari D, et al. (2006) Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon. Chem Mater 18: 3039-3045.
- Nakajima K, Michikazu H, Shigenobu H (2007) Environmentally benign production of chemicals and energy using a carbon-based strong solid acid. J Am Ceram Soc 90: 3725-3734.
- Kitano M, Arai K, Kodama A, Kousaka T, Nakajima K, et al. (2009) Preparation of a sulfonated porous carbon catalyst with high specific surface area. Catal Let 131: 242-249.
- Dawodu FA, Ayodele O, Xin J, Zhang S (2014) Application of solid acid catalyst derived from low value biomass for a cheaper biodiesel production. J Chem Technol Biotechnol 89: 1898-1909.
- Melero J, Bautista L, Morales G, Iglesias J, Briones D (2009) Biodiesel production with heterogeneous sulfonic acid-functionalized mesostructured catalysts. Energ Fuel 23: 539-547.
- Xing R, Liu Y, Wang Y, Chen L, Wu H, et al. (2007) Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mater 105: 41-48.
- Hara M (2010) Biodiesel production by amorphous carbon bearing SO3H, COOH and phenolic OH groups, a solid Brønsted acid catalyst. Top Catal 53: 805-810.
- Rolando Z (2001) Pyrolysis of biomass. Royal Institute of Technology, Stockholm.
- Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, et al. (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energ 87: 2589-2596.
- Satyarthia J, Srinivasa D, Ratnasamy P (2011) Hydrolysis of vegetable oils and fats to fatty acids over solid acid catalysts. Appl Catal A: General 391: 427-435.
Citation: Dawodu FA, Ayodele OO, Olatunde OC, Ibraheem AA, Adekunle AE, et al. (2019) Development of Solid Acid Catalyst from Biomass Obtained from Cake of Vitellaria paradoxa and Its Application in Biodiesel Production Via a Two-Step Reaction System. Ind Chem 4:129. DOI: 10.4172/2469-9764.1000129
Copyright: © 2019 Dawodu FA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3813
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2977
- PDF downloads: 836