Mini Review Open Access
Development of Opioid Tolerance and Endoplasmic Reticulum Stress
| Tomohiko Aoe* | |
| Department of Anesthesiology,Tokyo Women's Medical University,Yachiyo Medical Center, Chiba, Japan | |
| Corresponding Author : | Tomohiko Aoe Department of Anesthesiology Tokyo Women's Medical University Yachiyo Medical, Center, 477-96 Owada-Shinden Yachiyo, Chiba 276-8524, Japan Tel: +81-43-226-2573 Fax: +81-47-458-7047 E-mail: taoe@faculty.chiba-u.jp |
| Received January 20, 2015; Accepted February 06, 2015; Published February 16, 2015 | |
| Citation: Aoe T (2015) Development of Opioid Tolerance and Endoplasmic Reticulum Stress. J Pain Relief 4:174. doi: 10.4172/2167-0846.1000174 | |
| Copyright: © 2015 Aoe T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
Opioids are potent analgesics, widely used to control acute and chronic pain. While repeated administration of opioids, particularly morphine, induces tolerance that reduces the effectiveness of the analgesic, the precise molecular mechanism for the development of tolerance remains uncertain. Opioids bind to the μ opioid receptor (MOR) to activate various signaling molecules, leading to a decrease in neuronal excitability. Chronic morphine tolerance may be derived from adaptations in the intracellular signal transduction of post-MOR activation. Many physiological and pathological conditions, such as secretory demands, ischemia, hypoxia, and genetic mutations, can cause aberrant protein folding and the accumulation of misfolded proteins in the endoplasmic reticulum (ER). These insults lead to ER stress and initiate the unfolded protein response (UPR). Recent studies have suggested that chronic ER stress might modulate intracellular signaling pathways, resulting in several chronic disorders, such as type II diabetes. Binding immunoglobulin protein (BiP) is an ER chaperone that is central to ER functioning. Recently, our studies in mice suggest that BiP may play an important role in the development of morphine tolerance. We also found that a chemical chaperone, which improves ER protein folding capacity, attenuated the development of morphine tolerance. Thus, the modulation of ER functions by chemical chaperones and other drugs may lead to a new direction for the prevention of morphine tolerance.
| Keywords |
| Analgesics; Endoplasmic reticulum (ER); Immunoglobulin; Voltage; Phosphorylation |
| Introduction |
| Opioids like morphine have been widely used clinicaly as effective analgesics for acute and chronic pain. When opioids are used, the importance of care for side effects such as nausea, drowsiness and constipation is emphasized. In addition, continuous use of opioids developes tolerance in which the analgesic effect becomes attenuated. In this paper, we mainly discuss endoplasmic reticulum (ER) stress as one of the molecular mechanisms for the development of opioid tolerance. |
| ER stress response |
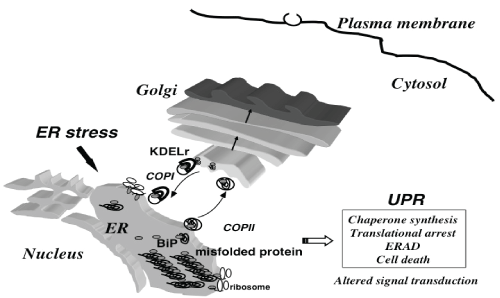
| The ER provides a folding environment for newly synthesized secretory and membrane proteins [1]. Secretory proteins are synthesized by ribosomes and translocated cotranslationally or posttranslationally to the ER. These newly synthesized proteins interact with ER molecular chaperones, such as immunoglobulin heavy chain-binding protein (BiP), calnexin, calreticulin and protein disulfide isomerase, to become properly folded and assembled into a mature protein complex for transport along the secretory pathway. Aberrant protein folding, due to extracellular stimuli such as ischemia and oxidative stress, or genetic mutations leads to the accumulation of misfolded proteins in the ER, which in turn evokes the unfolded protein response (UPR) [2]. The UPR reduces the amount of misfolded proteins [3] by inducing the production of ER chaperones that promote protein folding, reducing general protein synthesis, and enhancing the degradation of misfolded proteins via a ubiquitin-proteasome system, termed ER-associated degradation (ERAD) [4]. |
| A further overload of misfolded proteins initiates apoptosis, leading to diverse human disorders [5,6]. such as neurodegenerative diseases [7-9] and cardiomyopathies [10]. Another distinct mechanism for human disorders caused by ER stress is the alteration of signal transduction pathways during the UPR. Obesity causes ER stress that induces UPR, which may disturb insulin receptor signaling through hyperactivation of c-Jun N-terminal kinase (JNK) and subsequent serine phosphorylation of insulin receptor substrate-1 (IRS-1), resulting in type II diabetes (Figure 1). |
| Recent studies suggest that ER stress is involved in pain disorders such as diabetic peripheral neuropathy [11] and orofacial inflammatory pain [12]. Our previous studies in mice suggest that an ER chaperone, BiP, may play an important role in the development of morphine tolerance. We also found that a chemical chaperone, which improves ER protein folding capacity, attenuated the development of morphine tolerance [13]. |
| Analgesic mechanism and tolerance formation of opioid |
| Morphine is the main component of opium alkaloids from opium poppy. While morphine had been thought to exert an analgesic effect by acting on nerve system, it became clear that there are opioid receptors in the brain [14-16]. Subsequently, δ-opioid receptor gene was first identified [17,18], followed by μ, κ and ORL1 (opioid receptor-like 1) opioid receptor genes. Since analgesic effects of morphine were lost in mice deleted with μ opioid receptor (MOR) gene, MOR was confirmed to be responsible for morphine analgesic signaling [19]. |
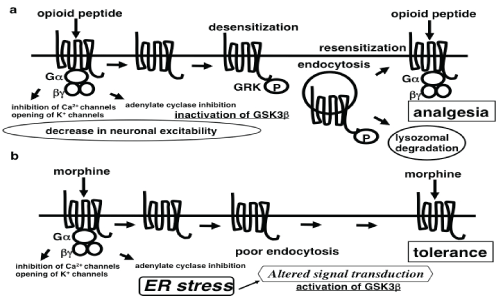
| Opioid receptors are cell surface receptors with seven transmembrane, belonging to the heterotrimeric guanine nucleotidebinding protein (G protein)-coupled receptor superfamily. The homology of amino acid sequences of transmembrane region among μ, δ, and κ receptors has been maintained, whereas the carboxyl terminal of intracellular domain and the amino terminal of extracellular domain are very different. The main endogenous ligand for MOR is β-endorphin that binds to MOR to activate various signaling molecules through Gα subunit of inhibitory Gi proteins, leading to a decrease in neuronal excitability by the inhibition of voltage-dependent calcium channels and the activation of inwardly rectifying potassium channels [20]. Activation of MOR also induces the phosphorylation of MOR by G-protein-coupled receptor kinases [21,22]. Phosphorylated MOR is recognized by arrestins [23], and internalized by clathrin-coated vesicles. The transient uncoupling of MOR from signaling pathways due to the phosphorylation and intracellular trafficking of MOR causes opioid desensitization. Most of the internalized MORs return to the cell surface, resulting in resensitization [24-26] (Figure 2). |
| Signal transduction upon MOR activation |
| Chronic morphine tolerance may be derived from adaptations in the intracellular signal transduction of post-MOR activation, as morphine does not induce effective MOR phosphorylation and internalization [27]. Persistent MOR activation on the cell surface may alter signal transduction, including changes in MOR-coupled G proteins from Giα to Gsα [28], increased activity of protein kinase C [29], and the upregulation of N-methyl-D-aspartate receptor signaling [30]. These changes may contribute to the development of morphine tolerance. Chronic morphine treatment also activates the cyclindependent kinase 5 and glycogen synthase kinase 3β (GSK3β) signaling pathway, while the inhibition of them diminishes morphine tolerance and restores analgesia in rats [31] (Figure 2b). |
| GSK3β is expressed ubiquitously and is one of the central molecules in intracellular signal transduction [32]. It may play an important role in diverse physiological and pathological states [33]. We focused on GSK3β as a key signaling molecule in the MOR signaling pathway. GSK3β is a serine/threonine kinase. The kinase activity is inactivated by the phosphorylation of Ser9 and enhanced by the dephosphorylation of Ser9 and the phosphorylation of Tyr216. The p90 ribosomal S6 kinase [34], Akt [35], protein kinase C [36] and protein kinase A [37] have been demonstrated to phosphorylate GSK3β at Ser9. MOR activation also phosphorylates GSK3β at Ser9 through the PI3K/Akt pathway [32]. On the other hand, the regulatory mechanism for the activation of GSK3β remains uncertain in comparison to that for its inactivation. ZAK1 [38], Fyn tyrosine kinases [39] and transient increases in intracellular Ca2+ [40] have been reported to phosphorylate GSK3β at Tyr216 to activate the kinase. In addition, ER stress has been also reported to induce the activation of GSK3β [41,42]. |
| Possible crosstalk between mor analgesic signal transduction and the UPR |
| Chronic morphine administration may cause altered signal transduction through persistent MOR activation on the cell surface. A mechanism similar to that occurring in type II diabetes would be possible in the crosstalk between MOR analgesic signal transduction and the UPR. We speculate that the UPR signaling might attenuate the MOR signaling, thus causing the development of morphine tolerance. |
| BiP, (or GRP78) is an ER chaperone that is central to ER functioning. Our studies in mice suggest that BiP may play an important role in the development of morphine tolerance, possibly through the modulation of GSK3β signaling. We have previously produced knockin mice expressing a mutant BiP in order to elucidate the physiological processes that are sensitive to BiP function in adulthood [43]. The mutant BiP protein lacks the retrieval carboxyl-terminal KDEL sequence [44,45] that normally functions to return BiP to the ER from the secretory pathway by the KDEL receptor in the Golgi complex. This mutant allows us to examine the effects of a defect in ER function without completely eliminating BiP function. |
| The kinase activity of GSK3β is regulated by its phosphorylation status. Phosphorylation of residue Ser9 inactivates the activity, whereas dephosphorylation of Ser9 and phosphorylation of Tyr216 enhance the activity [32]. We evaluated the phosphorylation status of GSK3β in the brain stems of wild-type and heterozygous mutant BiP mice using specific antibodies against phosphorylated Tyr216 GSK3β and phosphorylated Ser9 GSK3β [13]. After chronic morphine injection intraperitoneally for 5 days, the wild-type mice developed morphine tolerance, whereas the mutant BiP mice remained less tolerant to morphine. Because we injected morphine intraperitoneally, both spinal and supraspinal neurons were supposed to be affected. Neurons with MOR expression in the periaqueductal gray (PAG) matter contribute to morphine tolerance [46-48]. With repeated morphine treatment, the mutant BiP brain stems showed low levels of phosphorylation of Tyr216 in GSK3β, in contrast to the prominent phosphorylation in wild-type mice by western blotting. These brains were also sectioned and double-immunostained with antibodies raised against MOR and tyrosine-phosphorylated GSK3β. MOR-immunopositive neurons in the PAG region of wild-type brains showed more enhanced expression of tyrosine-phosphorylated GSK3β significantly than those in the mutant BiP brains. |
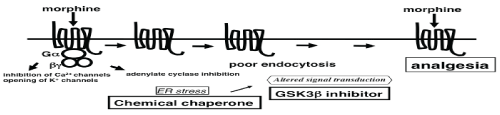
| These observations suggest that chronic MOR stimulation by repetitive morphine injection may activate GSK3β and that the activation of GSK3β may be related to the development of morphine tolerance. Mice with the mutant BiP may be defective in the activation of GSK3β and show less less tolerant to morphine. In fact, we showed that co-administration of morphine and a GSK3β inhibitor in wild type mice did not develope the tolerance [13] (Figure 3). |
| Chemical chaperone attenuates the development of Morphine tolerance |
| In order to confirm that an ER chaperone like BiP may mediate the development of morphine tolerance, we examined the effect of a chemical chaperone on morphine tolerance [13]. Tauroursodeoxycholic acid (TUDCA) is a derivative of endogenous bile acids that is thought to increase ER folding capacity and suppresses the expression of BiP [49,50]. We administered TUDCA together with morphine twice a day for 5 days in wild-type mice, and hot plate tests were performed at the first and the tenth treatments. The response latencies of the mice receiving both TUDCA and morphine were significantly longer than those of control mice with morphine alone after the tenth treatment. Thus, TUDCA prevented the development of morphine tolerance, suggesting a mechanistic relationship between an ER chaperone and morphine tolerance. The modulation of morphine analgesia by TUDCA reveals a potential clinical application of chemical chaperones that can modulate ER functions for the prevention of morphine tolerance. |
| Conclusion |
| Studies above suggest that morphine tolerance may be related to ER stress. Thus, the modulation of ER functions by chemical chaperones and other drugs may lead to a new direction for the prevention of morphine tolerance. |
| Acknowledgements |
| I thank Drs. Serabi Tanabe, Tamae Dobashi, Yota Okuyama and Hisayo Jin for their contributions to this work. |
References
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181-191
- Patil C, Walter P (2001) Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. CurrOpin Cell Biol 13: 349-355.
- Winnay JN, Kahn CR (2011) PI 3-kinase regulatory subunits as regulators of the unfolded protein response. Methods Enzymol 490: 147-158.
- Bonifacino JS, Weissman AM (1998) Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell DevBiol 14: 19-57.
- Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389-1398.
- Zhao L, Ackerman SL (2006) Endoplasmic reticulum stress in health and disease. CurrOpin Cell Biol 18: 444-452.
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, et al. (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol 1: 479-485.
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, et al. (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105: 891-902.
- Jin H, Mimura N, Kashio M, Koseki H, Aoe T (2014) Late-onset of spinal neurodegeneration in knock-in mice expressing a mutant BiP. PLoS One 9: e112837
- Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, et al. (2004) Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol 24: 8007-8017.
- O'Brien PD, Hinder LM, Sakowski SA, Feldman EL (2014) ER stress in diabetic peripheral neuropathy: A new therapeutic target. Antioxid Redox Signal 21: 621-633
- Yang ES, Bae JY, Kim TH, Kim YS, Suk K, et al. (2014) Involvement of endoplasmic reticulum stress response in orofacial inflammatory pain. ExpNeurobiol 23: 372-380.
- Dobashi T, Tanabe S, Jin H, Mimura N, Yamamoto T, et al. (2010) BiP, an endoplasmic reticulum chaperone, modulates the development of morphine antinociceptive tolerance. J Cell Mol Med 14: 2816-2826.
- Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179: 1011-1014.
- Simon EJ, Hiller JM, Edelman I (1973) Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. ProcNatlAcadSci U S A 70: 1947-1949.
- Terenius L (1973) Stereospecific interaction between narcotic analgesics and a synaptic plasm a membrane fraction of rat cerebral cortex. ActaPharmacolToxicol (Copenh) 32: 317-320.
- Evans CJ, Keith DE Jr, Morrison H, Magendzo K, Edwards RH (1992) Cloning of a delta opioid receptor by functional expression. Science 258: 1952-1955.
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG (1992) The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. ProcNatlAcadSci USA 89: 12048-12052.
- Gaveriaux-Ruff C, Kieffer BL (2002) Opioid receptor genes inactivated in mice: the highlights. Neuropeptides 36: 62-71.
- Dickinson P, Kimber WL, Kilanowski FM, Webb S, Stevenson BJ, et al. (2000) Enhancing the efficiency of introducing precise mutations into the mouse genome by hit and run gene targeting. Transgenic Res 9: 55-66.
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, et al. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. ProcNatlAcadSci U S A 95: 7157-7162
- Johnson EE, Christie MJ, Connor M (2005) The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals 14: 290-302.
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, et al. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2.286: 2495-2498.
- Gintzler AR, Chakrabarti S (2006) Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci 79: 717-722.
- Martini L, Whistler JL (2007) The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. CurrOpinNeurobiol 17: 556-564
- Zöllner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, et al. (2008) Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest 118: 1065-1073.
- Finn AK, Whistler JL (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32: 829-839.
- Chakrabarti S, Regec A, Gintzler AR (2005) Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Brain Res Mol Brain Res 135: 217-224.
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85: 395-404.
- Trujillo KA, Akil H (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251: 85-87.
- Parkitna JR, Obara I, Wawrzczak-Bargiela A, Makuch W, Przewlocka B, et al. (2006) Effects of glycogen synthase kinase 3beta and cyclin-dependent kinase 5 inhibitors on morphine-induced analgesia and tolerance in rats. J PharmacolExpTher 319: 832-839
- Grimes CA, Jope RS (2001) The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. ProgNeurobiol 65: 391-426.
- Jope RS, Yuskaitis CJ, Beurel E (2007) Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 32: 577-595.
- Sutherland C, Leighton IA, Cohen P (1993) Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 296: 15-19.
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785-789
- Goode N, Hughes K, Woodgett JR, Parker PJ (1992) Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J BiolChem 267: 16878-16882.
- Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett JR, et al. (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. ProcNatlAcadSci U S A 97: 11960-11965.
- Kim L, Liu J, Kimmel AR (1999) The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell 99: 399-408.
- Lesort M, Jope RS, Johnson GV (1999) Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem 72: 576-584.
- Hartigan JA, Johnson GV (1999) Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J BiolChem 274: 21395-21401.
- Song L, De Sarno P, Jope RS (2002) Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J BiolChem 277: 44701-44708.
- Qu L, Huang S, Baltzis D, Rivas-Estilla AM, Pluquet O, et al. (2004) Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev 18: 261-277.
- Mimura N, Hamada H, Kashio M, Jin H, Toyama Y, et al. (2007) Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ 14: 1475-1485.
- Munro S, Pelham HR (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899-907.
- Lewis MJ, Pelham HR (1990) A human homologue of the yeast HDEL receptor. Nature 348: 162-163.
- Yaksh TL, Yeung JC, Rudy TA (1976) Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114: 83-103.
- Bagley EE, Chieng BC, Christie MJ, Connor M (2005) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146: 68-76.
- Morgan MM, Fossum EN, Levine CS, Ingram SL (2006) Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. PharmacolBiochemBehav 85: 214-219
- Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, et al. (2002) Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592-601.
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E , et al. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137-1140.
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 14451
- [From(publication date):
March-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9919
- PDF downloads : 4532
