Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Development of Novel HIV Vaccine by Nano-biomaterials
| Md Salahuddin1*, Md Sayfullah2, Mahjabin Rashid3, Jay Prakash Sah4, Md Abdul Momin5, Syfullah Shahriar6, Chandra Kant Yadav4, Bhupendra Sharma4, Mohammad Showkat Mahmud7 and Mohammad Alam Miah1 | |
| 1Department of Physiology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh | |
| 2College of Medicine, Shaheed Ziaur Rahman Medical College Hospital, Bogra, University of Rajshahi, Bangladesh | |
| 3College of Medicine, Mymensingh Medical College and Hospital, University of Dhaka, Mymensingh, Bangladesh | |
| 4Department of Medical Laboratory Science, School of Health and Allied Sciences, Pokhara University, Lekhnath- 12, Kaski, Nepal | |
| 5Department of microbiology and hygiene, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh | |
| 6Department of Soil Science, Sher-e-Bangla Agricultural University, Dhaka-1207, Bangladesh | |
| 7Bangladesh Livestock Research Institute, Animal Health Research Division, Savar, Dhaka- 1341, Bangladesh | |
| Corresponding Author : | Md Slaahuddin Department of Physiology Bangladesh Agricultural University Mymensingh-2202, Bangladesh Tel: +8801745001875 E-mail: ssdin23@gmail.com |
| Received: May 14, 2015; Accepted: May 15, 2015; Published: May 22, 2015 | |
| Citation: Salahuddin M, Sayfullah M, Rashid M, Prakash Sah J, Abdul Momin M, et al. (2015) Development of Novel HIV Vaccine by Nano-biomaterials. Biochem Physiol 4:e136. doi:10.4172/2168-9652.1000e136 | |
| Copyright: © 2015 Salahuddin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Human immune deficiency virus is a life threatening immune deficiency disease causing virus. Nowadays Nano-Medicine is a promising field for manufacturing vaccines for various bacterial and viral diseases. FPCN containing specific antigen are capable to stimulate T-cell very well to kill the viruses. P24 is the capsid protein of HIV virus which can stimulate immune response. So FPCN containing p24 will be a good choice for HIV vaccination. The present hypothesis aimed to quantify the challenges of making a novel HIV vaccine using FPCN nano-particles.
| Keywords |
| HIV; FPCN; Nano-medicine; HIV vaccine; Nanobiomaterials |
| Editorial |
| HIV is a great challenge to rollout of treatment for HIV/AIDS in the developing world. There is still lack of effective and specific medication. Scientists are trying to find out the possible remedy against this virus. Usually HIV virus induces immunodeficiency through direct infection to CD4 T-cells, then replicate within these cells and produces huge copies of virion which later attack the other T- cell [1-10]. The unique feature of HIV virus is that its nucleus poses 2 copies of ssRNA, each of them consists of nine genes as gag, pol, env, tat, rev, nef, vif, vpr and vpu gene. The immunogenic part of HIV capsid consists of viral protein p24. Three genes (gag, pol and env) contain information needed to make the structural proteins for new virus particles. The six remaining genes like tat, rev, nef, vif, vpr, and vpu (or vpx in the case of HIV-2), are regulatory genes for proteins that control the ability of HIV to infect cells, produce new copies of virus [11,12]. The transcription co-activator of tat (p16 or p14) causes the apoptosis of cell by arresting cell division. The Nef protein (p27) down-regulates CD4 (the major viral receptor). In the same way, the MHC class I and class II molecules are introduced into cell through β (CCR5) and α (CXCR5) Chemokine receptors or co-receptor or by forming of viral synapses. HIV virus anchors to cell surface by spike like glycoprotein, gp120 that binds with integrin α4β7 and penetrates cell surface by ccr5 route [13-16]. |
| HIV Super-infection |
| Super-infection is the main problem for HIV which is rather uncommon in other viral infection. HIV shows error in reverse transcription which leads to its rapid mutation and thus increases the chance of super-infection. Super or reinfection occurs in patients after a second infection of other strain of this virus. The second strain infection is more dangerous and causes severe destruction of T-cell leading to drug resistance. There are also some controversial reports showing that super-infection is somehow beneficial of patient because a first infection creates anti-antibody against the second strain. A study published in the new England journal of medicine showed that coinfection in patient with HIV I and HIV II has slower progression of immunodeficiency in comparison to either HIV I or HIV II infection [17-21]. To cure any type of infection either normal or super infection, we need a cargo which can deliver the antigen against rapid or coinfection thereby raising the anti-HIV antibody to eliminate or reduce the risk of these pandemic diseases [22,23]. Whether CTL restricts the replication of virus but HIV controls the CTL and uses for selfreplication is really a interesting phenomenon [1]. |
| p24 |
| p24 is the capsid protein of HIV. Usually each capsid consists of more or less 1200 p24. Capsid protein p24 (HIV-1) plays an essential role in the production of infectious virus particles. Its interactions with itself and with neighboring structural proteins certainly play a crucial role in capsid assembly and the maturation steps leading to infectious particles, both during and after budding from the cell membrane. It is the antigenic part responsible for the antibody production in HIV infection [24,25]. |
| Ferritin Protein Nano particles |
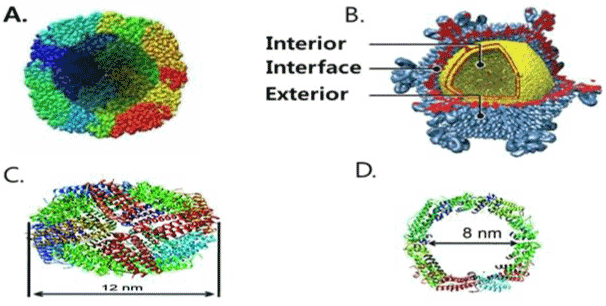
| Ferritin protein cage is the first protein cage which was used as a template for the synthesis of inorganic particles. It is a nano-biocontainer or cargo that can carry nano-scaled particles to deliver into the target cell or organ. FPCNs are faster than other biological carrier in term of releasing of particles into target cell. FPCs are present in all domains of life as Fe storage. The inner cavity of FPCs cage stores about 4000 iron atoms. FPCs have three distinct interfaces (interior cavity, inter phase and exterior surface) to carry the peptides or other biological substances. It is still unknown which one gives the better response. FPCNs are composed of protein subunits having highly symmetrical structures which can tolerate harsh chemical environments and have wide range of biomedical applications. (Figure 1 [23]). |
| Confirmative way of incorporation and expression of p24 in dendritic cell and development of HIV vaccine |
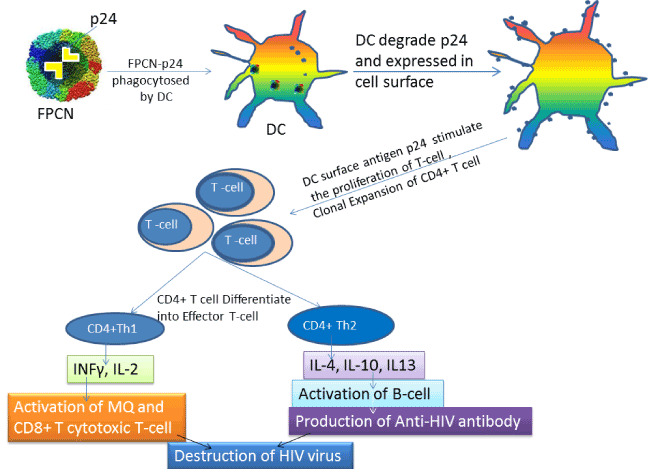
| The first things we need to consider that p24 protein are really incorporated into dendrite cells or not. To confirm this phenomenon, we need to collect dendrite cell lysate administered with p24, followed by western blot to confirm the expression of intentionally delivered p24 into dendrite cell. We can also prove it by FACS analysis of intentionally expressed p24 into dendritic cell by either surface or intracellular staining with p24 specific antibody. When FPCN-P24 is delivered to the dendritic cell, DCs phagocytize and degrade the p24 and then this degraded p24 antigens are expressed onto their cell surface. Due to highly immunogenic property of p24, its stimulation will greatly enhance surface phenotype of DC which later stimulates T-cell proliferation (especially CD4+ T-cell activation and differentiation). Finally the activated CD4+ T cell will differentiate into different subclasses of helper T cell depending on the specific expression of surface markers and cytokines secretion. Usually CD4+ T cells are differentiated into CD4+ Th1 and CD4+Th2. And the CD4+ Th1 cell will secrete INFγ and IL2. These cytokines later activate macrophage and CD8+ Cytotoxic T-cell. Both of them take part in direct destruction of HIV virus. On the other hand, when CD4+ T cell differentiate into CD4+ Th2 cells, they secrete IL4, IL 13 and IL10 thereby stimulate B-cell activation that will secrete p24 antigen specific anti-HIV antibody which will work against HIV viral antigen (Figure 2). |
| Precise Discussion |
| The Protein p24 derived from the Capsid of HIV virus genetically introduced into the internal cavity or external surface of ferritin protein cage nano-particles. Successfully introduced p24 into dendritic cell and this transfected dendritic cell sequentially stimulate T-cell specifically CD4+ T cell to Th1 and Th2 cells. Finally secreted INFγ, IL2, IL10, IL13 from these T-cells destroy the HIV virus. |
References
|
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13940
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9521
- PDF downloads : 4419
