Research Article Open Access
Development of Microcantilever Sensors for Liver Cancer Detection
Jingjing Wang1,2, Yinfang Zhu1,2, Jinying Zhang1 and Jinling Yang1,2*1Institute of Semiconductors, Chinese Academy of Sciences, Beijing 100083, P. R. China
2State Key Laboratory of Transducer Technology, Shanghai 200050, P. R. China
- *Corresponding Author:
- Jinling Yang
Institute of Semiconductors, Chinese Academy of Sciences
Qinghua Donglu A 35, Beijing 100083, P. R. China
Tel: +86 10 8230 4700
Fax: 86 10 8230 5141
E-mail: jlyang@semi.ac.cn
Received date: November 18, 2015 Accepted date: January 01, 2016 Published date: January 10, 2016
Citation: Wang J, et al. (2016) Development of Microcantilever Sensors for Liver Cancer Detection. Adv Cancer Prev 1:103. doi:10.4172/acp.1000103
Copyright: © 2016, Wang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Advances in Cancer Prevention
Abstract
Recently, microcantilever-based technologies are playing more and more important roles in early diagnosis of cancer due to their high sensitivity, fast response, low cost, small reagent consumption, portability, real-time, labelfree detection, and so on. However, in conventional cantilever sensors working on mass-loading principle, the change of stiffness coefficient k is neglected. This results in distinct error for mass detection. Some researchers tried a local immobilization method to eliminate the undesired effect of k. But the change of k in this method still brings unexpected error. An accurate theoretical model is needed to take the effect of k change into account in the local immobilization approach. A micro-cavity was designed in the free end of the cantilever for local antibody immobilization in our work, thus the adsorption-induced variation of k can be dramatically reduced compared to that caused by adsorption of the whole lever. In addition, an analytical model has been established to eliminate the effect of adsorption-induced lever stiffness change and has been applied to precise mass detection of cancer biomarker AFP, the detected AFP antigen mass (7.6 pg/ml) is close to the calculated one (5.5 pg/ml), two orders of magnitude better than the value by the fully antibody-immobilized cantilever sensor. These approaches will promote clinical application of the cantilever sensors in early diagnosis of cancer.
Keywords
MEMS cantilever; Liver cancer biomarker; Mass detection; Adsorption-induced stiffness change
Introduction
Microcantilever-based biosensors have been widely used to detect DNA/RNA, antigen, enzyme, mycotoxin, and drug monitoring [1-5]. Nowadays, researchers have been attracted to contribute to early diagnosis of cancer based on microcantilever biosensors due to its high sensitivity, reliability and low cost. It has been demonstrated that microcantilever has great potential for clinical application in early diagnosis of cancer.
Generally, cantilevers have two working modes, static mode and dynamic mode. In dynamic mode, the lever stiffness k was neglected, thus the resonance frequency shift only depended on the variation of mass m. However, the detection error was substantial due to the adsorption-induced surface stress [6]. Some research groups have found the ineligible difference between the theoretical and measured values. For example, Jeong et al. reported that the theoretical frequency shift was 2 Hz calculated by mass loading of C-reactive protein, while the measured result was 184 Hz, Amit et al. [7] found that the measured frequency shift was 132 kHz, 5 times larger than the calculated 24 kHz. The experimental results indicated that the change of lever stiffness k played an important role in the frequency shift due to adsorption of biomolecules on the cantilever [8].
The effect of surface stress on the cantilever frequency has been investigated with theoretical and experimental studies. Hwang et al. reported that due to the joint action of mass loading and surface stress caused by molecular interactions, the frequency shift was 100 Hz - 600 Hz corresponding to the antigen concentrations of 10 - 100 ng/ml, but the accurate frequency shift just caused by surface stress was difficult to distinguish [9]. Wang et al. tried to apply an ultra-thin microcantilever to investigate the effect of surface stress. They found that the gas adsorbed by the ultra-thin microcantilevers could induce surface stress, which changed the mechanical properties. But they couldn’t give the quantitative effect of the induced stress on the frequency shift [10].
We have reported a novel local reaction cavity structure in our previous cantilever arrays [11]. The local reaction at the free end of the lever can effectively minimize the impact on lever stiffness k. In order to increase the reaction area, the pillar arrays were fabricated. Moreover, we have established an analytical model to reduce the effect of k change on mass detection accuracy. Our model integrates the adsorption-induced surface stress and Young’s modulus, and consequently includes the impacts of k change and m change. The local reaction structure and the accurate theoretical model help to dramatically improve the accuracy in liver cancer biomarker detection with microcantilever biosensors.
Method and Discussion
Fabrication and functionalization of the cantilever
The microcantilever arrays were fabricated with MEMS technologies, and then functionalized with antibody. The details were given in published paper [11]. Cantilever arrays with five levers are designed to jointly detect multi-biomarkers. One of them served as reference lever, which was designed to remove the influence of environment noise and non-specific adsorption. The others were used to specifically react with the biomarkers. Furthermore, the novel local micro-cavity structures were designed to obtain local reaction in the tip of the lever. To increase the reaction areas of antigen, the pillar arrays were fabricated.
The mechanical properties of the cantilevers were characterized by laser Doppler vibration system. The quality factors (Q) of the cantilever are 587 in air and 49434 in vacuum, good enough for achieving high sensitivity.
Analytical model for multilayer adsorption and surface stress
The change of the resonance frequency depends on the changes of k and m. The change of k is generally caused by two aspects. The first one is the change of Young’s modulus due to the multilayer on cantilever [11-15]. It can be calculated according to the multilayer beam theory. The other one is the change of adsorption-induced surface stress which can be obtained based on the theoretical models in reported work [11,16-19]. For the AFP antigen solution of 18 pg/mL, the change of k could result in a frequency change of about 40 Hz, the same order of magnitude as that resulting from mass loading of AFP antigen [20].
AFP detection
To verify the performance of the fabricated cantilever-based mass sensor, a 5.5 pg/mL AFP antigen solution is prepared and detected with two different sensors. The first sensor, named as fully immobilized lever, has the antibody immobilized on full cantilever surface. And the second sensor, named as locally immobilized lever, has the antibody locally printed in the micro-cavity. The frequency shift is 830 Hz, about two orders of magnitude larger than the theoretical one 6 Hz corresponding to 5.5 pg/mL. This deviation could mainly be attributed to the lever k variation caused by the AFP adsorption on the whole cantilever.
The effect of the k variation on the frequency shift is greatly decreased by locally immobilizing the AFP antibody. Using the mentioned analytical model to further reduce the deviation from k change caused by the multilayer organic and adorption-induced surface stress, the experimental frequency shift was modified to 8.4 Hz, corresponding to AFP antigen mass of 7.6 pg/mL, close to 5.5 pg/mL.
The same method has been applied to detect the AFP antigen solution with various concentrations of 15 pg/mL and 18 pg/mL. The corresponding AFP antigen mass are 4.4 pg and 5.2 pg, respectively, the detected mass with the sensor are 4.5 pg and 5.35 pg, respectively, quite close to the theoretical values.
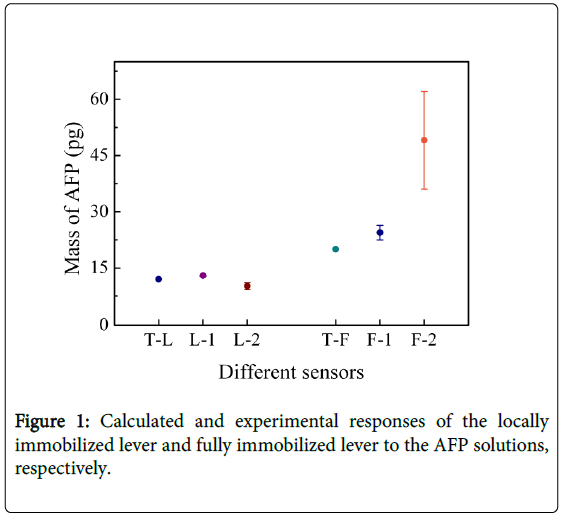
The same results were obtained by using difference micro cantilevers. Two different size of cantilever array were used. The experiment results were shown in Fig. 1, T-L denotes the calculated response to AFP solution for the locally immobilized sensor L-1 and L-2, and the value is 12 pg. On the horizontal axis L-1 and L-2 denote the experimental response to AFP solution for the locally immobilized lever of 200 µm × 70 µm × 5 µm and 180 µm × 50 µm × 5 µm, respectively. T-F denotes the calculated response to AFP solution for the fully immobilized sensor F-1 and F-2, and the value is 20 pg. F-1 and F-2 denote the experimental response to AFP solution for the fully immobilized lever of 200 µm × 75 µm × 3 µm, 300 µm × 100 µm × 3 µm, respectively. The result indicates that the locally immobilized sensors are less influenced by k variation compared with the fully immobilized sensors, and the response result of the locally immobilized sensor (200 µm × 70 µm × 5 µm) has a deviation of 0.95 ± 0.125 pg from the theoretical value of 12 pg. Whereas the fully immobilized sensors (300 µm × 100 µm × 3 µm) has a deviation of 29 ± 13 pg from the theoretical value of 20 pg. As shown in Figure 1, the deviation from the theoretical value increases for the levers with smaller stiffness coefficient k.
Various concentrations of 0-50 pg/ml AFP antigen solutions were prepared and detected by the locally immobilized anti-AFP cantilevers [21]. Figure 2 depicts the measured frequency shift of locally immobilized anti-AFP levers to the various concentrations of antigen AFP, and a linear regression was obtained with a relative uncertainty less than 5%. Furthermore, the result of the sensor response to 0 pg/ml anti-AFP is nearly 0, indicating that the binding between AFP antigens to antibodies was specific.
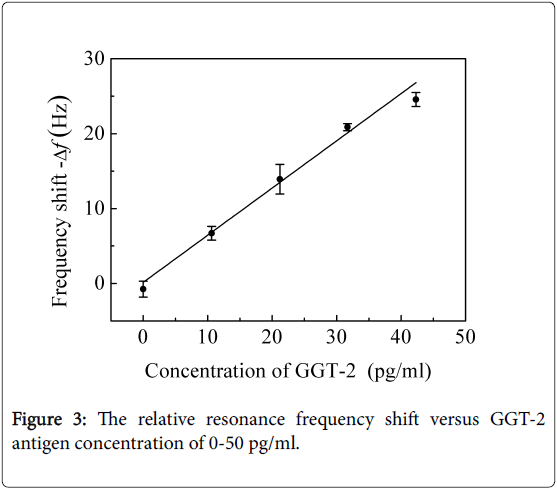
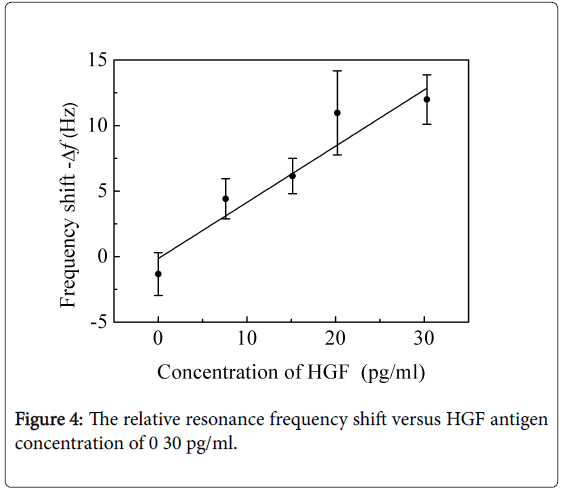
And the various concentrations of 0-50 pg/ml GGT-2 antigen solutions and 0-30 pg/ml HGF antigen solutions were detected with the locally immobilized anti-HGF cantilevers and locally immobilized anti-GGT-2 cantilevers, respectively. As shown in Figures 3 and 4, the relative frequency shift linearly changed with the antigen concentration.
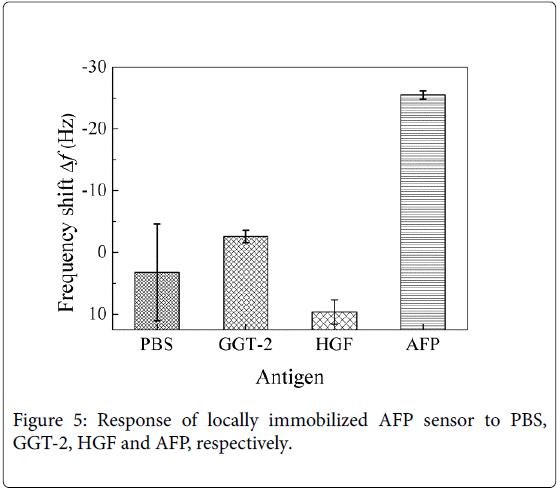
The cantilever arrays locally immobilized with anti-AFP was utilized to detect PBS, AFP, HGF, and GGT-2, respectively. Figure 2 shows the response of sensor to the different antigens, AFP, HGF, and GGT-2. It indicates that the reaction of antigen and antibody was specific, and the cross reaction between HGF, GGT-2, and AFP was slight.
Above all, the adsorption induced resonance frequency shift of the cantilever-based sensor working in a dynamic mode depends not only on mass loading but also on variation of the lever stiffness. Therefore, it is important to eliminate the interference of the stiffness change for precise mass detection. The newly developed cantilever-based sensor with the micro-cavity for local reaction and the analytical model for the inter-molecule interaction and multilayer cantilever have effectively eliminated the influence of k variation and significantly improved the detection accuracy and performance of the cantilever-based biosensor. This approach can promote clinical application of the cantilever-based sensors. The PCB circuit for processing the output signal of the Wheatstone bridge will be integrated with the cantilever chip to build up a portable cancer diagnosis system in near future.
Conclusion
A local immobilization method with an analytical model is designed to eliminate the effect of k change and precise mass detection of cancer biomarker AFP. These structural features offer several advantages: high sensitivity, high throughput, high mass detection accuracy, small volume, and low cost. Cantilever arrays are batch fabricated using MEMS technologies and used to detect the concentration of AFP antigen using whole lever method and local immobilization method, respectively. The measured frequency shift is around two orders of magnitude larger than the theoretical one 6 Hz using the whole lever method. While the local immobilization method exhibits a measured frequency shift of 8.4 Hz, close to theoretical 6 Hz. These results indicate that the local immobilization method with an analytical model is promising in clinical application of the cantilever-based sensors.
Acknowledgements
This work was supported by the project from MOST of China (2013YQ16055103), NSFC projects (61234007 and 61404136) and State Key Laboratory of ASIC & System, Fudan University (2015KF001).
References
- Huber F, Lang HP, Backmann N, Rimoldi D, Gerber C (2013) Direct detection of a BRAF mutation in total RNA from melanoma cells using cantilever arrays. Nat Nano 8: 125-129.
- Lu L, Si JC, Gao ZF, Zhang Y, Lei JL, et al. (2015) Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNAzyme-amplified electrochemical detection. Biosensors and Bioelectronics 63: 14-20.
- Park J, Karsten SL, Nishida S, Kawakatsu H, Fujita H (2012) Application of a new microcantilever biosensor resonating at the air-liquid interface for direct insulin detection and continuous monitoring of enzymatic reactions. Lab on a Chip 12: 4115-4119.
- Bai X, Hou H, Zhang B, Tang J (2014) Label-free detection of kanamycin using aptamer-based cantilever array sensor. Biosensors and Bioelectronics 56: 112-116.
- Huang LS, Pheanpanitporn Y, Yen YK, Chang KF, Lin LY, et al. (2014) Detection of the antiepileptic drug phenytoin using a single free-standing piezoresistivemicrocantilever for therapeutic drug monitoring. Biosensors and Bioelectronics 59: 233-238.
- Johnson BN, Mutharasan R (2012) Biosensing using dynamic-mode cantilever sensors: a review. BiosensBioelectron 32: 1-18.
- Lee JH, Kim TS, Yoon KH (2004) Effect of mass and stress on resonant frequency shift of functionalized Pb (Zr0. 52Ti0. 48) O3 thin film microcantilever for the detection of C-reactive protein. ApplPhysLett 84: 3187-3189.
- Gupta AK, Nair PR, Akin D, Ladisch MR, Broyles S, et al. (2006) Anomalous resonance in a nanomechanical biosensor. PNAS 103: 13362-13367.
- Hwang KS, Eom K, Lee JH, Chun DW, Cha BH, et al. (2006) Dominant surface stress driven by biomolecular interactions in the dynamical response of nanomechanicalmicrocantilevers. ApplPhysLett 89: 173905.
- Wang DF, Ono T, Esashi M (2004) Thermal treatments and gas adsorption influences on nanomechanics of ultra-thin silicon resonators for ultimate sensing. Nanotechnology 15: 1851.
- Wang SP, Wang JJ, Zhu YF, Yang JL, Yang FH (2015) A new device for liver cancer biomarker detection with high accuracy. Sensing and Bio-Sensing Research 4: 40-45.
- Hwang KS, Lee SM, Kim SK, Lee JH, Kim TS (2009) Micro-and nanocantilever devices and systems for biomolecule detection. Annu Rev Anal Chem 2: 77-98.
- Albrecht T, Grütter P, Horne D, Rugar D (1991) Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J ApplPhys 69: 668-673.
- Thundat T, Warmack R, Chen G, Allison D (1994) Thermal and ambient induced deflections of scanning force microscope cantilevers. ApplPhysLett 64: 2894-2896.
- Snis N, Edqvist E, Simu U, Johansson S (2008) Monolithic fabrication of multilayer P (VDF-TrFE) cantilevers. Sensors and Actuators A Physical 144: 314-320.
- Sony P, Puschnig P, Nabok D, Ambrosch-Draxl C (2007) Importance of van der Waals interaction for organic molecule-metal junctions: Adsorption of thiophene on Cu (110) as a prototype. Phys Rev Lett 99: 176401.
- Yi X, Duan H (2009) Surface stress induced by interactions of adsorbates and its effect on deformation and frequency of microcantilever sensors. JMPS 57: 1254-1266.
- Chen G, Thundat T, Wachter E, Warmack R (1995) Adsorption-induced surface stress and its effects on resonance frequency of microcantilevers. J ApplPhys 77: 3618-3622.
- Wormer PE (2000) Intermolecular potentials, internal motions, and spectra of van der Waals and hydrogen-bonded complexes. Chem Rev 100: 4109-4144.
- Wang SP, Wang JJ, Zhu YF, Yang JL, Yang FH (2014) Cantilever with immobilized antibody for liver cancer biomarker detection. Journal of Semiconductors 35: 104008.
- Wang JJ, Wang SP, Wang X, Zhu YF, Yang JL, Yang FH (2014) Cantilever array sensor for multiple liver cancer biomarkers detection. SENSORS IEEE 343-346.
Relevant Topics
- 3D Mammograms
- Alternative Cancer Medicines
- Aromatase Inhibitors
- Breast Reconstruction Surgery
- Cancer and Nutrition
- Cancer Prevention from Nuts
- Cancer Screening
- Chemoprevention
- Dietary Supplements
- Exercise and Cancer
- Naturopathic Treatments
- Oncoplastic Surgery
- Scintimammography
- Stem Cell Transplants for Cancer Prevention
Recommended Journals
Article Tools
Article Usage
- Total views: 12404
- [From(publication date):
January-2016 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 11308
- PDF downloads : 1096