Development of Artificial Reefs Using Environmentally Safe Ceramic Material
Received: 19-Mar-2018 / Accepted Date: 26-Mar-2018 / Published Date: 31-Mar-2018 DOI: 10.4172/2157-7625.1000253
Abstract
To develop artificial reefs, that are environmentally friendly and inexpensive, prototype artificial reefs made of ceramic material have been studied. The properties of the reefs (chemical constituents, surface texture, water absorbability, mechanical strength, erosion rate, and sustainability) were examined. The reefs were compared with natural coral rubbles. The effects of the reefs on nutrient availability, toxic chemical diffusion, and the propagation of plankton (bacteria, diatom) and other benthic organisms were examined, for which the reefs were set in aquaria for a long time. It was shown that the reefs were nontoxic, pH-neutral, mechanically strong, and sustainable in a hostile shallow sea environment. This study suggests that artificial reefs made from the ceramic material are not hazardous to small fish.
Keywords: Artificial reef; Shallow sea; Ceramic material; Environmentally safe; Propagation of fish
Introduction
The development of artificial fish reefs has been studied worldwide to enhance fish abundance and diversity, recover coastal and marine habitats, and restore damaged coral reefs [1-5]. Various materials have been used in the construction of artificial reefs worldwide. They include bamboo, concrete blocks, ceramic structures, fibre glass, pipes, reinforced plastic, metal, and marine alloys [6]. Every material, however, has some drawbacks when used as artificial reefs. Bamboo and wood are easily scattered by water currents, lose their fish-aggregating integrity, and cause further degradation of the sea bed [7]. Concrete blocks are durable and can be structured in various configurations, but have limited applications because of their cost [7]. In fact, placing any type of material in the marine environment poses a risk of causing pollution, which would have harmful effects on marine ecosystems [7]. Therefore, by considering detrimental effects apart from benefits, it is necessary to choose materials that have minimal negative impacts on marine environments and can enhance fish colonization. In this regard, ceramic materials are one of the best candidates, because they are economical, environmentally safe, and suitable for hostile marine environments. Santos et al. [8] studied the fish colonization of artificial reefs made of ceramic, concrete, and polyvinyl chloride (PVC) pipes, and they found that ceramic reefs were more effectively colonized than concrete and PVC reefs. Although there have been some studies on the colonization of fish in ceramic reefs, few studies have shown effective reef design and functionality of ceramic materials in a shallow sea environment.
In this research, ceramic artificial reefs with a hexagonal column structure were designed from a ceramic material. They would be set on sandy sea beds to obtain hard and flat sea beds. The effectiveness of the hexagonal structure was investigated by determining the mechanical strength of the reefs and their sustainability in an adverse shallow sea environment. Ceramic materials were analyzed by measuring chemical constituents, surface texture, water absorption, erosion rate, and durability. These qualities were compared with those of natural coral rubbles. The effect of the ceramic materials on water quality and bioproductivity was investigated by observing changes in nutrient availability, toxic chemical diffusion, and the propagation of bacteria, diatom, and other benthos by putting them in an artificial marine environment produced in aquaria.
Materials and Methods
Preparation of artificial reefs
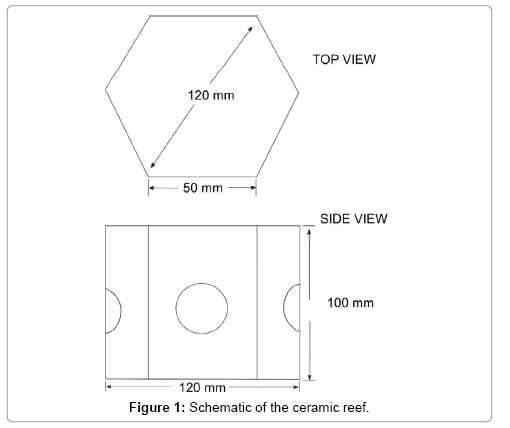
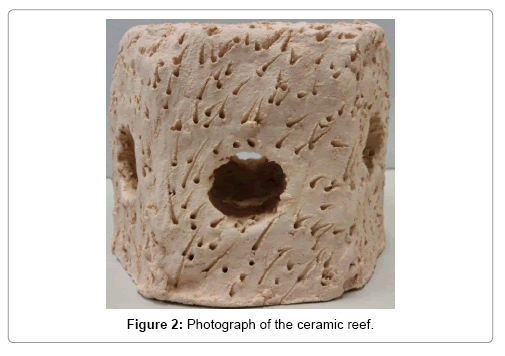
Two styles of ceramic material were prepared: hexagonal column type reefs (diagonal length 120 mm and height 100 mm), and ceramic rods (length 40 mm and diameter 10 mm). A schematic of the reef is shown in Figures 1 and 2. First, the reefs and rods were made by hand from clay containing a high density of iron species (Noyaki-koudo, Mino-nendo Co., Japan), then dried for one week at room temperature, and baked for 4 h in an electric kiln (DAC-OIT, Shimpo Co., Japan) by gradually increasing the temperature to 800°C (400°C for 1 h, 600°C for 1 h, 800°C for 2 h). After heating, they were cooled naturally. The mass density of the ceramic was about 1.62 g/cm3. Natural branching coral rubbles (Montipora sp.) (Platinum reef sand, No. 25, JUN Co., Japan) about 35 mm length and 10 mm diameter and massive coral (Favia sp.) (Sangoishi-L, Ryuka Shoji Co., Japan) (about 8 × 8 × 4 cm3) were used for comparison with the reef samples. The mass density of the coral rubbles was about 2.20 g/cm3.
Two aquaria (40 × 30 × 25 cm3, glass boxes) with the same experimental conditions were prepared in the laboratory, one of which is shown in Figure 3. Pure water and sea salt (Red sea salt, Red Sea Co.) were poured, and sand (grain diameter of about 0.25 mm) and abundance of bacteria (Bicom 78 and Bicom 21 PD, BIOCOM Co.) were set in the aquaria. Then, the samples, named the ceramic reefs, ceramic rods, and coral rubbles, and a water filtering system (Tetra. Ex Power, VX-60, Tetra Co., Japan), were set. The temperature of the water was maintained at about 25°C throughout the experiment by a heating system (Tube heater-100 W, Tetra Co., Japan) in winter and a cooling system (Tetra Cool Tower CR-2N, Tetra Co., Japan) in summer. The salinity of the water was kept at approximately 1.022. Finally, sufficient light was introduced (white LED, 26 W, 1.7 × 104 lux), which was turned ON for 12 hours every day. We tried to make the warm shallow aquatic environment in the aquarium similar to shallow sea areas.
Measurements
First, the chemical compositions of the ceramic were measured using energy dispersive X-ray spectroscopy (EDX, EDX-800, Shimadzu Co.) in air. The surface texture of the ceramic was measured with a scanning electron microscopy (SEM, JSM-6510LV, JEOL Co.) apparatus and an optical microscope (BX60, Olympus Optical Co.), and then compared with that of the natural coral rubbles.
The amounts of water absorption for both the ceramic rods and coral rubbles were measured. 50 g each of the ceramic rods and coral rubbles were selected, heated in an electric kiln (DAC-OIT, Shimpo Co.) for 1 h at 300°C, and then their weights were measured. These samples were immersed in pure water for 1 h, and then taken out, and their weights were measured again. From the weight difference, the amount of absorbed water was obtained.
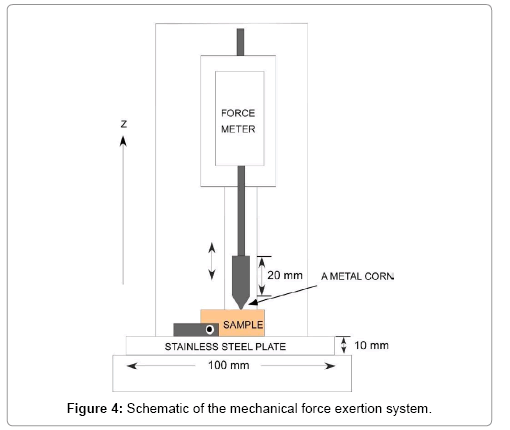
A mechanical force exertion system (Model 1308, Aikoh Engineering Co.) was used to examine the mechanical strength of the ceramic rods and coral rubbles. A schematic of the system is shown in Figure 4. A metal column (steel, 10.0 mm in diameter and 20.0 mm in length) with a sharp cone of about 0.7 mm tip curvature and 45° angle was used. The force was applied perpendicularly to the surface of the center of the ceramic rod or the coral rubble at the moving speed of about 0.1 mm/s until a crack appeared on the sample or the sample broke down. The maximum force at the breaking point, the diameter of the hole made by the cone tip, and the hole depth were recorded.
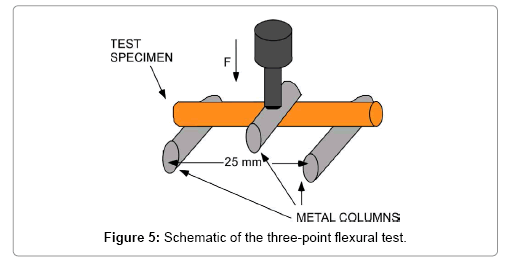
A three-point flexural test was also carried out to estimate the mechanical strength of the ceramic materials, the schematic of which is shown in Figure 5. The sample was placed on two metal columns (stainless steel, 10.0 mm in diameter, and 20.0 mm in length). The distance between the two columns is 25.0 mm. A loading bar of the same size, which was lowered to the center of a sample at a constant speed (about 0.1 mm/s) and the load was measured. When a beam is in bending, flexural strength σfM [9];
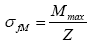 (1)
(1)
Where Mmax is the maximum flexural moment, Z is the crosssection factor. In this case, it is assumed that the material behavior is in consistent with Hook’s law. For a beam of circular cross section, Z=πd3/32 where d is the diameter of the sample. From Figure 5, the bending moment Mmax=Fmax L/2, where Fmax is the total applied force and L is the length of the beam between the support points. Therefore,
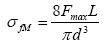 (2)
(2)
A hydraulic press (Riken Power, Riken Seiki Co.,) was used to measure the mechanical hardness of the ceramic reefs with different wall thickness. In this system, a cylinder of 48.0 mm diameter with a circular plane surface was used. Pressure was applied vertically and slowly in a cylinder until the ceramic reef was crushed. The maximum pressure and force were recorded.
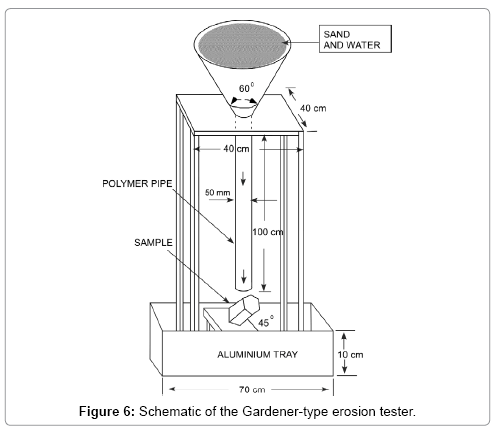
To measure the erosion characteristics of the ceramic reefs, a Gardener-type sand-water erosion tester [10] as shown in Figure 6 was developed in our laboratory and used. The main part of the system consists of a funnel (8 inch opening with an angle of 60°) and a polymer pipe of 40 mm inner diameter and 100 cm length connected at the collar of the funnel. The reef sample was supported by an aluminum base at an angle of 45° from the horizontal bottom base so that the opening of the pipe is set above the area of the reef to be eroded, and the distance of the pipe from the target surface is about 5.0 cm. The erosion of the apex part, which is considered to be the weakest part of the ceramic reef, was examined. Four types of experiment were performed. First, water was continuously poured into the top funnel of the tester with a 500 mL beaker until 50 L (500 mL, 100 times) impacted the sample. Each 500 mL of water was allowed to flow for 1-1.5 min. Then, the sample was visually inspected to check for cracking, grazing, or volume-ersion. Secondly, quartz sand (small grains of about 0.25 mm diameter and 2.61 g/cm3 mass density; Sand King Co.) and water (500 mL sand+500 mL water) were continuously poured to the top funnel. In this case, 100 L of the sand-water mixture (50 L of sand+50 L of water) was used. The sand-water mixture was allowed to flow through the pipe for approximately 1-1.5 min, impacted the sample surface, and was deposited on an aluminium tray. This process was continued until 100 L (1000 mL, 100 times) of the sand-water mixture impacted the sample surface. Then, the impacted part of the sample was observed. Thirdly, the erosion test was carried out with other sand grains of about 1.5 mm diameter and 2.59 g/cm3 mass density for the falling height in intervals of 7-130 cm. Finally, the erosion test was carried out with coral sand grains of about 1.2 mm diameter, 2.58 g/cm3 mass density for the falling height of 130 cm and the sand-water mixture volume in intervals of 50-200 L. In each case, the thickness and volume of the erosion were measured. For ensuring the accuracy of the experiment, the erosion test was conducted several times for each case, and average results were obtained. The total kinetic energy of the falling sand-water mixture was calculated using the principle of conservation of kinetic energy [11].
The diversity and abundance of plankton in the aquaria were studied. After setting the artificial reefs, the ceramic rods and coral rubbles, and about 5 L Sea water collected at Nagaizaki beach in Numadzu in the aquaria, the normal sea water conditions (water temperature ~25°C, salinity 1.022, and continuous circulation of water through the filtering system) were maintained. It was observed that some brownish parts developed on the wall of the aquarium and on both of the ceramic reefs, the rods, and coral rubbles. Later, the brownish part was collected and observed using the optical microscope. Then many algae, planktons and animals were found in this part. For further investigation, new ceramic samples were set in the aquaria and kept for 1 year, and then analyzed following the same procedure.
After 3 months of keeping the first ceramic reefs and coral rubbles in the aquaria, 50 mL of water was taken from the aquaria and set in test tubes to observe and enumerate bacteria and diatoms. The samples were processed for microscopic observation according to the procedure [12]. Bacteria and diatom were determined by counting under UV excitation within 15 to 30 randomly selected fields using an epifluorescence microscope (Nikon Eclipse E6000, Japan). The abundance of the bacteria and diatom was estimated by the following equation [13]:
Abundance of bacteria /mL in the sample, (Vt):
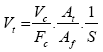 (3)
(3)
Where Vc is the total number of bacteria counted, Fc is the total number of fields counted, At is the surface area of the filter in μm2, Af is the area of each field in μm2, and S is the volume of the sample filtered in μL.
To estimate the amount of carbon biomass of bacteria, diatom and other benthic organisms on the reef, the ceramic reef was taken out from the aquarium carefully after 3 months of first setting ensuring no removal of plankton and other benthic organisms and its weight was measured. Then, the reef was heated in an electric kiln (DACOIT, Shimpo Co.) for 1 h at 300°C to remove water, and the weight was measured. Afterwards, the ceramic reef was burned to 800°C by gradually increasing the temperature. After cooling, the weight was measured again and the decrease in weight was measured. During the heating from 300°C-800°C, all the animals, plants, bacteria, diatom, and benthic organisms are considered to have burned out, and the amount of sublimated carbon biomass was estimated.
The nutrient content in the water was estimated. The water from both aquaria was collected in 125 mL polyethylene bottles and preserved for the analysis of the nutrient content in the water, such as ammonium (NH4+), nitrite such as ammonium (NH4+), nitrite (NO2-) and nitrate (NO3-). Nutrients were analyzed according to procedure [14] using an autoanalyzer (TRAACS 200, BL Tek, Germany). The available nutrients were compared with the optimum levels in the marine environment.
Sheltering of small fish around the reef was observed. Two sets of ceramic reefs having holes of 3 cm diameter on the wall and 6 cm diameter on the top were set in the aquarium. To observe the sheltering of fish, a small fish (Chrysiptera cyanea) was placed in the aquarium. We suddenly put a rod on the outer surface when the fish was outside of the ceramic reefs, and the fish’s motions were recorded using a digital camera (EX-ZR700, Casio Co.). Many types of fish motion were analyzed and classified, where the role of reef is studied. The sheltering test was performed at day time (11 A.M.) for 45 minutes.
Results and Discussion
The elemental compositions of the ceramic reefs and coral rubbles were measured by EDX and are shown in Table 1. The ceramic reef material was mainly composed of aluminum (Al2O3) and silica (SiO2), while the coral rubbles were mostly composed of calcium oxide (CaO). All these elements and their oxides are nontoxic and thus favorable for the marine environment. Since diatoms and other silica-secreting organisms utilize silicon (SiO3) [15] and the major component of the reefs is silicon (63.7%), they can be set in the coral areas to be used as bodies of small animals. The amount of iron in the reef is also considerable.
| Compounds | Atomic content (wt %) | |
|---|---|---|
| Ceramic reef | Coral rubble | |
| Na2O | 2.105 | 1.110 |
| MgO | 0.844 | 1.440 |
| Al2O3 | 32.6 | 0.139 |
| SiO3 | 60.6 | 1.443 |
| P2O5 | 0.024 | 0.717 |
| SO3 | 0.047 | 0.818 |
| Cl | 0.180 | 0.639 |
| K2O | 0.976 | 0.159 |
| CaO | 0.131 | 80.59 |
| Sc2O3 | 0.007 | 10.23 |
| TiO2 | 0.511 | 0.018 |
| Cr2O3 | 0.008 | 0.007 |
| MnO | 0.011 | 0.006 |
| Fe2O3 | 0.902 | 0.198 |
| Co2O3 | 0.020 | 0.011 |
| NiO | 0.002 | 0.042 |
| CuO | 0.005 | 0.008 |
| As2O3 | 0.003 | 0.010 |
| Rb2O | 0.003 | 0.005 |
| SrO | 0.002 | 2.340 |
| CdO | 0.005 | 0.046 |
| BaO | 0.939 | 0.018 |
Table 1: Atomic percentage of different elements in the ceramic reef and the coral rubbles measured by the EDX method.
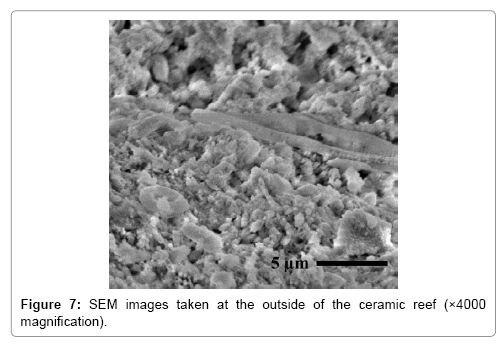
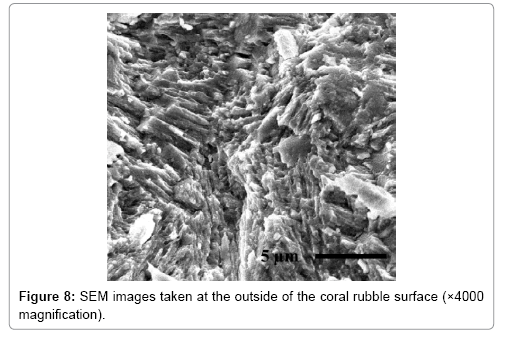
Figures 7 and 8 shows the images of the surfaces of the ceramic reef and coral rubbles taken by SEM. It is clearly shown that the ceramic surfaces have a varied texture and micro porous structures similar to those of coral rubbles. Because of the texture of the ceramic surfaces, it is conjectured that the reefs are preferable for the propagation of organisms [6].
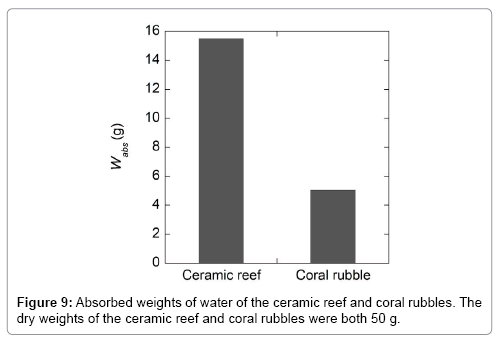
Results of the water absorption measurement of the ceramic reef and coral rubbles are shown in Figure 9. It is apparent that the water absorbability of the ceramic reef is about 4 times higher than that of coral rubbles. The higher water absorbability indicates that there are many small empty spaces inside the material, which can provide habitats for bacteria and aquatic microorganisms. The other advantage is that by absorbing much water, it becomes more stable against the forces in the water [6].
The hydrographical contents of the aquarium water with the ceramic reefs were measured. The salinity of the aquarium water was 1.022 and the pH was 7.8 which are lower than the pH of Ocean surface water (about 8.1). It can be conjecture that due to continuous circulation of water through the filter which involved three layers of different sizes of sand, the pH of the aquaria water decreased. The pH indicates that aquaria water quality is slightly alkaline [15]. The temperature of the water was kept at about 25°C.
Several mechanical tests of the ceramic reef were carried out. In case of the cone hardness test, the ceramic rods broke at a force of 118 N and cracks appeared around the circumference of the ceramic rod. In this case, the remaining force was about 53 N. The cone tip made a half-sphere dent of about 0.80 mm diameter and 0.25 mm depth before the crack appeared. In the case of coral rubbles, breakdown occurred after applying a force of about 170 N. They were separated at the point of critical force. It was also found that the cone tip made a hole of about 0.92 mm diameter and 0.27 mm depth before the breakdown occurred. From this test, it can be concluded that the ceramic materials have comparable mechanical hardness to coral rubbles.
The flexural stress of the ceramic rod and coral rubbles were calculated using eqn. (2) in the three-point flexural test. Flexural strength indicates the highest stress in the material at its moment of rupture or fracture. It is seen that the average flexural stress of the ceramic rod is around 4.5 MPa, and that of the coral rubble is around 13.8 MPa. This measurement implies that the ceramic rod is weaker than coral rubbles. Thus, it is necessary to improve the hardness of the ceramic reefs. The selection of ceramic materials and the processing should be studied.
Table 2 shows the results of the pressure test performed using the hydraulic press. A maximum pressure of 57.0 N/cm2 (1031 N) was recorded to crush the ceramic reef with a wall thickness of 24.0 mm. This result shows that the hexagonal ceramic reefs with thick walls are stronger and stable.
| Reef | Pressure(N/cm2) | Force(N) | Wall thickness(mm) |
|---|---|---|---|
| 1 | 37 | 669 | 13 |
| 2 | 37 | 669 | 13 |
| 3 | 57 | 1031 | 24 |
Table 2: Critical pressure and force required to crushing down the ceramic reef using the hydraulic press.
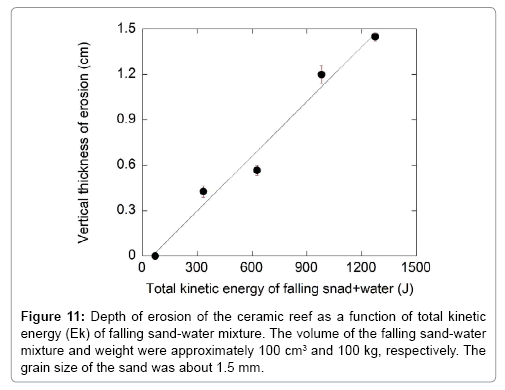
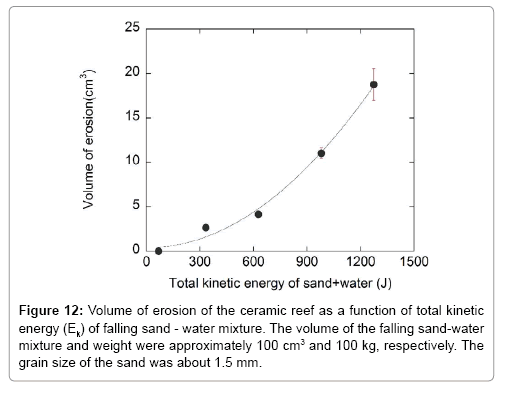
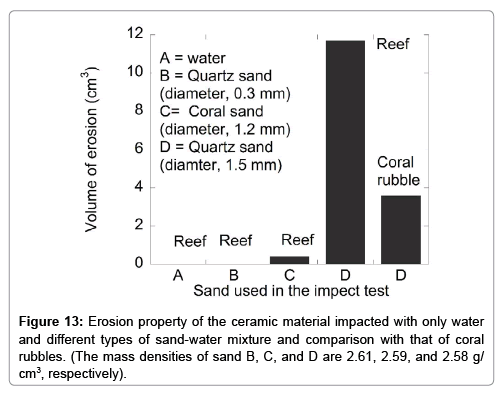
The erosion rate of the ceramic reefs was measured by exploiting the kinetic energy of the sand-water mixture falling from different heights, and the erosion property was compared with that of coral rubbles. Figure 10 shows the reef after the sand-water mixture impact test. This figure shows that the ceramic reef was eroded when it was impacted with the sand-water mixture. Figures 11 and 12 are the vertical depth and volume of erosion, respectively, of the ceramic reef after impacting the sand-water mixture as a function of kinetic energy when sand of about 1.5 mm diameter was used. From Figure 11, it is found that the vertical depth of erosion of the ceramic reef increases almost linearly with increasing kinetic energy of sand-water mixture, indicating the local erosion and stability of the material. Figure 12 shows that the volume of erosion of the reef is increased parabolically with increasing the kinetic energy of the sand-water mixture. Figure 13 shows the erosion characteristics of the ceramic reef and coral rubbles when they were impacted with only water and with the sand-water mixture. It is revealed that in the case of only water and the sand-water mixture (sand diameter, ~0.25 mm), no erosion was observed. In the case of the sand-water mixture (sand diameters ~1.2 and 1.5 mm), slight erosion was recorded. The sand-water erosion test confirms that the ceramic reefs and coral rubbles would be eroded in the shallow sea environment when 1.5 mm sized sands repeatedly hit the surfaces. It is found that erosion rate of the ceramic reef is higher than that of the coral rubbles. To avoid the erosion, a harder ceramic material should be studied.
The growths of plankton and small animals in the aquaria were determined by the microscopic observation of the surfaces of the ceramic reefs and coral rubbles. It was observed that some brownish parts which are microalgae propagated on the ceramic reefs and coral rubbles, one year after setting them in the aquarium. The brownish part was observed using the optical microscope and SEM. Microalgae collected from the ceramic reef, coral rubbles, and the glass wall were separately analyzed using the optical microscope. It was observed that many planktons and small animals propagated in both the ceramic reefs and coral rubbles surfaces one year after setting them in the aquarium. Microalgae analysis revealed that plant and animal growths gradually increased in the aquaria. This result indicates that the ceramic reef is a good material for plankton and small animal growths and their propagation.
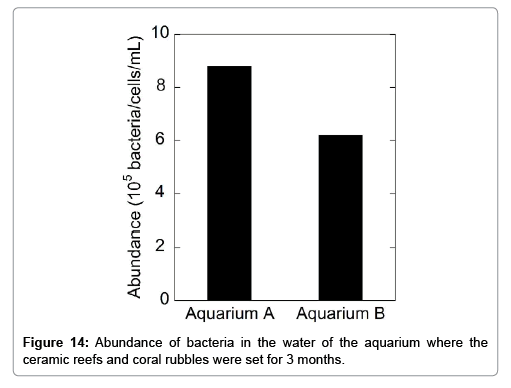
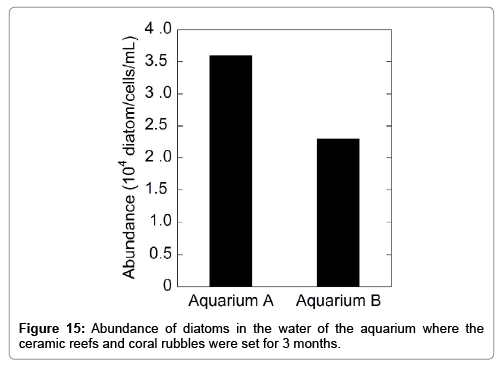
The abundance of micro benthos (bacteria and diatom) was investigated for both aquaria water 3 months after their operation. The results are shown in Figures 14 and 15. The presence of a significant number of bacteria and diatom in the aquaria implies that the ceramic reefs do not affect water quality and are not harmful to micro benthos evolution. Almost the same abundances of bacteria and diatoms in both aquaria were observed.
The mass of deposited organisms and their carbon biomass were measured as 143.6 and 8.41 mg, respectively on 400 cm2 of the ceramic reef surface. In terms of carbon biomass, the data revealed that there were significant numbers of bacteria, diatom, small animals, and other micro benthos in the aquaria water with the ceramic reefs. This result favors the use of ceramic reefs.
The nutrient concentration of the water in the aquaria shows that there were significant amounts of nutrients available in the water for up-taken by plankton and bacteria. The measured nutrient concentrations were compared with the accepted levels in the marine [15], which is shown in Table 3. From these nutrient data, it can be confirmed that the existing levels of nutrients in the aquarium are good for phytoplankton and bacteria which will be consumed by fishes and other organisms.
| Nutrients | In the aquarium water with ceramic reef (µg/L) | Accepted levels in marine environment (µg/L) |
|---|---|---|
| Nitrate | 265.98 | 1-600 |
| Nitrite | 10.85 | 1-50 |
| Ammonium | 8.39 | 5-50 |
Table 3: Comparison of nutrients measured in the aquarium water where the ceramic reef and the coral rubbles were set and accepted level in the marine [15].
The sheltering effects of the ceramic reefs were examined. It was observed that the fish used different routes to enter the ceramic reefs to obtain shelter when an action was applied. The probability of sheltering in the ceramic reef was 78% and that of outside of the reef was 22% in the aquarium. It was also revealed that the fish often used the shortest route to enter the ceramic reef. The fish sheltering test confirms that ceramic reefs will provide good shelters in the sea for small fishes when predators (big fishes) are present.
Conclusions
The prototype of artificial ceramic reefs made of the low-cost clay material has been studied. This study suggests that the ceramic reef is nontoxic and pH-neutral. The mechanical strength and erosion rate indicate that the ceramic reef with a hexagonal structure is stable and has long-term sustainability in a shallow sea environment. They can be set to make a hard and flat sea bed. However, the mechanical strength and the erosion rate should be improved by studying the ceramic material and the fabrication method. The biological analysis of the reef revealed that it has no harmful effects on the biological productivity. It is found that the production of bacteria, diatom and other benthic organism are sustained around the reefs and in the porous structures. The sheltering test for small fish shows that the ceramic reefs will be a good shelter to escape from predators. Therefore, it can be concluded that in the shallow sea areas the ceramic reefs are good candidates for small fish to grow, providing shelter from predators, and allowing escape from fishing nets used by fishermen. The larger and harder ceramic reefs will be studied.
Acknowledgements
We would like to express our gratitude to Professor Y. Suzuki of Shizuoka University, and thank to the members of the Casareto Marine Biochemistry Laboratory of Shizuoka University for their helping in the enumeration of bacteria and nutrients.
References
- Bohnsack JA, Sutherland DL (1985) Artificial reef research: A review with recommendations for future priorities. Bull Mar Sci 37: 11-39.
- Bohnsack JA (1990) Habitat structure and the design of artificial reefs. In habitat structure: The physical arrangements of objects in space. InHabitat Structure. Springer, Dordrecht.
- Bohnsack JA, Johnson DL, Ambrose RF (1991) Ecology of artificial reef habitats and fishes. In: Artificial habitats for marine and freshwater fisheries. Accademic Press Inc, New York,pp: 66-107.
- Seaman W (1996) Does the level of design influence success of an artificial reef. InProceedings of the 1st Conference of the European Artificial Reef Research Network, Ancona, Italy, pp: 13.
- Spieler RE, Gilliam DS, Sherman RL (2001) Artificial substrate and coral reef restoration: What do we need to know what we need. Bull Mar Sci 69: 1013-1030.
- Chou LM (1997) Artificial reefs of southeast Asia-do they enhance or degrade the marine environment? Environ Monit Assess 44: 45-52.
- Cognetti G (1987) Artificial reefs: Myth or reality? Mar Pollut 18: 367-368.
- Santos NL, Berthou EG, Agostinho AA, Latini DJ (2011) Fish colonization of artificial reef in a large neotropical reservoir: Material type and successional changes. Ecol Appl 21: 251-262.
- TemenoffJS,Mikos AG (2008) Biomaterials: The intersection of biology and material science.Pearson Prentice Hall Bioengineering, New Jersy, USA.
- Resnick R, Halliday D, Walker P (2006) Fundamentals of Physics. (8th edition), McGraw-Hill, New Jersy, USA,p: 526.
- Sultana R, Casareto BE, Sohrin R, Alam MS, Fujimura H, et al. (2016) Response of subtropical coastal sediment systems of Okinawa, Japan, to expermental warming and high pCO2. Front Mar Sci 3: 1-14.
- Suttle CA, Fuhrman JA (2010) Enumeration of virus particles in aquatic or sediment samples by epifluorescence microscopy. In: Wilhelm SW, Weinbauer MG, Suttle CA editors.Manual of Aquatic Viral Ecology. Wiley Online Library, New Jersy,pp: 154-153.
- Hansen HP, Koroleff F (2007) Determination of nutrients. In: Methods of seawater analysis.Wiley-VCH Verlag GmbH and Co. Weinheim, Germany,pp: 159-228.
- Sverdrup HU, Johnson MW, Fleming RH (1942) The oceans: Their physics, chemistry and general biology,Prentice-Hall. New York,pp: 181-182.
Citation: Kalam MA, Mieno T, Casareto BE (2018) Development of Artificial Reefs Using Environmentally Safe Ceramic Material. J Ecosys Ecograph 8: 253. DOI: 10.4172/2157-7625.1000253
Copyright: © 2018 Kalam MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12768
- [From(publication date): 0-2018 - Feb 07, 2025]
- Breakdown by view type
- HTML page views: 11589
- PDF downloads: 1179