Research Article Open Access
Development of a Thin-Film Microextraction Device based on ZSM-5/Tenax TA for VOC Detection in Liquid Samples
Shigemi Goda1, Roman Selyanchyn1, Takuma Nozoe1, Hidetaka Matsui2 and Seung Woo Lee1*1Department of Chemical Processes and Environments, Graduate School of Environmental Engineering,The University of Kitakyushu, Japan
2Shinkou Seiki Co. Ltd., Fukuoka, Japan
- *Corresponding Author:
- Seung Woo Lee
Department of Chemical Processes and Environments
Graduate School of Environmental Engineering
The University of Kitakyushu
1-1 Hibikino, Wakamatsu
Kitakyushu 808–0135, Japan
Tel: +81-93-695-3384
Fax: +81-93-695-3293
E-mail: leesw@kitakyu-u.ac.jp
Received date: November 22, 2013; Accepted date: January 15, 2014; Published date: January 17, 2014
Citation: Goda S, Selyanchyn R, Nozoe T, Matsui H, Lee SW (2014) Development of a Thin-Film Microextraction Device based on ZSM-5/Tenax TA for VOC Detection in Liquid Samples. J Anal Bioanal Tech S12:004. doi: 10.4172/2155-9872.S12-004
Copyright: © 2014 Goda S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The zeolite material ZSM-5 was combined with Tenax TA, a porous polymer adsorbent, to form a thin film microextraction (TFME) device that was used as a novel alternative tool for headspace (HS) volatile organic compound (VOC) extraction and preconcentration. The ZSM-5/Tenax TA film deposited on a cylindrical aluminium rod (AR) substrate exhibited superior properties for the adsorption and preconcentration of chloroform, hexane, cyclohexane, benzene, and toluene compared with those of a conventional Tenax TA film when applied in both the direct and HS extraction modes. The advantages of the fabricated device include its enhanced chromatographic performance and consequently lower detection limits for certain VOCs, the improved retention of compounds in the film (possibly enabling its application for both HS and direct extractions from aqueous solutions), the exceptional simplicity of its fabrication, and its robustness. The use of the film for HS extraction leads to increased application lifetime, film stability and shorter preparation times, because drying step is not necessary. Desorption of the adsorbed VOCs was achieved by heating in a conventional Curie point injector for less than 2 min.
It should be noted that the catalytic properties of the zeolite can be disadvantageous at high VOC concentrations (e.g., 100 μM); abundant background peaks as a result of a range of saturated and unsaturated hydrocarbons generated via catalytic degradation of adsorbed compounds at high temperature in the presence of ZSM-5 appear in the gas chromatograph. This effect is still visible at concentrations as low as 10 μM, but does not influence the measurement results. Thus, safe and accurate analyses are achievable at the liquid VOC concentrations in the submicromalar range, which is sufficient for a number of important analytical applications (e.g., detection of VOCs in waste water).
Keywords
ZSM-5; Tenax TA; Thin film microextraction; VOC
Introduction
Solid-phase microextraction (SPME) is a sample preparation technique possessing several advantages over traditional analytical methods such as the convenient integration of extraction, preconcentration, and sample introduction. This technique is rapid and solvent-free in some cases; however, SPME may be quite expensive when it is automated. Since its introduction, SPME has been successfully applied in many fields such as environmental, food, and drug analyses [1].
The principle of SPME is based on the difference in the interactions of analytes with the sample matrix and the extraction phase (coating) via absorption or adsorption (depending on the nature of the coating). The extraction selectivity and efficiency of SPME mainly depend on the coating’s physical properties (porosity and specific surface area) and size, as well as its interactions with the analytes (chemical properties). For large sample volumes, the amount of analyte extracted, n can be expressed by the following equation:
n = KesVeCs (1)
where Kes is the distribution constant of the analyte between the extraction phase and the sample matrix, Ve is the volume of the extraction phase, and Cs is the initial concentration of the analyte in the matrix [2].
Because the amount of analyte extracted using SPME is proportional to the volume of the extraction phase (eq. 1), the sensitivity of a method can be improved by increasing the volume of the extraction phase. Thus, an increase in the coating thickness can increase the volume of the extraction phase and therefore improve the sensitivity of the method; however, a longer equilibration time (depending on the diffusion of the analyte in the coating material) is needed. As a result, the best option for increasing the volume of the extraction phase and the sensitivity of the method is to use a thin extraction phase with a larger surface area [3]. In other words, the extraction phase should have a large surface area-to-volume ratio to provide enhanced sensitivity without sacrificing analysis time [3]. It is also possible to increase the sensitivity using highly porous materials for which a change in the thickness will not significantly reduce the mass transport and diffusion of analytes in the coating.
To achieve effective detection of the analytes that are present at trace levels, a preconcentration step is typically introduced during sample preparation for gas chromatography–mass spectrometry (GC–MS)- based analytical methods. The performance of any preconcentration technique is generally characterized by the term known as the preconcentration factor (PF) that can be defined as ratio between the analyte concentration in extraction phase after extraction finished (Cf) and in initial concentration in sample (C0).
Tenax TA, poly(2,6-diphenyl-p-phenylene oxide), is a well-known porous polymeric material that is widely used as an adsorbent for analyte preconcentration prior to gas chromatography analysis. This linear polymer is soluble in lower chlorinated hydrocarbons and insoluble in alkanes, alcohols, ketones, etc. Tenax TA has also been used as a stationary phase in gas chromatography [4] because it has various outstanding features, such as high thermal stability (up to 350°C), relatively low water retention, and resistance to oxygen (does not require purification of the carrier gas) [5]. The majority of chromatographic applications use the material in the form of microsized beads packed in volatile organic compound (VOC) preconcentration tubes. Alfeeli et al. however, recently demonstrated that Tenax TA can also be used in the form of a thin film [6,7] for the same preconcentration purposes.
ZSM-5 zeolite, initially developed by Mobil Oil Company, belongs to high-silica crystalline alumosilicates with wide application as zeolite-based catalysts and sorbents [8]. On the molecular level, ZSM-5 has a tetrahedral structure of [SiO4]4− and [AlO4]5− forming porous, three-dimensional crystals with pore sizes of 0.56×0.54 nm in the axial direction “a” and 0.55×0.51 nm in the axial direction “b” [9]. Therefore, molecules with sizes smaller than the pores of ZSM-5 can enter and be adsorbed by the zeolite with equilibrated retention. Many different application of ZSM-5 and its composites were reported in scientific literature, mainly related to the catalytic applications such as bulky conversion, isomerization of organic molecules [10,11], enzyme carriers [12] also for selective adsorbtion and filtration, for instance, recovery of alcohols from complex mixtures [13] gas separation [14], and for other relevant areas.
To the best of our knowledge, in this study, a composite film thin film of ZSM-5 and Tenax TA was employed for the first time as an extraction phase for the equilibrium-based extraction [15] and preconcentration of organic solvents from aqueous media. Films deposited via dip coating onto homemade aluminum rods (ARs) were tested in both direct and headspace (HS) extraction modes. Hydrophobic organic solvents that are common hazardous pollutants in the chemical laboratory and often the subject of environmental exposure were chosen as model analytes.
Experimental
Materials
Tenax TA beads were purchased from GL Science (cat. 1002- 31106). According to the manufacturer information on Tenax TA, a specific surface area is 35 m2/g, a density is 0.25 g/cm3, a pore volume is 2.4 cm3/g, and an average pore size is 200 nm [5].
Chloroform (purity=99%, Mw=119.38 g/mol) purchased from Tokyo Chemical Industry Co., Ltd. was used as the solvent for film preparation without additional purification. ZSM-5 powder (SiO2/Al2O3=30, Lot 110421) was purchased form JGC Catalysts and Chemicals Ltd. Methanol (99.8%, purchased from Wako Pure Chemical Industries Ltd.) was used to prepare a dispersion of ZSM- 5. All of the chemicals used to prepare the VOC solutions and their important parameters are listed in Table 1. As a stock solution, the 10 organic solvents in Table 1 were mixed in equal molar amounts (0.1 M). Acetone (99.5%, Cat.No. 01026-00), dichloromethane (99%, Cat. No. 10158-00), diethyl ether (99%, Cat.No. 14134-01), 1-pentanol (98.5%, Cat.No. 01335-00) and 1-octanol (99.5%, Cat.No. 31013-08) were purchased from Kanto Chemical Co. Hexane (97%, 17922-65) and toluene (99%, 34121-25) were purchased from Nacalai Tesque. Benzene (99.8%, 027-14525) and benzaldehyde (98%, 025-12206) were purchased from Wako Pure Chemical Industries, Ltd. Cyclohexane (99.5% Cat. No.000-19243) was purcahsed from Kishida Chemical Co. All of the compounds were used as purchased without purification.
Adsorbtion device fabrication
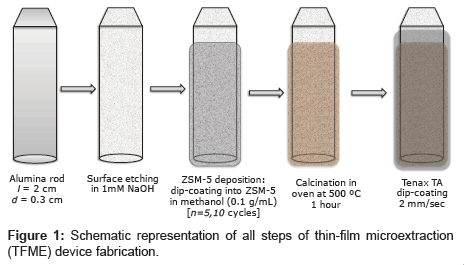
Homemade ARs with diameter d=0.3 cm and length l=2 cm were fabricated by cutting cylinders from a thick aluminium wire purchased from a local store. The rods were cleaned via sonication in ethanol (10 min) with subsequent plasma treatment (4 min). Prior to film deposition, the ARs were subjected to etching in 1 M sodium hydroxide (20 min) and then rapidly washed with pure water and dried in nitrogen gas. Thin films were deposited on the obtained cylindrical ARs, and the surface area of the rod and respectively thin film deposited on it was approximately 2 cm2.
Figure 1 schematically shows all of the steps required for fabrication of the adsortption device. ZSM-5 films were deposited in a manner similar to described in reference [16] via sequential dip coating of the AR (immersion speed: 100 mm/min, start-up time: 0 s, lifting speed: 100 mm/min) in 0.1 g/mL zeolite in a methanol solution (which was slowly stirred during deposition). The films were dried at room temperature in air between each dipping, and the operation was repeated several times (5 or 10 cycles) to prepare a thick coating. The ARs with the fine ZSM-5 coating were then placed in an electric furnace and baked at 500°C for 3 h to strenthen the zeolite film via sintering.
A 100 mM (1.89 wt%) solution of Tenax TA in chloroform was used to apply thin flat films of Tenax TA via dip coating with a nano dip-coater device (SD1, immersion speed: 2 mm/s, start-up time: 10 s, lifting speed: 2 mm/s, room temperature in air). Tenax TA coatings directly applied to theARs were used as controls. Composite films were also prepared by dip coating the ARs with ZSM layers into the same Tenax TA solution in chloroform. Before using the ARs for VOC adsorbtion, they were baked three times in a Curie Point Injector (CPI) device at 220°C to remove any residual solvent from the Tenax TA coating.
VOC sample collection
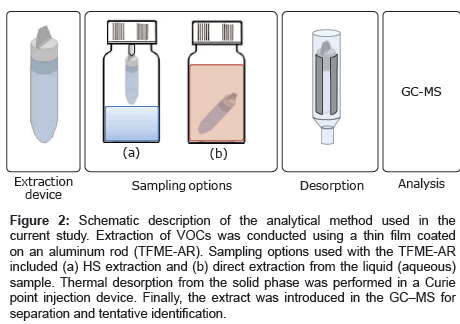
The stock solution containing 10 VOCs was diluted with water to prepare different aqueous concentrations (100 μM, 50 μM, 10 μM, 1 μM, 500 nM, 100 nM, 50 nM, 10 nM, 5 nM, 1 nM, and 500 pM), and 15 mL of each solution was placed in a 50 mL sample bottle. AR with the composite ZSM-5/Tenax TA film was attached to the bottle cap for HS extraction, as shown in Figure 2a. VOC collection was conducted at room temperature for 30 min with stirring the solution. After the collection, the AR was inserted into a quartz tube wrapped in pyrofoil, and the VOCs were thermally desorbed in the CPI by flushing with extra pure He and heating to 220°C. The gas extract was then directly introduced into the GC–MS inlet for analysis. In addition, the same adsorption device was tested in direct extraction mode. In this case, the rod was placed inside the sample at room temperature without stirring, as shown in Figure 2b. After extraction, the rod was washed with water and dried with nitrogen. Thermal desorption and analysis was conducted as described above.
Figure 2: Schematic description of the analytical method used in the current study. Extraction of VOCs was conducted using a thin film coated on an aluminum rod (TFME-AR). Sampling options used with the TFME-AR included (a) HS extraction and (b) direct extraction from the liquid (aqueous) sample. Thermal desorption from the solid phase was performed in a Curie point injection device. Finally, the extract was introduced in the GC–MS for separation and tentative identification.
GC–MS conditions
GC–MS program: the injector and GC–MS interface temperatures were maintained at 220°C and 210°C, respectively. The oven temperature program is as follows: 40°C for 1 min, increased at 3°C/ min to 105°C, further increased at 15°C/min to 200°C, maintained for 5 min (total GC program time: 34 min). He gas was used as the mobile phase at a flow-rate of 1.5 mL/min. The capillary column was AQUATIC (25% phenyl-, 75% methylpolysiloxane middle-polarity stationary phase, 60 m length, 0.25 mm inner diameter, with 1.4 μm film thickness) purchased from GL Sciences.
The temperature of the MS ion source was maintained at 200°C. The initial cut-off time for MS recording was 1 min, and the mass spectrometer was operated in electron impact (EI) ionization mode at 70 eV. Data acquisition was performed in the full scan mode from m/ z=24–220 with a scan time of 300 ms. Selected ion chromatograms with m/z=characteristic ions of each compound were used for quantitative information extraction. Compounds were identified by both retention time and comparison to the National Institute of Standards and Technology (NIST) mass spectral library using JEOL software (Version 1.0.3208.25600).
Results and Discussion
Film morphology
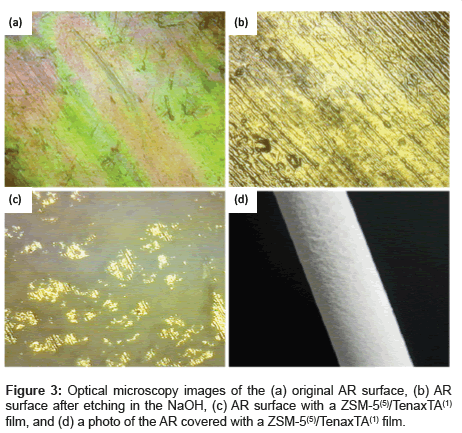
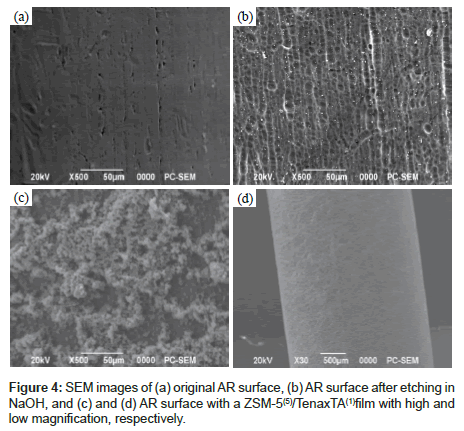
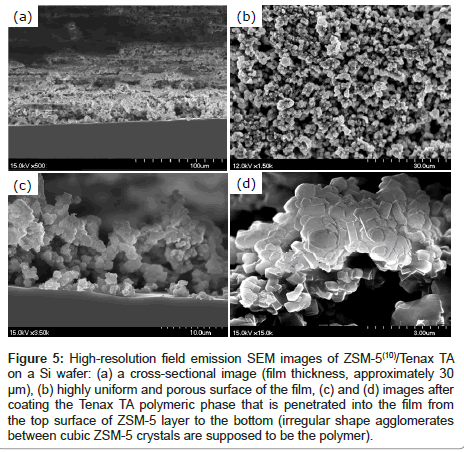
Figure 3 shows optical microscopy images of ARs surface before preparation, after etching, and with a deposited film. The unevenness that developed after etching is assumed to enhance the physical attachment of the ZSM-5 [11]. The adhesion of the ZSM-5 to the surface roughness’s of the AR for a 5-cycle ZSM-5 coating can be seen in optical microscopy and ordinary photography images in Figures 3c and 3d, respectively. Figure 4 shows analogous scanning electron microscopy (SEM) images of ARs before treatment, after etching, and with a deposited film. As can be seen in Figures 4c and 4d, the ZSM-5 is quite uniformly attached to the surface, and a very rough and macroporous surface is obtained. A finished device with a ZSM- 5(5)/Tenax TA(1) film is shown in Figure 4d; however, the Tenax TA coating cannot be seen because the polymer readily penetrates into the ZSM-5 coating and appears to cover the ZSM-5 powder nanoparticles with a nanoscale polymeric layer. Figure 5 shows high-resolution field emission SEM images of a ZSM-5/Tenax TA film on a Si wafer. In Figure 5a, it can be seen that the surface of the Si wafer is uneven, which suggests the presence of the Tenax TA film, considering the typical flat surface of silicon wafers is expected. The surface of the entire film can be seen in Figure 5b; the thickness of the film is approximately 30 μm, and there are numerous irregularities at the nanoscale level. Notably, in Figures 5c and 5d, it can be seen that the quasi-cubic ZSM-5 crystals are covered with the Tenax TA film.
Figure 5: High-resolution field emission SEM images of ZSM-5(10)/Tenax TA on a Si wafer: (a) a cross-sectional image (film thickness, approximately 30 μm), (b) highly uniform and porous surface of the film, (c) and (d) images after coating the Tenax TA polymeric phase that is penetrated into the film from the top surface of ZSM-5 layer to the bottom (irregular shape agglomerates between cubic ZSM-5 crystals are supposed to be the polymer).
Influence of the preparation parameters on the film thickness and mass
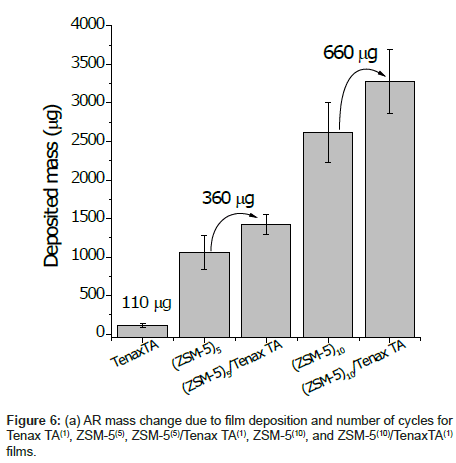
The amount of deposited ZSM-5 is also significantly important to increase the amount of absorbed Tenax TA. Figure 6 shows the increase in weight of the coating after deposition of ZSM-5 or Tenax TA alone and when both were applied. As can be seen from the figure, when the film was prepared without ZSM-5, the amount of Tenax TA deposited after one dip-coating cycle was 110 μg, but when 5- and 10-cycle ZSM-5 coatings were first deposited, the amount of Tenax TA employed by the same dip coating was 330 and 660 μg, respectively. Thus, the thicker the zeolite coating, the bigger Tenax TA loading in the composite can be achieved. This result indicates that more polymers can be loaded onto a thicker macroporous ZSM-5 coating because the polymer adheres not only to the surface but also penetrates into the pores of the entire ZSM-5 coating.
Analytical performance of the TFME device
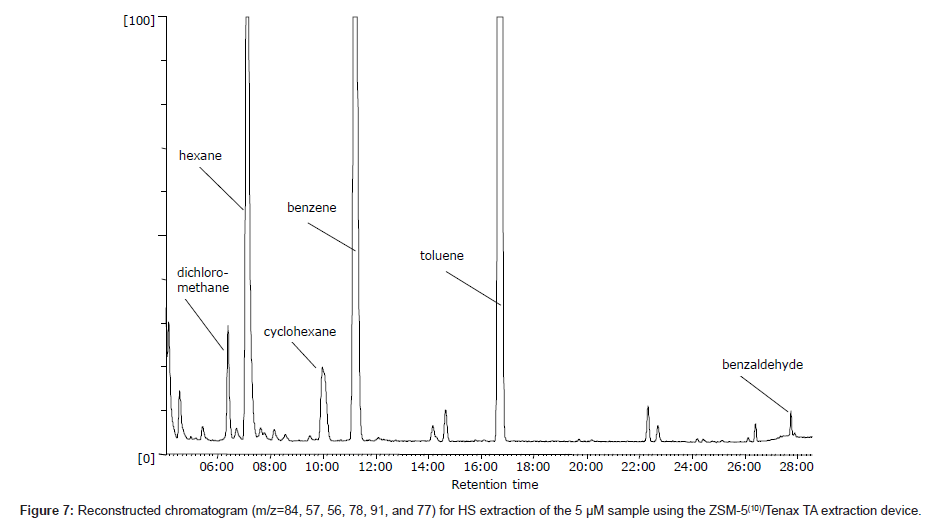
Ten VOCs were evaluated to determine the possibility of using the fabricated adsorption device for extraction. First, chromatographic separation of all of the compounds was confirmed via direct injection into the GC. Next, it was determined what of the compounds could be detected at low concentrations in aqueous solutions. Figure 7 shows a fragment of the reconstructed ion chromatogram obtained after HS extraction of a 5 μM aqueous solution of the ten VOCs listed in Table 1. As can be seen in the figure, six compounds were clearly detected at this concentration. It should be noted that all of the compounds were highly hydrophobic. Non-hydrophobic compounds (ethyl ether, acetone, and 1-pentanol) were not detected effectively because of their high mobility inside the column used and partially good solubility in water. Zeolites generally operate by two possible adsorption mechanisms: physical adsorption into the pores and electrostatic adsorption due to the negative charge on the zeolite. For the selected compounds, the only possible mechanism is adsorption into zeolite pores; however, in the presence of Tenax TA, adsorption by the polymer is also possible. Therefore, it is believed that the combination of ZSM-5 with Tenax TA enhances the adsorption because the polymer creates a protective layer and helps retain the VOCs in the ZSM-5 pores.
| # | VOC | MW | Density | b.p. | Water solubility | Polarity index* | m/z | Retention time |
|---|---|---|---|---|---|---|---|---|
| g/mol | g/mL | °C | wt.% (at 25°C) | min:s | ||||
| 1 | Acetone | 58.08 | 0.7844 | 56.067 | infinite | 0.355 | 58 | 05:27 |
| 2 | Hexane | 86.117 | 0.65484 | 68.736 | 0.00123 | 0.009 | 86 | 07:07 |
| 3 | Cyclohexane | 84.161 | 0.77389 | 80.73 | 0.010 | 0.006 | 56 | 09:53 |
| 4 | Dichloromethane | 84.933 | 1.31678 | 39.64 | 1.30 | 0.309 | 84 | 06:22 |
| 5 | Diethyl ether | 74.122 | 0.70782 | 34.431 | 6.04 | 0.117 | 74 | 05:05 |
| 6 | Benzene | 78.113 | 0.8736 | 80.094 | 0.1791 | 0.111 | 78 | 11:10 |
| 7 | Toluene | 92.14 | 0.86219 | 110.63 | 0.0515 | 0.099 | 91 | 16:41 |
| 8 | Benzaldehyde | 106.124 | 1.04013 | 178.75 | 0.3 | n.a. | 106 | 27:45 |
| 9 | 1-Pentanol | 88.149 | 0.8108 | 137.983 | 2.19 | 0.568 | 55 | 27:46 |
| 10 | 1-Octanol | 130.23 | 0.82157 | 195.156 | 0.0538 | 0.537 | 55 | 28:35 |
*Relative polarity index values were extracted from Christian Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Publishers, 3rd ed., 2003
Table 1: List of VOCs tested in the adsorption experiment.
Comparison of the GC–MS performance of ZSM/Tenax TA and Tenax TA films
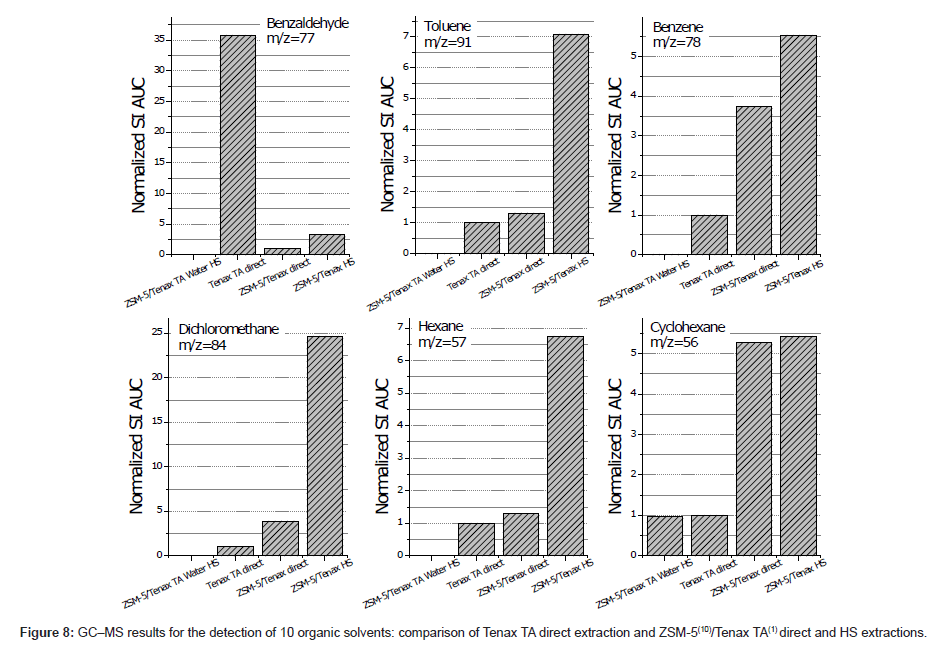
GC–MS analysis results for ZSM-5/Tenax TA-coated ARs were compared with those for ARs with only a Tenax TA coating. It was found that in direct extraction mode, when with the ZSM-5/Tenax TA system was more effective for dichloromethane, hexane, cyclohexane, benzene and toluene than that with the Tenax TA-coated AR device. However, the adsorption of diethyl ether, acetone, 1-pentanol, 1-octanol, and benzaldehyde was reduced for the hybrid device. Therefore, it can be concluded that the fabricated material possesses a certain level of chemical selectivity regarding the tested VOCs. In addition, the adsorption phase was observed to be highly hydrophobic, and as a result, adsorption from the HS was even greater as indicated by an increase in the peak areas in the GC–MS chromatograms for the mentioned compounds. Using the ratios of the peak areas obtained from the ZSM-5/Tenax TA and Tenax TA direct extractions, the relative adsorption factor for each compound was calculated, and the results are presented in Figure 8. In particular, an increase of 24.7, 6.7, and 7.1 with the ZSM-5/Tenax TA hybrid thin film was observed for CHCl3, C6H14, and C6H6, respectively.
The GC–MS results for the AR with only a ZSM-5 film also provided interesting information. Namely, other compounds were observed in the gas chromatogram (e.g., the small peaks in Figure 7) that could not be assigned as contaminants in the zeolite or Tenax TA or impurities in the sample matrix. Specifically, various hydrocarbons, including both saturated chains from C4 (e.g., butane) to C12 (e.g., methyl undecane) and benzene derivatives, were detected. However, this finding was relevant only when the concentration of VOCs in the sample was relatively high, i.e., the peaks significantly increased both qualitatively and quantitatively. Zeolite is known as an effective thermal catalyst, and it is therefore believed that the catalytic activity of ZSM-5 leads to the thermal degradation (transformation) of adsorbed compounds.
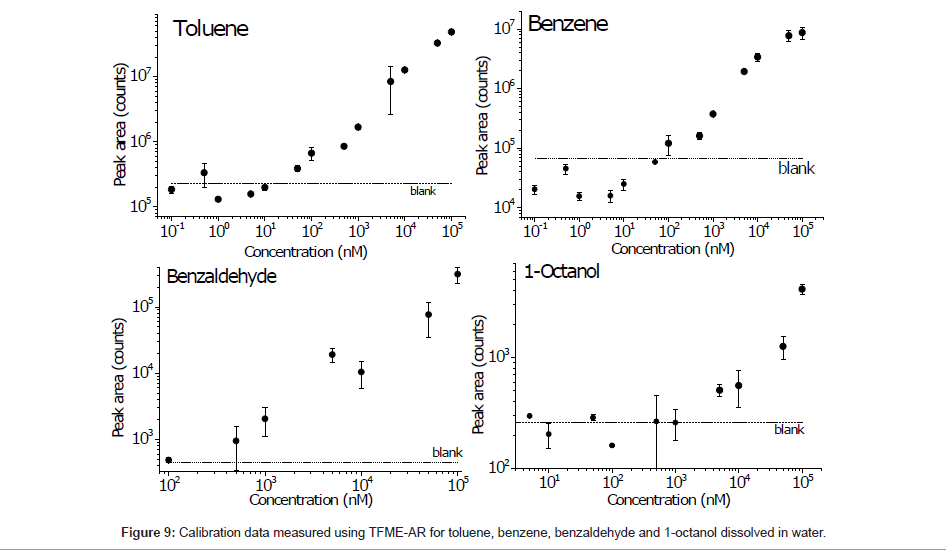
Finally, the calibration data for compounds that were well detected using the described method are shown in Figure 9. Using the peak areas, the limits of detection (LODs) were calculated from the calibration curve cross section for a blank signal, and the highest LODs were found for benzene and toluene (151 and 146 nM, respectively). This result suggests that the ZSM-5 pores are particularly favorable for loading these compounds and thus exhibit selective adsorption.
Conclusions
SPME has proven to be effective for many analytical tasks, particularly those related to the VOC analysis of environmental samples. However, the conventional geometry of the SPME devices in the form of thin glass fibers with deposited sorptive coatings has certain drawbacks. Patented technology is not yet sufficiently cheap or robust to be applicable for harsh matrices, and single-use options have not yet been demonstrated. In addition, new materials are needed to extend the applicability of sorptive devices to a larger array of analytes. In this study, it was demonstrated that a gas sorptive coating can be readily applied onto a cheap aluminum substrate. The combined use of ZSM- 5 zeolite with Tenax TA further improves the sensitivity of the Tenax TA for thin-film extraction. This was achieved by the extraction phase volume increase not altered diffusion because of the high porosity of the ZSM-5. As shown on the example of model VOCs, it is possible to preconcentrate and detect certain compounds present in aqueous matrices at very low concentrations, meeting the needs of real-life applications.
Acknowledgement
This work was supported by a grant-in-aid for scientific research (B) (25293429) from the Japan Society for the Promotion of Science. Shinkou Seiki Co. Ltd. also supported us financially as a special research project.
References
- Pawliszyn J (2002) Sampling and sample preparation for field and laboratory: Fundamental and new directions in sample preparation. Volume 37, Elsevier: Amsterdam.
- Pawliszyn J (1997) Solid-Phase Microextraction: Theory and Practice. Wiley-VCH: New York.
- Bruheim I, Liu X, Pawliszyn J (2003) Thin-film microextraction. Anal Chem 75: 1002-1010.
- Dettmer K, Engewald W (2002) Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Anal Bioanal Chem 373: 490-500.
- SIS Catalog (2008) Adsorbent Resins for Trapping Volatiles. Ringoes, NJ, USA: Scientific Instrument Services, SIS Catalog: C10–C11.
- Alfeeli B, Hogg D, Agah M (2010) In Solid-phase microextraction using silica fibers coated with tenax-TA films. 5: 1152-1155.
- Alfeeli B, Taylor LT, Agah M (2010) Evaluation of Tenax TA thin films as adsorbent material for micro preconcentration applications. Microchemical Journal 95: 259-267.
- Cejka J, van Bekkum H (2005) Zeolites and Ordered Mesoporous Materials: Progress and Prospects: the 1st FEZA School on Zeolites, Prague, Czech Republic. Volume 157, Elsevier.
- Kokotailo GT, Lawton SL, Olson DH, Meier WM (1978) Structure of synthetic zeolite ZSM-5. Nature 272: 437-438.
- Dedecek J, Sobalík Z, Wichterlová B (2012) Siting and Distribution of Framework Aluminium Atoms in Silicon-Rich Zeolites and Impact on Catalysis. Catalysis Reviews - Science and Engineering 54: 135-223.
- Tukur NM, Al-Khattaf S (2011) Comparison studies of xylene isomerization and disproportionation reactions between SSZ-33, TNU-9, mordenite and ZSM-5 zeolite catalysts. Chemical Engineering Journal 166: 348-357.
- Mitchell S, Pérez-Ramírez J (2011) Mesoporous zeolites as enzyme carriers: Synthesis, characterization, and application in biocatalysis. Catalysis Today 168: 28-37.
- Saravanan V, Waijers DA, Ziari M, Noordermeer MA (2010) Recovery of 1-butanol from aqueous solutions using zeolite ZSM-5 with a high Si/Al ratio; suitability of a column process for industrial applications. Biochemical Engineering Journal 49: 33-39.
- Ohlin L, Bazin P, Thibault-Starzyk F, Hedlund J, Grahn M (2013) Adsorption of CO2, CH4, and H2O in zeolite ZSM-5 studied using in situ ATR-FTIR spectroscopy. Journal of Physical Chemistry C 117: 16972-16982.
- Ouyang G, Pawliszyn J (2008) A critical review in calibration methods for solid-phase microextraction. Anal Chim Acta 627: 184-197.
- Chang N, Gu ZY, Wang HF, Yan XP (2011) Metal-organic-framework-based tandem molecular sieves as a dual platform for selective microextraction and high-resolution gas chromatographic separation of n-alkanes in complexes. Anal Chem 83: 7094-7101.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18262
- [From(publication date):
specialissue-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13694
- PDF downloads : 4568