Research Article Open Access
Development and Validation of Spectrophotometric Method for Determination of Finasteride in Pharmaceutical Formulation Using 1,2-Naphthoquine-4-sulfonate (NQS)
Sally Mohammed Ahmed and Abdalla Ahmed Elbashir*
University of Khartoum, Faculty of Science, Chemistry Department, Khartoum, Sudan
- *Corresponding Author:
- Abdalla Ahmed Elbashir
University of Khartoum, Faculty of Science
Chemistry Department
Khartoum, Sudan
Tel: 5661431-431
E-mail: aaelbashir@uofk.edu
Received date: May 04, 2015; Accepted date: May 22, 2015; Published date: May 29, 2015
Citation: Ahmed SM and Elbashir AA (2015) Development and Validation of Spectrophotometric Method for Determination of Finasteride in Pharmaceutical Formulation Using 1,2-Naphthoquine-4-sulfonate (NQS). J Anal Bioanal Tech 6:247. doi: 10.4172/2155-9872.1000248
Copyright: © 2015 Ahmed SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid, simple and sensitive method for the determination of finasteride using sodium 1,2-naphthoquine-4- sulfonate (NQS) has been developed. The method is based on the fact that a brown product can be formed by the reaction between finasteride and sodium NQS in a buffer solution of a pH 13.0. Beer’s law is obeyed in the range 2-14 μg/ml of finasteride at maximum wavelength of 447 nm. The linear regression equation of the calibration curve is y=0.062857x+0.069857, with a linear regression correlation coefficient of 0.999. The limit of detection (LOD) and limit of quantification (LOQ) were found to be 0.03 and 0.09 μg/ml, respectively. The method has been successfully applied to the determination of finasteride in pharmaceutical formulation. The results were in good agreement with those obtained with the official high performance liquid chromatography (HPLC) method. The proposed method is useful for routine analysis of finasteride in quality control laboratories.
Keywords
Finasteride; Spectrophotometric; Pharmaceutical formulation; Method validation; Sodium 1,2-naphthoquine-4- sulfonate (NQS)
Introduction
Finasteride is a synthetic drug for the treatment of benign prostatic hyperplasia (BPH) in low doses and in protest cancer in higher doses. It’s chemically known as N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4- azaandrost-1-ene-17-carboxamide is an antiandrogen which acts by inhibiting 5α-reductase, the enzyme that converts testosterone to dihydrotestosterone (DHT) [1]. Finasteride is widely used for the treatment of benign prostatic hyperplasia (BPH), prostate cancer, and androgenetic alopecia [2-8]. A daily dose of 5 mg has been used for the treatment of BPH and prostate cancer, and a 1 mg dose has been used for the treatment of androgenetic alopecia.
Literature survey reveals that various methods have been reported for the analysis of finasteride in pharmaceutical preparations and biological samples such as gas-chromatography (GC) [9], and GCMS [10] high performance liquid chromatography (HPLC) with various detectors [11-13], micellar electrokinetic chromatography [14] polarography [15], voltammetry [16] and spectrophotometry [17,18]. Different official methods were described in Pharmacopoeias for analysis of finasteride in raw materials and tablets United States [19], European [20], and British [21]. However, some of these methods take longer than 40 min for the chromatographic run.
Spectrophotometry is considered the most convenient analytical technique, because of its inherent simplicity, low cost, and wide availability in most quality control laboratories. Only few spectrophotometric methods were reported for determination of finasteride in pharmaceutical formulation [17,18]. However these methods were associated with some major drawbacks such as laborious multiple extraction steps in the analysis by ion-pair based methods [18].
NQS has been used as a color-developing reagent in the spectrophotometric determination of many pharmaceutical amines [22-34]. In depth review on the applications of NQS for determination of pharmaceutical bearing amine group have been reported by Elbashir et al. [24]. The reaction between NQS and finasteride has not been investigated yet. Therefore, the present study was devoted to investigate the reaction between NQS and finasteride, and use this color reaction in the development of simple rapid spectrophotometric method for determination of finasteride in its dosage form.
Experimental
Apparatus
Absorbance was carried out by using UV-visible spectrophotometer model Shimadzu 1800 with quartz cells of 1 cm optical path length, pH meter was used for pH measurements, analytical balance and ultrasonic bath.
Material and reagent
Finasteride, (Azal pharmaceutical industries company Ltd, Khartoum, Sudan) were obtained and used as received; its purity was 99.15%. A solution of sodium-1,2-naphthoquinone-4-sulfonate (NQS) 0.3% (w/v) was prepared by dissolving 0.3 g in distilled water, transferred into a 100 ml volumetric flask and diluted to the mark with distilled water and mixed well. The solution was freshly prepared and protected from light during use; buffer solution of pH 13.0 was prepared by solution of 0.1M disodium hydrogen phosphate adjusted to pH 13.0 with 1M sodium hydroxide. All other chemicals were of analytical grade.
Preparation of standard and sample solution
Stock standard solution of finasteride (400 μg/ml): An accurately 20 mg of finasteride standard was dissolved in a mixture of equal volume of methanol and distilled water, transferred into 50 ml volumetric flask, diluted to the mark with same solvent and mixed well. This stock solution was further diluted to obtain working solutions in the range of 2-14 μg/ml.
Sample solution: Preparation of finasteride tablets by preparing in a local compounding pharmacy the mixing of 20 mg of finasteride and 600 mg of the placebo compounds, all in a solid state, dissolved by sonication in a mixture of equal volume of methanol and distilled water into a 50 ml volumetric flask, diluted to the mark with the same solvent (400 μg/ml), diluted this solution to obtain concentration 100 μg/ml.
Procedure: A 1.0 ml of 100 μg/ml of finasteride was transferred into 10 ml volumetric flask, 1.0 ml of 0.3% sodium-1,2-naphthoquinone-4- sulfonate was added and followed by 1.0 ml pH 13.0 Na2HPO4 buffer solution. The reaction was completed to volume with distilled water, and the resulting solution was measured at 447 nm against reagent blank treated similarly.
Job’s method: The Job’s method of continuous variation was employed. Master equimolar (1 × 10-3 M) aqueous solution of finasteride and NQS were prepared. Series of 10 ml portions of the master solution of finasteride and NQS were made up comprising different complementary proportions (1:9,…9:1, inclusive) in 10 ml volumetric flask containing 1 ml of buffer solution (pH=13.0). The solution was further manipulated as described under the general recommended procedures.
Results and Discussion
Absorption spectra
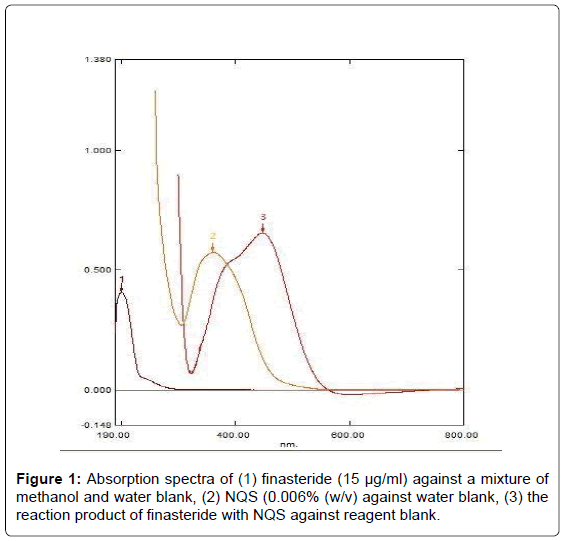
The absorption spectrum of finasteride was recorded against water (Figure 1), it was found that finasteride exhibits a maximum absorption peak (λmax) at 201 nm. Because of highly blue shifted λmax of finasteride, its determination in the dosage form based on the direct measurement of its absorption for ultraviolet is susceptible to potential interferences from the common excipients. Therefore, derivatization of finasteride red-shifted light-absorbing derivative was necessary. The reaction between finasteride and NQS was performed, and the absorption spectrum of the product was recorded against reagent blank (Figure 1). It was found that the product is brown colored exhibiting λmax at 447 nm, and the λmax of NQS was 361 nm. The λmax of finasteride-NQS derivative was red-shifted, eliminating any potential interference. Therefore, the measurements were carried out at 447 nm.
Optimization of the reaction conditions
The optimum conditions for the development of method were established by varying the parameters one at a time while keeping the others fixed and observing the effect produced on the absorbance of the colored product. In order to establish experimental conditions, the effect of various parameters such as pH, time, buffer volume and concentration of NQS were investigated.
Effect of pH
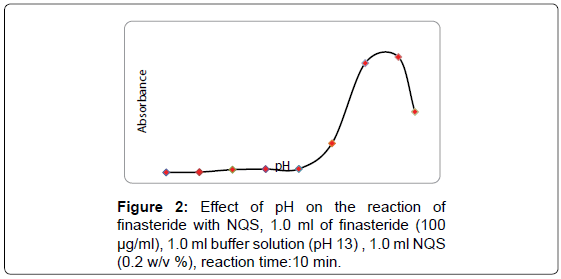
The effect of pH on the reaction between finasteride and NQS was examined by varying pH form 6.0 to 13.5. As shown in Figure 2, the absorbance of the product is low at pH 6.0, indicate that finasteride has difficulty to react with (NQS) in slightly acidic media. This was possibly due to the existence of the amino group of finasteride in the form of hydrochloride salt, thus it loses its nucleophilic substitution capability. As the pH increased from 10–13, the readings increased rapidly, as the amino group of finasteride turns into the free amino group, thus facilitating the nucleophilic substitution. The maximum readings were attained at pH values of 13.0. At pH values more than 13.0 decrease in the readings occurred. This was attributed probably to the increase in the amount of hydroxide ion that holds back the reaction of finasteride with NQS. Reaction of NQS with compound bearing primary amines at pH 13.0 was reported [35].
Effect of time
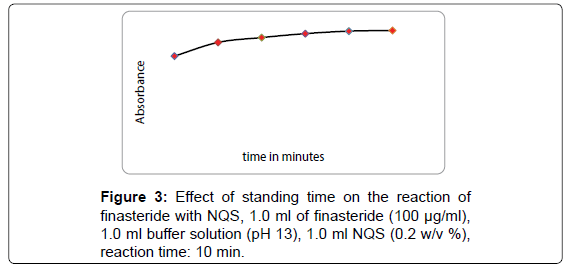
The absorbance of the reaction product was determined at different time (Figure 3). Keeping other conditions unchanged, the absorbance of the reaction product was measured after standing for different time periods at 25°C. The results show that finasteride react with NQS at 25°C and the absorbance begins to increase instantly and becomes constant after 10 min. Furthermore, it is also observed that the absorbance remain constant for 30 min.
Effect of amount of the buffer
Keeping pH at 13.0, the effect of amount of buffer solution on the absorbance of reaction product was also studied. It shows that the absorbance of the reaction product enhances rapidly with the rise of amount of buffer solution, and becomes maximal when the amount of buffer solution is 1.0 ml. Therefore, the amount of 1.0 ml buffer solution was selected to ensure the highest absorbance.
Effect of NQS concentration
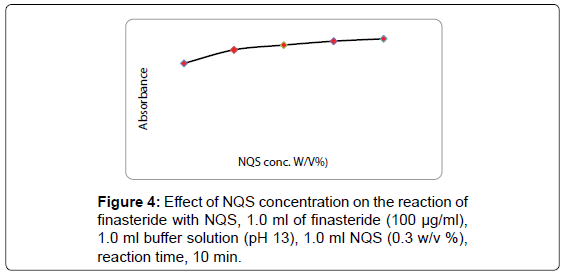
The studying of NQS concentrations revealed that the reaction was dependent on NQS reagent. The highest absorption intensity was attained at NQS concentration of 0.3% (w/v), and higher concentration of NQS (0.5%, w/v) had no effect on the absorption values, as shown in Figure 4.
From the above experiments, the optimized conditions used for the assay were: pH 13.0, NQS concentration 0.3% w/v, volume of the buffer 1.0 ml, reaction time 10 min and temperature 25°C.
Validation of method
The method was validated for the following parameters: specificity, linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), and robustness according to the International Conference on Harmonization (ICH) guidelines [35].
Linearity, limit of detection (LOD) limit of quantification (LOQ)
The linearity was evaluated by linear regression analysis determined by constructing seven concentrations of finasteride, in the range of 2-14 μg/ ml, which was calculated by the least square regression method to calculate the calibration equation and the correlation coefficient. The calibration curves were constructed by plotting concentration versus absorbance, using linear regression analysis. The regression equation for the results was y=0.062857x+0.0698571 (r2=0.999), where y is the absorbance at 447 nm, x is the concentration of finasteride in μg/ml in the range of 2-14 μg/ml, and r is correlation coefficient (Table 1). The limit of detection (LOD) and limit of quantification (LOQ) were determined according to the following formula LOD=3.3 × SDa/b, and LOQ=10 × SDa/b, SDa is the standard deviation of the blank, b is the slope under the ICH guidelines. The LOD and LOQ were found to be 0.03 and 0.09 μg/ml, respectively (Table 1).
| Parameter | Value |
|---|---|
| Measurement wavelength (nm) | 447 |
| Linear range ( μg/mL) | 2-14 |
| Intercept | 0.069857 |
| Standard deviation of the Blank | 0.0006 |
| Slope | 0.062857 |
| Correlation coefficient (r) | 0.999 |
| Limit of detection, LOD ( µg/mL) | 0.03 |
| Limit of quant., LOQ ( µg/mL) | 0.09 |
Table 1: Parameters for the performance of the proposed method.
Accuracy
The accuracy of the proposed method was carried out by applying 3 different concentrations 3, 7, and 11 μg/ml of finasteride drug within linear range calculated as the percentage of the drug recovered from the samples (Table 2).
| Sample No | Sample content (µg/mL) | Finasteride amount | Amount found | Recovery |
|---|---|---|---|---|
| 1 | 3 | 3 | 3.01 | 100.18 |
| 2 | 7 | 7 | 6.96 | 99.37 |
| 3 | 11 | 11 | 11.01 | 100.08 |
Table 2: Recovery studies for the determination of finasteride, by the proposed method.
Robustness
Robustness was examined by evaluating the influence of small variation in the method variables on its analytical performance. In these experiments, one parameter was changed whereas the others were kept unchanged, and the recovery percentage was calculated each time. It was found that small variation in the method variables did not significantly affect the procedures; recovery values were recorded in Table 3. This indicated the reliability of the proposed method to routine application for the analysis of finasteride.
| Recommended conditions | 101.02 |
|---|---|
| NQS concentration (%, w/v) 0.28 | 103.48 |
| NQS concentration (%, w/v) 0.32 | 99.90 |
| Buffer solution (pH) 12.8 | 101.78 |
| Buffer solution (pH) 13.2 | 105.33 |
| Reaction time (min) 8 | 99.21 |
| Reaction time (min) 12 | 100.59 |
Table 3: Influence of small variation in the assay condition on the analytical performance of the proposed spectrophotometric method for determination of finasteride using NQS reagent.
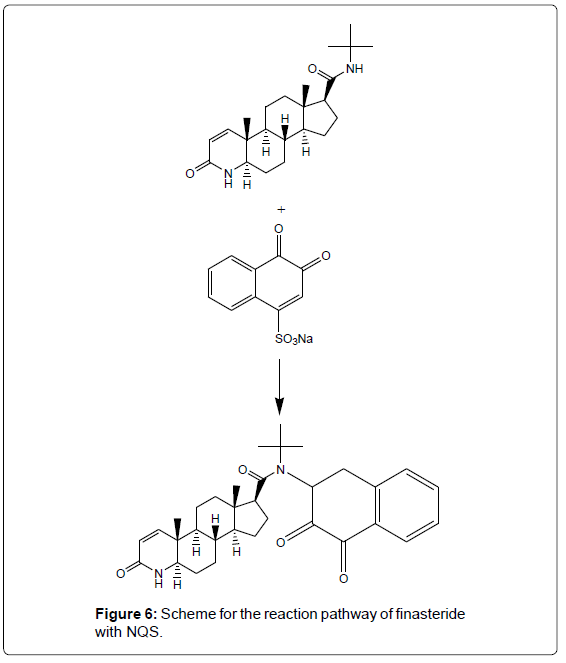
Reaction mechanism
It has been reported that NQS could react with amino group of Secondary amine derivative. Similarly, amino group of finasteride, taking on nucleophilicity due lone electron pair of nitrogen atom, trend to attack on the electron–deficient center in NQS, namely no.4 carbon atom (3,4-C=C carbon bond conjugate with 2-C=O, as a result 4-C of NQS becomes electron lacking center). At the same time, it has been proved that the composition of product I is 1:1 of finasteride and NQS (Figure 5). So it is concluded that amino group of finasteride react with 4-sodium sulphonate of NQS molecule respectively, to form brown N-alkyl-amino-naphthoquinone. The reaction equation is shown in Figure 6.
Application of the proposed method to analysis of finasteride dosage form
Finasteride tablets were subjected to the analysis by the proposed as well as with the official HPLC method (United States pharmacopeia) and the obtained results were statistically compared with each other. The label claim percentage was 101.02 ± 1.69 and 102.92 ± 1.18 for batch 5-06105 and batch 5-06107 respectively (Table 4). With respect to t- and F-tests, no significant differences were found between the calculated and theoretical values of both the proposed and the reported methods at 95% confidence level. This indicated similar accuracy and precision in the analysis of finasteride in tablets. The proposed method has the advantage of being virtually free from interferences by excipients.
| Dosage form | Recovery % +RSD (n =5) | t-value | f-value | |
|---|---|---|---|---|
| Proposed | Official | |||
| Prostasafe batch 5-06105 | 101.02 ± 1.69 | 100.17 ± 1.048 | 0.67 | 2.60 |
| Prostasafe batch 5-06107 | 102.92 ± 1.18 | 101.64 ± 0.80 | 2.43 | 3.77 |
Table 4: Analysis of finasteride containing dosage forms by the proposed and official HPLC methods (United States Pharmacopoeia).
Conclusion
The present paper described the evaluation of NQS as analytical reagents in the development of simple, sensitive, and accurate spectrophotometric methods, for the determination of finasteride in pharmaceutical formulation. The proposed method is simple, reliable, specific, accurate, reproducible, and highly sensitive, for the determination of finasteride in commercially available dosage forms. The procedure presented here does not need necessitate any expensive apparatus; therefore the proposed method can be used advantageously as a routine method for the determination of finasteride in quality control and industry, our method may be applied to the determination of other secondary amine derivatives as well.
References
- (2005) Current Index of Medical Specialities(CIMS),updated prescriber’s Hand Book.CMP Media India Pvt Ltd., Bangalore, India,p. 288.
- Schmidt LJ,Tindall DJ (2011) Steroid 5α-reductase inhibitors targeting BPH and prostate cancer. J Steroid BiochemMolBiol 125: 32-38.
- Cha EK, Shariat SF (2011) The use of 5α-reductase inhibitors for the prevention and treatment of prostate cancer.EurUrol 59: 515-517.
- Lucas JK (1995) Finasteride in the treatment of hirsutism. JWomens Health 4: 655-661.
- Mella JM,Perret MC, Manzotti M, Catalano HN, Guyatt G (2010) Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 146: 1141-1150.
- Tully AS,Schwartzenberger J, Studdiford J (2010) Androgenic Alopecia. JMen’s Health 7: 270-277.
- Yamazaki M,Miyakura T, Uchiyama M, Hobo A, Irisawa R, et al. (2011) Oral finasteride improved the quality of life of androgenetic alopecia patients. J Dermatol 38: 773-777.
- Almeida HM, Marques CH (2011) Physicochemical characterization of finasteride:PEG 6000 and finasteride:Kollidon K25 solid dispersions, and finasteride: β-cyclodextrin inclusion complexes. J InclPhenom Macro 70: 397-406.
- Saglik S, Tatar Ulu S (2006) Development and validation of a new gas flame ionization detector method for the determination of finasteride in tablets. Anal Biochem 352: 260-264.
- Guarna A, Danza G, Bartolucci G, Marrucci A, Dini S, et al. (1995) Synthesis of 5,6,6-[2H3]finasteride and quantitative determination of finasteride in human plasma at picogram level by an isotope-dilution mass spectrometric method. J Chromatogr B Biomed Appl 674: 197-204.
- Constanzer ML, Chavez CM, Matuszewski BK (1994) Picogram determination of finasteride in human plasma and semen by high-performance liquid chromatography with atmospheric-pressure chemical-ionization tandem mass spectrometry.J Chromatogr B Biomed Appl658: 281-287.
- Matuszewski BK,Constanzer ML, Chavez-Eng CM (1998) Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem 70: 882-889.
- Demir H, Cucu A, Sakarya S (2006) Determination of finasteride in the tablet form by liquid chromatography and its analytical method validation. Anal Chim Acta 557: 252-255.
- Caglayan MG, Palabiyik IM, Onur F (2013) Stability-indicating micellarelektrokinetic chromatography method for determination of finasteride in pharmaceutical preparations. Journal of Liquid Chromatography and Related Technologies 36: 781-791.
- Amer SM (2003) Polarographic behavior and determination of finasteride. Farmaco 58: 159-163.
- Alvarez-Lueje A, Brain-Isasi S, Núñez-Vergara LJ, Squella JA (2008) Voltammetric reduction of finasteride at mercury electrode and its determination in tablets. Talanta 75: 691-696.
- Ilango K, Valentina P, Lakshmi KS (2002) Spectrophotometric determination of Finasteride in tablet formulation. Indian J Pharm Sci 64: 174-175.
- Ulu ST (2007) A new spectrophotometric method for the determination of finasteride in tablets. SpectrochimActa A MolBiomolSpectrosc 67: 778-783.
- The United States Pharmacopeia Commision (2011) The United States Pharmacopeia (USP) (34thedn.) The United StatesPharmacopeial Convention, Rockville, MD, USA, pp. 2833.
- European Pharmacopoeia Commission (2008)European Pharmacopoeia sixth edition, Council of Europe, Stuttgart, Germany, p. 1891.
- British Pharmacopoeia Commission (2009) The British Pharmacopoeia, volume I, The Stationary Office Ltd., London, England, p. 852.
- Hasani M,Yaghoubi L, Abdollahi H (2007) A kinetic spectrophotometric method for simultaneous determination of glycine and lysine by artificial neural networks. Anal Biochem 365: 74-81.
- Elbashir AA, Elwagee AHE (2012) Spectrophotometric determination of pyrimethamine (PYM) in pharmaceutical formulation using 1,2-naphthoquinone-4-sulfonate (NQS). Journal of the Association of Arab Universities for Basic and Applied Sciences 11: 32-36.
- Elbashir AA, Abir AA, Shazalia MAA, Aboul-Enein HY (2012) 1,2-naphthoquinone-4-sulphonic acid sodium salt (NQS) as an analytical reagent for the determination of pharmaceutical amine by spectrophotometry. Spectroscopy Review 47: 219-232.
- Li QM, Yang ZJ (2007) Spectrophotometric determination of aminomethylbenzoic acid using sodium 1,2-naphthoquinone-4-sulfonate as the chemical derivative chromogenic reagent.SpectrochimActa A MolBiomolSpectrosc60: 656-661.
- Xu L, Wang H, Xiao Y (2004) Spectrophotometric determination of ampicillin sodium in pharmaceutical products using sodium, 2-naphthoquinone-4-sulfonic as the chromogentic reagent. SpectrochimActa A MolBiomolSpectrosc 60: 3007-3012.
- EbraheemSAM, Elbashir AA, Abou-Enein HY (2011) Spectrophotometric methods for the determination of gemifloxacin in pharmaceutical formulations. ActaPharmaSinica 4: 248-253.
- Ahmed SMA, Elbashir AA, Aboul-Enein HY (2015) New spectrophotometric method for determination of cephalosporins in pharmaceutical formulations. Arabian Journal of Chemistry 8: 233-239.
- Elbashir AA, Ahmed SM, Aboul-Enein HY (2012) Newspectrofluorimetric method for determination of cephalosporins in pharmaceutical formulations. J Fluoresc 22: 857-864.
- Elbashir AA, Awad SF (2013) A New Spectrophotometric Method for Determination of Penicillamine in Pharmaceutical Formulation using 1,2-naphthoquine-4-sulfonate (NQS). J Pharmacovigilance 1:105.
- Elbashir AA,Ebraheem SA, Elwagee AH, Aboul-Enein HY (2013) New spectrophotometric methods for the determination of moxifloxacin in pharmaceutical formulations. Acta Chim Slov 60: 159-165.
- AMN Altigani, AA Elbashir (2014) Spectrophotometric Method for Determination of Primaquine in Pharmaceutical Formulations via Derivatization with, 2-Naphthoquinone-4-Sulfonate. Austin J Anal Pharm Chem 1: 1019.
- Abdalla FAA, Elbashir AA (2014) Development and Validation of Spectrophotometric Methods for the Determination of Mesalazine in Pharmaceutical Formulation. Med Chem 4:361-366.
- Li Q, Zhang H (2008) A novel spectrophotometric method for the determination of aminophylline in pharmaceutical samples in the presence of methanol. SpectrochimActa A MolBiomolSpectrosc 70: 284-289.
- ICH Expert Working Group (2005) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Validation of analytical procedures: Text and methodology Q2 (R1).
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 21160
- [From(publication date):
June-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 16454
- PDF downloads : 4706