Development and Validation of RP-UHPLC Method for Determination of Sertraline in Bulk Drug and Dosage Form
Received: 27-Jun-2022 / Manuscript No. jabt-22-70526 / Editor assigned: 29-Jun-2022 / PreQC No. jabt-22-70526(PQ) / Reviewed: 13-Jul-2022 / QC No. jabt-22-70526 / Revised: 17-Jul-2022 / Manuscript No. jabt-22-70526(R) / Published Date: 24-Jul-2022 DOI: 10.4172/2155-9872.1000469
Abstract
Objective: The new, rapid, sensitive, simple, precise and accurate Reversed- Phase Ultra High Performance Liquid Chromatography (RP-UHPLC) method was developed and validated for determination of Sertraline in bulk drug and Pharmaceutical dosage form.
Methods: The UV Spectrum of Sertraline in diluent showed maximum wavelength at 273nm. In RP-UHPLC method separation achieved by Agilent C18 (75mm×3.9mm, 2μm particle size) column using Acetonitrile :( 0.1%OPA) Water (80:20v/v) as mobile phase at flow rate 0.7ml/min. Injection volume is 20μl. RP-UHPLC detection carried out at 273nm.
Results: In RP-UHPLC method retention time was found to be 3.75min. The Calibration curve was found to be linear (r2=0.999) with concentration range of 10-50μg/ml. The Accuracy (% recovery) for Sertraline was found to be 99-100%. The % RSD (intra-day and inter precision) values are not more than 2% hence the developed methods are accurate and precise. The LOD and LOQ were found to be 0.2085μg/ml and 0.6321μg/ml respectively.
Conclusion: The developed method was validated with respect to linearity, accuracy, precision, robustness, ruggedness, LOD and LOQ as per ICH guidelines. The proposed method was used for routine analysis of Sertraline in Bulk drug and Solid dosage Form.
Keywords
Sertraline; Antidepressant agent; Method development; Method validation; UV-Spectrophotometer; RP-UHPLC
Introduction
Ultra-high performance liquid-chromatography (UHPLC) covers liquid chromatography separations implementing columns enclose particles smaller than the 2.5–5 μm sizes typically used in highperformance liquid chromatography (HPLC). UHPLC work on the same assumption as that of HPLC and of which governing principle is that, as column packing particle size decrease, efficiency and thus resolution increases. High strength silica (HSS) is another type of column used in UHPLC. In UHPLC, high pore volume UHPLC particles do not acquire the mechanical stability necessary to hold up the high pressure innate of UHPLC separations. For that, there is established a novel silica particle and appropriate morphology required to give long and lifetime efficiency UHPLC column at high pressure likely 1000 bars.
Analytical method development followed by method validation is an important process in the drug discovery. Although the drug shows good potency, lack of validated analytical method will not allow the drug to enter into the market. This is to ensure the quality and safety of the drug [1].
Validation is process of establishing documentary evidence demonstrating that a procedure, process or activity, carried out in testing and then production maintains the desired level of compliance at all stages. Validity of a specific method should be demonstrated in laboratory experiments using samples or standards that are similar to the unknown samples analyzed in the routine. The preparation and execution should follow a validation protocol, preferably written in a step by step instruction format [2].
Sertraline hydrochloride is a selective serotonin reuptake inhibitor (SSRI) for oral administration. Chemical name is (1S-cis)-4-(3, 4-dichlorophenyl)-1, 2, 3, 4-tetrahydro-Nmethyl-1-naphthalenamine hydrochloride. The empirical formula is C17H17NCl2.HCl. Molecular weight of sertraline is 306.229g/mol. Sertraline is soluble in organic solvent like methanol, ethanol, DMSO. It is also soluble in water and acetonitrile. Sertraline is a popular anti-depressant medication commonly known for its selective serotonin reuptake inhibitor (SSRI) activity, and is similar to drugs such as Citalopram and Fluoxetine [3]. Sertraline inhibits the reuptake of serotonine (5-HT) at the presynaptic neuronal membrane, thereby increasing serotonergic activity. This result in an increased synaptic concentration of serotonine in the CNS, which leads to numerous functional changes associated with enhanced serotonergic neurotransmission. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder, and obsessive compulsive disorder (OSD). The chemical structure of sertraline is shown in Fig-1. (Figure-1)
The review of literature includes RP-HPLC [4-7], UV-Spectroscopy [8,9], HPTLC, [10,11], UPLC [12], UPLC MS-MS [13] ,LC MS-MS [14], Stability indicating methods for determination of sertraline either in individually or in combination with other drugs also in pharmaceutical dosage form. From the literature survey observed that UPLC method was developed for determination of sertraline in biological fluid and not in mobile phase hence , the aim of the present work is to develop a simple, precise, and economical RP-UHPLC method for determination of sertraline in bulk drug and dosage form. The RP-UHPLC method used the column having particle size 2μm which having high resolution power than HPLC method. The developed method was validated for linearity, accuracy, precision, repeatability, robustness, ruggedness, LOD and LOQ.
Materials and Methods
Materials
Chemical and Reagents Used:
Sertraline pure drug was a gift sample from Swapnroop drugs and Pharmaceuticals, Aurangabad. For UV-Spectroscopic determination water (HPLC grade) was used as solvent. HPLC grade acetonitrile, methanol and 0.1% OPA was used for RP-UHPLC determination of sertraline. Marketed formulation of Sertraline (SERTA-50 50mg) was purchased from local pharmacist.
Instruments Used:
A double beam UV-Spectrophotometer (Systronic-2201) was used for recording of spectrum and absorbance. The light source used is Deuterium lamp of Spectrophotometer, a computer is attached which help in data processing. A Quartz cuvette with path length 1cm was used. The HPLC analysis carried out on instrument (Agilent Technologies® Gradient System with Auto injector) with Reverse phase (Agilent) C18 column (75mm×3.9mm, 2μm particle size), SP930D pump, and 20μl injection loop were used. Weighing balance (WENSAR™ High Resolution Balance), Sonicator (Ultrasonic electronic instrument) were used.
Method Development
Solubility Studies:
This study was carried out to find an ideal solvent in which drugs are completely soluble. Various solvents were tried for checking solubility of Sertraline. From solubility studies it was concluded that of Sertraline is freely soluble in Acetonitrile, methanol and poorly soluble in water hence adjusted with 0.1% Orthophosphoric Acid, Buffer pH 3. (Table-1)
| Column used | Mobile phase, Flow Rate, Wavelength | Injection Volume | Observation | Conclusion |
|---|---|---|---|---|
| C18 (Agilent) (3.9 mm x 75mm, 2.0μ) | Methanol+ (0.1%OPA)Water, pH 3 (80+20%v/v),273 nm , Flow rate. 0.5ml, |
20 μl | Sharp Peaks were not obtained | Hence rejected |
| C18 (Agilent) (3.9 mm x 75mm, 2.0μ) | Acetonitrile + (0.1% OPA)Water(80+20%v/v) pH 3.0, 273 nm, Flow rate 1ml | 20 μl | Sharp Peaks were not obtained | Hence rejected |
| C18 (Agilent) (3.9 mm x 75mm, 2.0μ) | Acetonitrile + (0.1% OPA)Water (90+10 %v/v) pH 3.0, 273 nm, Flow rate 0.7ml | 20 μl | Sharp Peaks were not obtained | Hence rejected |
| C18 (Agilent) (3.9 mm x 75mm, 2.0μ) | Acetonitrile + (0.1% OPA)Water (80+20 %v/v) pH 3.0, 273 nm, Flow rate 0.7ml | 20 μl | Sharp and well resolved Peaks were obtained | Hence selected |
Chromatographic Conditions:
The method was developed by using Agilent C18 (75mm×3.9mm, 2μm particle size) column with mobile phase Acetonitrile :( 0.1% OPA) water (80:20). Flow rate was maintained 0.7ml/min. The sample injection volume was 20μl and detected at 273 nm. The Optimized chromatographic conditions are shown in Table-2. (Table-2, 3) Preparation of 0.1% Orthophosphoric Acid Water: 0.1% OPA acid prepared by transferring 0.1 ml of Orthophosphoric acid in 100 ml of volumetric flask and volume made up to the mark with water to obtained 0.1 % Orthophosphoric acid
Name |
RT (min) | Area[mAU*s] | Symmetry | Height | Width | TP | Resolution |
|---|---|---|---|---|---|---|---|
| Sertraline | 3.751 | 5179.55713 | 0.73 | 524.126828 | 0.1456 | 4678 | 0.00 |
Parameters |
Description |
|---|---|
| Stationary phase | Agilent C18 (75mm×3.9mm,2µm particle size) |
| Mobile phase | Acetonitrile:(0.1%OPA)water 80:20v/v |
| Flow rate | 0.7ml/min |
| Detection wavelength | 273nm |
| Injection volume | 20µl |
| Run time | 10 min |
Preparation of Mobile phase:
Mobile phase was prepared by mixing the Acetonitrile and 0.1% OPA water in the ration of 80:20% v/v and the mixture degasified by vacuum filtration using 0.45μ filter and sonication. Mobile phase used as diluent for the preparation of standard stock and working solutions.
Preparation of Standard Stock Solution:
10 mg of Standard Sertraline was accurately weighed and transferred to 10ml volumetric flask. Methanol was added up to the mark and sonicated for 15 min to dissolve to obtained 1000μg/ml solution. It was filtered through 0.45μ membrane filter.
Determination of Absorption maxima:
From Standard stock solution (1000μg/ml) 0.2 ml was pipette out and transferred into 10 ml volumetric flask and volume made up to the mark with diluent to obtained 2μg/ml solution. It was filtered through 0.45μ membrane filter. This solution was scanned in the range of 200- 400nm for the analysis of absorption maxima of sertraline.
Assay of Marketed Formulation:
20 tablets of Sertraline were weighed and crushed into fine powder. A quantity of powder equivalent to 10 mg of sertraline 13.66mg transferred into 10ml volumetric flask and sonicated to dissolve completely then volume made up to the mark with diluent to obtained 1000μg/ml of solution. It was filtered through 0.45μ membrane filter. From above solution 0.3ml added to volumetric flask and made up to the mark with water to obtained 30μg/ml solution for assay.
Results
UV-Spectrometric Determination (Absorbance maxima):
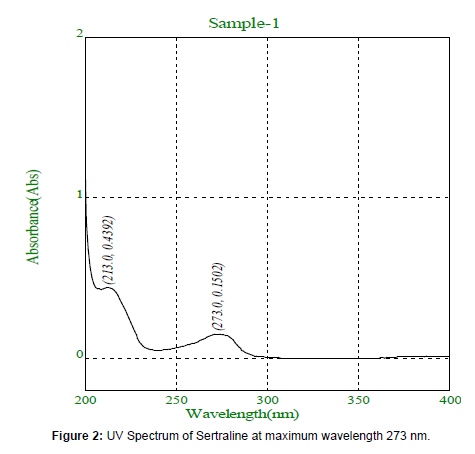
The Standard solution of Sertraline (2μg/ml) was prepared from standard stock solution and scanned in the range of 200-400nm and UV Spectrum was recorded. The absorption spectral analysis shows the maximum wavelength at 273 nm. (Figure-2)
Method Optimization:
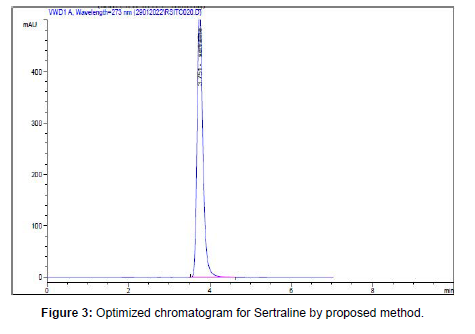
Many trials have been performed by various mobile phases, flow rate, and stationary phase. After observing theoretical plates and stability factor, various chromatographic parameters were chosen. To optimize the HPLC method parameters, mobile phase ratios of different solvents were tried. Good separation and peak symmetry for Sertraline were developed with combination of Acetonitrile and 0.1% OPA water in the ratio of 80:20% v/v. System flow rate was confirmed as 0.7 mL/min. The peak was eluted at the retention time of 3.75 min at 273 nm wavelength.
Method validation:
The method was validated for linearity, precision, accuracy, system suitability, robustness, ruggedness, LOD and LOQ according to ICH guidelines Q2.
Linearity and Range:
Linearity is the ability of the method to elicit test results that is directly proportional to the concentration within a given range. It is generally reported as variance of slope or regression.
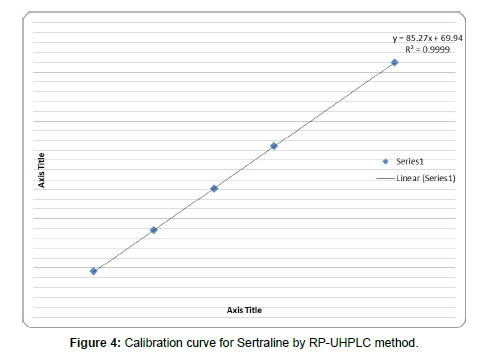
Accurately measured standard solution of sertraline (0.1, 0.2, 0.3, 0.4, and 0.5ml) were transferred to series of 10 ml volumetric flasks and diluted to the mark with mobile phase to obtained the concentration range of 10-50μg/ml. 20μl of sample solution injected into the chromatographic system and chromatogram were recorded. Calibration curve was constructed by plotting Area versus Concentration of Sertraline and regression equation was calculated shown in Table-5. (Table-4, 5) (Figure-3)
Concentration(µg/ml) |
Area Sertraline |
|---|---|
| 10 | 928.78 |
| 20 | 1773.78 |
| 30 | 2623.22 |
| 40 | 3477.225 |
| 50 | 5191.23 |
Regression Equation Data Y=mx+c |
|
|---|---|
| Slope(m) | 85.27 |
| Intercept(c) | 69.94 |
| Correlation Coefficient | 0.999 |
Accuracy:
The parameter accuracy is the extent to which the experimental results deviate from the expected results. The accuracy of method was determined by calculating recoveries of Sertraline by Standard addition method. A known amount of drug (80, 100, and 120%) was added to pre analyzed sample solution. Good recovery of the spiked drug was obtained at each added concentration, indicating that the method was accurate. The results for accuracy studies for Sertraline are shown in Table-6. The %RSD was less than 2. (Table-6)
| Method | Drug | Level (%) | Amount taken (μg/ml) | Amount Added (μg/ml) |
*Area |
*Amount. recovered (μg/ml) | *%Recovery ± S.D. |
%RSD |
|---|---|---|---|---|---|---|---|---|
| RP-UHPLC Method | Sertaline | 80% | 10 | 8 | 1605.40 | 8.0 | 100.06±0.98 | 0.97 |
| 100% | 10 | 10 | 1772.64 | 9.97 | 99.73 ±0.15 | 0.15 | ||
| 120% | 10 | 12 | 1941.34 | 11.94 | 99.97±0.27 | 0.27 | ||
| *Mean of two observations | ||||||||
Precision:
The precision of the method is degree of agreement among individual test results when the procedure is applied repeatedly to the multiple samplings. Precision of the method was studied as Inter-day precision and Intra-day precision. Intra-day precision was determined by analyzing 20, 30 and 40μg/ml of Sertaline solution for RP-UHPLC method for two times in the same day. And Inter-day precision was determined by analyzing 20, 30 and 40μg/ml of Sertaline solution for two days daily and results were recorded.
Intraday and Interday precision for Sertraline shows the high precision % amount in between 99% to 100%. Results are shown in Table-7. (Table-7)
|
Method |
Drug | Concentration (µg/ml) |
Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|---|---|
| * Peak Area ± SD | *%Amt Found | % RSD | *Peak Area± SD | *%Amt Found | %RSD | |||
| RP-UHPLC Method | Sertaline | 20 | 1778.58 ±3.86 | 100.27 | 0.21 | 1774.80 ±2.21 | 99.95 | 0.12 |
| 30 | 2611.15±11.40 | 99.33 | 0.44 | 2619.10 ±4.41 | 99.63 | 0.17 | ||
| 40 | 3461.64±2.10 | 99.43 | 0.06 | 3465.77 ±4.54 | 99.55 | 0.13 | ||
| *Mean of two observations | ||||||||
Repeatability (System suitability):
The repeatability of method was assessed by two replicates analysis of Sertraline at concentration of 20μg/ml prepared from Stock solution and the results are reported in Table-8. Different parameters such as number of theoretical plates, peak symmetry, and tailing factor were calculated from obtained data. (Table-8)
Concentration of Sertraline (µg/ml) |
Peak area | Amount found (mg) | % Amount found | Retention time | Theoretical plate |
|---|---|---|---|---|---|
| 20 | 1771.500 | 19.96 | 99.81 | 3.70 | 3581 |
| 20 | 1773.530 | 19.98 | 99.83 | 3.75 | 3663 |
| Mean | 19.97 | 99.82 | 3.72 | 3622 | |
| SD | 1.44 | ||||
| %RSD | 0.08 | ||||
Robustness:
Robustness is the measurement of capacity of analytical method to remain unaffected by small variations in method parameters. To evaluate the robustness of the proposed method, experimental conditions were deliberately altered and the response of the drug (10μg/ ml) was recorded. The mobile phase composition was changed in (±1 ml/min-1) proportion and the flow rate was varied by (±1ml/min-1), and wavelength changes (±1 ml/min-1) of optimized chromatographic condition. The results of minor variations in composition of mobile phase, wavelength, and flow rate are shown in Table-9. The reproducible results were obtained which proves that the method is robust. (Table-9)
Parameters |
Modification | Concentration (µg/ml) | Area(mean ±SD) | %RSD |
|---|---|---|---|---|
| Flow rate change(0.7ml/min) | 0.6ml/min | 10 | 853.69± 2.02 | 0.24 |
| 0.8ml/min | 10 | 695.25 ± 1.58 | 0.23 | |
| Wavelength 273nm | 272nm | 10 | 921.1±1.32 | 0.14 |
| 274nm | 10 | 796.60±2.02 | 0.25 | |
| Mobile phase composition-ACN:(0.1%OPA) water(80:20v/v) | (79:21v/v) | 10 | 920.35±1.75 | 0.19 |
| (81:19v/v) | 10 | 918.9±2.02 | 0.22 | |
Limit of Detection (LOD) and Limit of Quantificatication (LOQ):
Sensitivity of proposed method was estimated as LOD and LOQ. Limit of detection is the lowest concentration of analyte that can be detected. Limit of quantification is lowest concentration of analyte that can be quantified.
The LOD and LOQ of the method were determined by using the following equations:
LOD = 3.3 σ / S AND LOQ = 10 σ / S
Where
σ = standard deviation of the response and
S = slope of the calibration curve (Table-10)
Analysis of Sertraline in tablet formulation: (Assay)
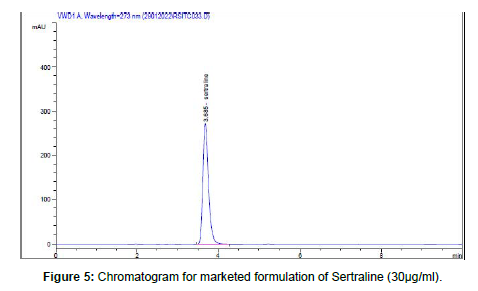
Amount of drug present in SERTA-50® 50 mg tablet was calculated. The Results was found to be for Sertraline by RP-UHPLC method was 98.97% shown in Table-11. (Table-11, 12) (Figure-4, 5)
Drug |
σ | Slope | LOD (µg/mL) | LOQ ( µg/mL) |
|---|---|---|---|---|
| Sertraline | 5.39 | 85.27 | 0.2085 | 0.6321 |
Assay |
Drug | Concentration (µg/ml) | Area | Amount Found | %Label claim |
|---|---|---|---|---|---|
| RP-UHPLC Method | Sertraline | 30 | 2599.470 | 29.66 | 98.97% |
| 30 | 2604.840 | 29.72 | |||
Parameters |
Results |
|---|---|
| Linearity range | 10-50 µg/ml |
| Regression equation | y=85.27x + 69.24 |
| Slope | 85.27 |
| Intercept | 69.24 |
| Correlation Coefficient | 0.9999 |
| Accuracy | 99-100% |
| Precision Intra day Inter day |
0.06-0.44 0.12-0.17 |
| LOD(µg/ml) | 0.2085 µg/ml |
| LOQ(µg/ml) | 0.6321 µg/ml |
Discussion
A precise and sensitive RP-UHPLC method using ACN: 0.1% OPA water (80:20v/v) and the flow rate of 0.7ml/min was developed and validated. The absorption maximum for Sertraline was found to be 273 nm. RP-UHPLC method was developed by using C18 (75mm×3.9mm) Column having particle size 2μm due to that method is rapid and have high resolution power than HPLC method. Linearity is the method ability to obtain test results, which are directly proportional to the concentration of analyte in sample. Sertraline showed a linear response curve for RP-UHPLC method. Correlation coefficient was found to be 0.9999 for Sertraline by RP-UHPLC method .To demonstrate the accuracy of method, standard addition and recovery experiments were conducted. The % recovery was in the range of 99-100% by RP-UHPLC method for Sertraline. The method precision determines the closeness of agreement between series of measurement of the same sample. The % RSD for intraday and inter precision for both methods were obtained NMT 2%. The LOD and LOQ were found to be 0.2085μg/ ml and 0.6321μg/ml respectively. The results obtained on validation parameters met the requirements. Validation of developed method was done as per ICH guidelines. Assay results found from the study show that the methods can be successfully applied for the estimation of Sertraline in tablet formulation. The proposed method was found to be simple, precise, Fand accurate and validated with respect to Linearity, Accuracy, Precision, Robustness, Ruggedness, LOD and LOQ which remained well within limit.
Acknowledgments
The authors would like to thanks principal and management of Poojya Sane Guruji Vidya Prasarak Mandals’s College of Pharmacy, Shahada (Maharashtra) India for providing the required facilities to carry out this research work.
References
- Sudha T, Kanth VK, Sainath NPC, Mishal Raja TS, Ganesan V (2012) Method Development and Validation- A Review. J Adv Pharm Educ Res 2:146-176.
- Lavanya G, Sunil M, Eswarudu MM, Chinna Eswaraiah M, Harisudha K, et al. (2013) Analytical method validation: An updated review. Int J Pharm Sci Res 4:1280-1286.
- Bairagi SH, Ghosh RS (2020) Development and Validation of RP-HPLC Method for Determination of Sertaline in Bulk drug and Dosage form. World J Pharm Pharm Sci 9: 1236-1246.
- Bais S, Bhavsar M, Singhvi I, Chandewar AV (2014) Analytical method development and validation for the estimation of Alprazolam and Sertraline Hydrochloride by HPLC. Int J Pharm Res 11: 0975-8216.
- Richards MP, Reddy ASK, Chowdary GV (2015) RP-HPLC method development and validation for simultaneous estimation of sertraline HCL and alprazolam in tablet dosage form. Int J Res Pharm Sci 6:285-290.
- Narkhede SP, Singh SK, Vidyasagar G (2016) A simple HPLC method for IN-VITRO dissolution study of sertraline hydrochloride in tablets. Int J Life Sci Pharma Res 6:ISSN 2250-0480
- Rani BV, Parthiban C, Sudhakar M (2014) Method development and validation for the sertraline and doxophylline in pharmaceutical dosage form by RP-HPLC. Int J Pharm Pharm Sci 7: 465-468.
- Nagaraju PT, Venugopal K, Murali Krishna NV, Ragalatha P, Mani P, et al. (2017) Analytical Method Development and Validation of Sertraline in Pure From by using UV Spectrophotometry. World J Pharm Pharm Sci 6:1594-1603.
- Ratnia R, Yadav V, Kumar A (2015) Method development and its validation for estimation of Sertraline Hydrochloride by using UV Spectroscopy. International Journal of Pharma Research and Health Science 3:616-620.
- Venkateshwarlu K, Venisetty RK, Yellu NR, Keshetty S (2007) Development of HPTLC-UV absorption Densitometry method for the analysis of Alprazolam and Sertraline in combination and its Application in the evaluation of Marketed preparations. J Chromatogr Sci 45:537-539.
- Gupta KR, Tajne MR, Wadodkar SG (2008) A validated High Performance Thin Layer Chromatography method for Quantification of Sertraline in tablets. J Planar Chromatogr 23:134-136.
- Bonchev G, Zleteva S, Marinov P, Yocheva M, Vazharov I (2017) A UHPLC Method for Sertraline determination. J of IMAB 23:1765-1768.
- Yue HX, Wang Z, Tian DD, Zhang JW, Zhu K, et al. (2016) Determination of Sertraline in human plasma by UPLC-MS/MS and its Application to a Pharmacokinetic Study. J Chromatogr Sci 54:195-199.
- Zhang M, Gao F, Cui X, Zhang Y, Sun Y, et al. (2011) Development and validation of an improved method for the Quantitation of Sertraline in Human plasma using LC-MS-MS and its application to Bioequivalence studies. J Chromatogr Sci 49:89-93.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Chaudhari V, Patil S, Pawar SP (2022) Development and Validation of RP-UHPLC Method for Determination of Sertraline in Bulk Drug and Dosage Form. J Anal Bioanal Tech 10: 469. DOI: 10.4172/2155-9872.1000469
Copyright: © 2022 Chaudhari V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1997
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1657
- PDF downloads: 340