Research Article Open Access
Development and Validation of a New HPLC Method for In-vitro Studies of Haloperidol in Solid Lipid Nanoparticles
Mohd Yasir1,2*, Udai Vir Singh Sara3, Iti Som2 and Lubhan Singh41Department of Pharmacy, Uttarakhand Technical University, Dehradun-248007, Uttarakhand, India
2Department of Pharmaceutics, I.T.S Pharmacy College, Delhi- Meerut Highway, Muradnagar, Ghaziabad-201206 (UP), India
3Department of Pharmaceutics, Dr. M C Saxena College of Pharmacy, Lucknow- 226101, (UP), India
4Ram-Eesh Institute of Vocational and Technical Education, Greater Noida, Gautam Budh Nagar-201310, Uttar Pradesh, India
- *Corresponding Author:
- Mohd Yasir
Department of Pharmaceutics, I.T.S Pharmacy College
Delhi- Meerut Highway, Muradnagar, Ghaziabad-201206 (UP), India
Tel: 91-9761131206
Fax: (01232) 225380
E-mail: mohdyasir31@gmail.com
Received Date: October 31, 2016; Accepted Date: November 16, 2016; Published Date: November 21, 2016
Citation: Yasir M, Sara UVS, Som I, Singh L (2016) Development and Validation of a New HPLC Method for in-vitro Studies of Haloperidol in Solid Lipid Nanoparticles. J Anal Bioanal Tech 7:339. doi: 10.4172/2155-9872.1000339
Copyright: © 2016 Yasir M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple and sensitive HPLC method was developed and validated for the study of in-vitro drug release from haloperidol loaded solid lipid nanoparticles (SLNs). The method was also used for the analysis of drug for detection of shelf life of developed SLNs. Chromatogram separation was achieved using C18 column as stationary phase. The mobile phase consisted of 100 mM/L potassium dihydrogen phosphate–acetonitrile-TEA (10:90:0.1, v/v/v) and the pH was adjusted with o-phosphoric acid to 3.5. Flow rate of mobile phase was 2 ml/minute and eluents were monitored at 230 nm using UV/VIS detector. The method was validated for linearity, precision, accuracy, reproducibility, limit of detection (LOD) and limit of quantification (LOQ). Linearity for haloperidol was in the range of 1-60 μg/mL. The value of LOD and LOQ was found to be 0.045 and 0.135 μg/ml respectively. The drug loaded SLNs showed sustained drug release with maximum value of 95.52 ± 5.21% in 24 h. The shelf life of SLNs formulation was found to be 2.31 years at 4°C.
Keywords
Haloperidol; HPLC; SLNs; Validation
Introduction
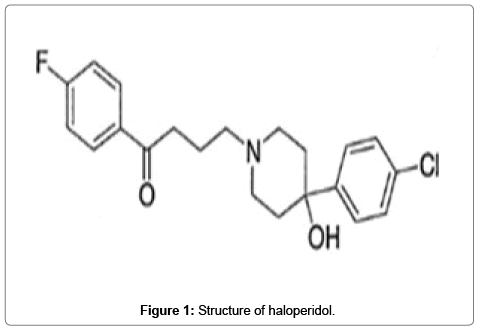
Haloperidol is an orally administered dopamine inverse agonist of the typical antipsychotic class of medications that chemically belongs to butyrophenone group [1]. Its mechanism of action is mediated by blockade of D2 dopamine receptors in brain [2]. It is used to treat certain psychiatric conditions including schizophrenia, maniac states, medicament induced psychosis and neurological disorders with hyperkinesias [3]. Haloperidol is chemically 4-[4-(4-chlorophenyl)-4- hydroxypiperidino] 4’-fluorobutyrophenone (Figure 1). The molecular formula of haloperidol is C21H23ClFNO2 and molecular weight is 375.86 g/mol.
Author developed the solid lipid nano particles of haloperidol for nose to brain delivery (data are not given here and already published by same author). SLNs have the several advantages including targeted drug delivery and controlled release delivery, and increase bioavailability so as to reduce the dose and adverse effects associated with drug. SLNs offer an improvement to traditional nose-to-brain drug delivery since they are able to protect the encapsulated drug from biological and/or chemical degradation and may also increase nasal retention time due to an occlusive effect, good application properties, and adhesion of the SLNs to mucous membranes [4].
Various analytical techniques have been used for determination of haloperidol in pharmaceutical formulations. These include high performance thin-layer chromatography (HPTLC) [5], 19F NMR spectroscopy [6], square-wave adsorptive stripping voltammetry at a mercury electrode [7], square-wave and cyclic voltammetry at hanging mercury drop electrode [8], cyclic voltammetry at multiwalled carbon nanotubes-modified glassy carbon electrode [9]. Nonaqueous titrimetric method has also been developed for haloperidol determination [10]. Several UV spectrophotometric assay procedures have been developed and described in official compendia [11-13]. The literature survey revealed that haloperidol was quantitatively assayed in pharmaceutical preparations as well as in biological fluids either individually or in the presence of other drugs using HPLC (Table 1) but procedures related to detection of haloperidol in solid lipid nanoparticles are not published in literature till today. Author tried to reproduce the previously reported methods (Table 1) but due to unknown reasons none of them could reproduced. Hence, the aim of the research work was to develop and validate a new HPLC method for haloperidol. The different analytical performance parameters such as linearity, precision, accuracy, reproducibility, limit of detection (LOD) and limit of quantification (LOQ) were determined as per ICH Q2 (R1) guidelines [14,15]. The significance and relevance of this method was in vitro dissolution assessment of solid lipid nanoparticles. This method was also applied for quantification of haloperidol in solid lipid nanoparticles stored at different temperature and humidity conditions. For the obtained data, the shelf life of haloperidol was determined. Additionally, the method can be used for quantification of haloperidol in other type nanoparticles.
| S. No. | Column | Mobile phase | Flow rate | Detector | References |
|---|---|---|---|---|---|
| 1 | Thermo C18-ODS column (250 × 4.6 mm× 5µm) | Methanol and monobasic potassium phosphate (pH=4) (60:40 v/v) | l mL/min | UV at 264 nm |
[16] |
| 2 | Thermo C18-ODS column (250 × 4.6 mm× 5µm) | Acetonitrile and water (50:50 v/v), pH adjusted to 2.5 using 0.05 M KHPO4) | 1.6 mL/min |
UV at 240 nm |
[17] |
| 3 | Chemito C18 column (250 × 4.6 mm× 5 µm). | Methanol and water (70:30 v/v) | 1 mL/min | UV at 220 nm | [18] |
| 4 | Merck RP-C18 column (100 × 4.6 mm×5µm) | Phosphate buffer (100 mm, pH 3.5)-acetonitrile (80:20, v/v) |
2 mL /min | UV at 230 nm |
[19] |
| 5 | Spheri RP-C18 column (25 ×4.6 mm× 5) | Phosphate Buffer (50 mm, pH 2.5)–acetonitrile–THF–TEA (63:34:3:0.1, v/v/v/v) |
1 mL/min | UV at 247 nm | [20] |
| 6 | Nucleosil RP- C18 column (25 cm×4.6 × 5µm) | Methanol-water (63:37 v/v) containing 0.2 M a ammonium acetate | 0.5 mL/min | UV at 250 nm | [21] |
Table 1: Reported HPLC methods for haloperidol determination.
Materials and Methods
Drug and other chemicals
Haloperidol was received as a gift sample from Vamsi Labs Ltd Solapur, Maharashtra, India.
Potassium dihydrogen phosphate was purchased from Qualigens fine chemicals, Mumbai, India. Sodium hydroxide was procured from Fisher scientific, Mumbai, India. Acetonitrile (ACN), Triethylamine (TEA) and o-phosphoric acid were purchased from Sigma-Aldrich, (Millipore, Molsheim, France) water was used to prepare solutions wherever required and it was filtered before use through a 0.22 μm membrane filter.
Instruments
Bath sonicator (Multitech Pvt. Ltd., N. Delhi), Balance (AUX 220, Shimadzu Corporation, Kyoto, Japan), Magnetic stirrer (Remi Instruments Pvt. Ltd., Mumbai, India), pH meter (Hicon Scientific Instruments, Delhi, India), Centrifuge (Remi Instruments Pvt. Ltd., India), Micro pipettes (Labnet, USA), Vortex mixture (S.M. Scientific instruments, Pvt. Ltd, Delhi) were used for the study.
HPLC System
The HPLC system consisted of a pump (Jasco PU 2080 plus), UV/ VIS detector (Jasco UV 2075 plus) and the software used was Jasco Borwin version (1.5, LC-Net II/ADC system). The column used was C-18 Cosmosil packed column (5 C18 MS-II, 250 mm × 4.6 mm with particle size of 5.0 μm). Syringe filters (Syringe-driven filters of 0.22 μm, HiMedia Laboratories Pvt. Ltd., Mumbai, India) were used to filter to sample.
Preformulation studies of haloperidol: Haloperidol was received as a gift sample from Vamsi Labs Ltd Solapur, Maharashtra, India. Authenticity of haloperidol was verified by conducting the preformulation studies like organoleptic properties, melting point, FTIR spectral analysis, DSC study, partition coefficient determination.
HPLC method
Several methods have been developed for the determination of haloperidol in pharmaceutical preparation.
The concentration of haloperidol had been earlier determined by HPLC method using methanol-water (63:37, v/v) containing 0.2 M ammonium acetate as mobile phase and diphenylamine as internal standard [21]. The method used here was slightly modified in the terms of mobile phase. The mobile phase consisted of 100 mM/L potassium dihydrogen phosphate-acetonitrile-TEA (10:90:0.1, v/v/v) and the pH was adjusted with o-phosphoric acid to 3.5. It was sonicated for 15 min and filtered through 0.22 μm membrane filter. Flow rate of mobile phase was maintained at 2 mL/min and eluents were monitored at 230 nm. The samples were injected using a 20 μL HPLC injector. All determinations were performed at ambient temperature for a run time of 5 min [22].
Preparation of calibration curve: The 1.0 mg/mL stock solution of haloperidol was prepared in mobile phase. An appropriate volume of stock solution was further diluted with mobile phase to obtain a standard solution having a final concentration 100 μg/mL. Different concentrations (1-60 μg/mL) were made for the preparation of calibration curve from the prepared standard solution [23]. The prepared dilutions were injected serially (20 μL) and area under peak was recorded for each dilution. The calibration curve was constructed by plotting the concentration of haloperidol on X-axis and peak area on Y-axis.
Method validation
HPLC method for Haloperidol was validated as per the ICH guidelines Q2 (R1) for linearity, precision, accuracy, repeatability, LOD and LOQ.
Linearity: The linearity of response for haloperidol was assessed in the range of 1-60 μg/mL for standard drug.
Accuracy as recovery: Accuracy was determined using standard addition method. The pre-analyzed samples were spiked with extra 50,100 and 150% of the standard haloperidol and the mixtures were analyzed by the proposed method. The experiment was performed in triplicate. The % recovery, % relative standard deviation (RSD), and standard error of mean (SEM) were calculated at each concentration level.
Precision: The precision was determined at two levels as per ICH, Q2 (R1) guidelines i.e., repeatability and intermediate precision.
Repeatability of drug sample was determined as intraday variation. Three replicates for each of the three concentrations were analyzed three times a day. Hence a minimum of 9 determinations were done covering the specified range for the procedure whereas intermediate precision was determined by interday variation (for three different days) for the determination of haloperidol at three different concentration levels of 4, 8 and 12 μg/mL in triplicate. The % relative standard deviation was calculated for absorbance to obtain the intraday and interday variation.
Reproducibility: Reproducibility of the method was investigated by obtaining precision of the method in another laboratory using the different instrument and analyzed by another person. Both intraday & interday precision were calculated at three different concentration levels of 4,8,12 μg/mL.
LOD and LOQ determination: LOD and LOQ of the drug were calculated using the standard deviation method with the help of following equations 1 and 2, respectively, as per ICH guidelines.
LOD=3.3 × σ/S (1)
LOD-10 × σ/S (2)
Where σ=standard deviation of the response; S=slope of the regression line.
Application of HPLC Method in in- vitro drug release study
Haloperidol loaded solid lipid nanoparticles (HP-SLNs) were prepared by modified solvent emulsification–diffusion technique and optimised by Boxbehken design [13].
The in vitro dissolution studies were carried out to evaluate the release of drug form optimized HP-SLN formulation and comparing it with the pure drug.
An accurate amount of freeze dried HP-SLNs and aqueous haloperidol suspension, each containing drug equivalent to 10 mg was transferred to a dialysis bag and sealed at both ends. The sealed bag was then suspended in a beaker containing 100 mL of dissolution medium (Phosphate buffer pH 7.4, corresponding to cerebrospinal fluid pH) and stirred at a constant speed at 37 ± 0.5°C. Aliquots were withdrawn at predetermined time intervals up to 24 h from receiver compartment (beaker) and replaced with an equal volume of fresh medium to maintain sink condition. The samples were analyzed by HPLC using the selected mobile phase. Comparison between release profile of drug suspension and optimized HP-SLN formulation was made by student’s t test at p< 0.05.
Analysis of haloperidol by HPLC method for the determination of shelf life
The shelf life of optimized HP-SLNs was determined by conventional method using Arrhenius equation. Optimized SLN formulations were kept at 25 ± 2ºC; 30 ± 2ºC and 40 ± 2ºC at ambient RH [24]. Samples were withdrawn after specified time intervals (0, 30, 90, and 180 days) and the drug content was determined using HPLC method. Amount of drug remaining was determined at each interval. Logarithm of percent drug remaining versus time (days) was plotted. The degradation rate constant ‘K’ was determined from the slope of the lines at each elevated temperature using the following equation (3).
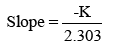 (3)
(3)
Arrhenius Plot was constructed between log K and 1/T to determine the shelf life of optimized SLNs. By extrapolation method K value at 4ºC was determined. The shelf life (T 0.9) at 4°C was calculated with the help of following equation (4).
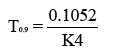 (4)
(4)
Results and Discussion
Preformulation studies of haloperidol
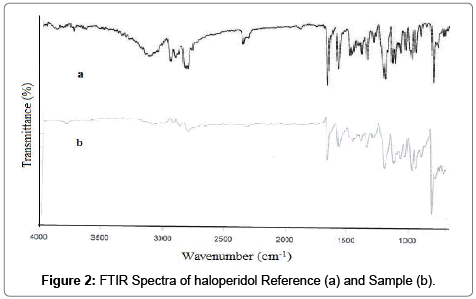
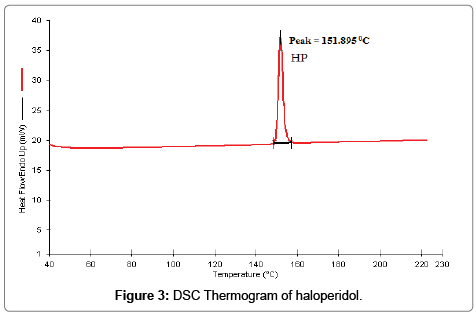
Haloperidol was found to be white, light yellow crystal or crystalline powder with non smell. The melting point was determined by capillary melting point apparatus and it was found to be 153 ± 1.23°C while reported melting point 147-152°C [25]. The partition coefficient of the haloperidol was determined by “Shake flask method” and It was found to be 3.87 ± 0.36 while reported value 4. FTIR spectrum of haloperidol was taken by FTIR spectrophotometer (Alpha model Bruker ATR-FTIR spectrophotometer) and compared with reference spectrum [26] and was found to be identical with reference spectrum as shown in Figure 2. DSC was performed to determine the melting point of haloperidol in temperature mode 40-230°C. The DSC thermogram of haloperidol showed a sharp melting peak at 151.895°C indicating the crystalline nature of drug (Figure 3). The result of DSC experiment was in good agreement with result obtained by melting point measurement. On the basis of physical characterization and spectral analysis like FTIR, and DSC, the drug sample was found to be pure and authentic.
Preparation of calibration curve
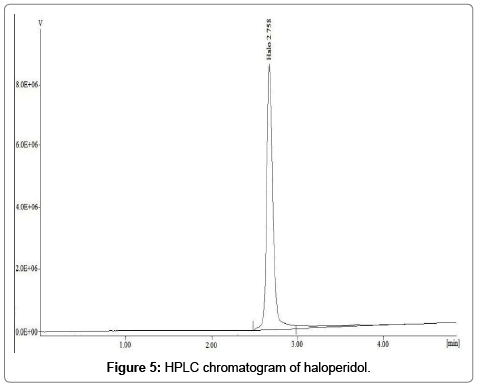
Calibration curve was constructed and a linear relationship was observed between peak area and concentration. The regression data for calibration curve showed a good linear relationship over concentration range of 1-60 μg/mL with respect to the peak area (Figure 4). The retention time of haloperidol was found to be 2.00 ± 0.12 min as shown in Figure 5.
Method validation
Using the optimized chromatographic conditions, the developed HPLC method was validated with respect to linearity, precision, accuracy, reproducibility, LOD and LOQ as per the ICH guidelines.
Linearity: The linearity range of haloperidol solution was obtained as 1-60 μg/mL. The linear regression equation was Y=101563X+7043.1 with correlation coefficient (R2) of 0.9995.
Accuracy as recovery studies: The proposed method afforded recovery of 99.55-100.42 % after spiking the additional standard drug solution to the previously analyzed test solution. The value of % recovery, % RSDs and SE are shown in Table 2, which indicated the accuracy of the proposed method.
| % of standard spiked to the sample |
Concentration | %drug recovered |
% RSD |
SE | ||
|---|---|---|---|---|---|---|
| Sample (µg/mL) |
Total including spiked sample (µg/mL) |
Spiked sample determined (µg /mL) ±SD (n=3) |
||||
| 50 | 10 | 15 | 15.03±0.29 | 99.55 | 1.93 | 0.17 |
| 100 | 10 | 20 | 19.97± 0.21 | 99.86 | 1.03 | 0.12 |
| 150 | 10 | 25 | 25.11±0.35 | 100.42 | 1.39 | 0.20 |
Table 2: Accuracy as recovery of proposed method.
Precision: The precision was assessed by analyzing haloperidol in three different concentration levels as 4, 8 and 12 μg/mL of in triplicate.
The results of repeatability (intraday precision) and intermediate (interday) precision were expressed in the terms of % RSD. The intraday and interday precision study of the developed method confirmed the adequate sample stability and method reliability where all RSDs were below 2% as shown in Table 3.
| Conc. (µg/ mL) |
Repeatability (intraday precision) | Intermediate precision (interday) | |||||
|---|---|---|---|---|---|---|---|
| Mean area±SD (n=3) | SEM | % RSD | Days | Mean area±SD (n=3) | SEM | % RSD | |
| 4 | 424842±2410 | 1393 | 0.56 | 1 | 425363±3293 | 1903 | 0.77 |
| 2 | 422999±5335 | 3084 | 1.26 | ||||
| 3 | 420934±3868 | 2236 | 0.91 | ||||
| 8 | 835070±9054 | 5234 | 1.08 | 1 | 840698±5981 | 3457 | 0.71 |
| 2 | 839759±4031 | 2330 | 0.48 | ||||
| 3 | 836843±3414 | 1973 | 0.41 | ||||
| 12 | 1256774±11926 | 6894 | 0.95 | 1 | 1249711±12335 | 7130 | 0.99 |
| 2 | 1251832±13451 | 7775 | 1.1 | ||||
| 3 | 1246394±13045 | 7540 | 1.05 | ||||
Table 3: Precision of proposed method.
Reproducibility: Reproducibility of the method was investigated by obtaining precision of the method in another laboratory using the different instrument and analyzed by another person. Both intraday and interday precision were calculated and there were no significant differences observed (p<0.05) in the % RSD values of intraday and interday precision, which indicated the reproducibility of the method. The results of intraday and interday precision are shown in Table 4.
| Conc. (µg/ mL) |
Repeatability (intraday precision) | Intermediate precision (interday) | |||||
|---|---|---|---|---|---|---|---|
| Mean area±SD (n=3) | SEM | % RSD | Days | Mean area±SD (n=3) | SEM | % RSD | |
| 4 | 421175±5048 | 2918 | 1.19 | 1 | 424363±7371 | 4261 | 1.74 |
| 2 | 417599±6567 | 3796 | 1.57 | ||||
| 3 | 425268±5608 | 3241 | 1.32 | ||||
| 8 | 836737±11564 | 6684 | 1.38 | 1 | 838365±10388 | 6004 | 1.24 |
| 2 | 837659±8401 | 4856 | 1.00 | ||||
| 3 | 834843±12296 | 7108 | 1.47 | ||||
| 12 | 1253774±14150 | 8179 | 1.12 | 1 | 1250378±16891 | 9763 | 1.35 |
Table 4: Reproducibility of proposed method.
LOD and LOQ determination: LOD and LOQ of this method were determined by the standard deviation method. The value of LOD and LOQ were found to be 0.045 and 0.135 μg/mL, respectively, which indicated that the proposed method can be used for the detection and quantification of haloperidol even in very low concentration.
In- vitro release of haloperidol from HP-SLNs
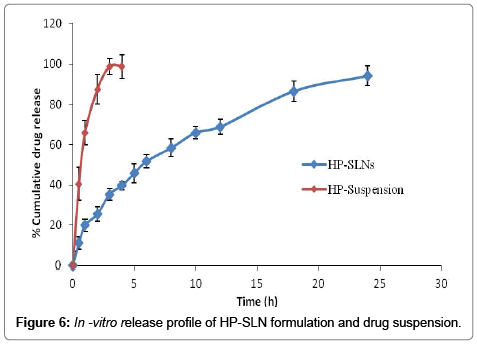
The comparative in vitro drug release study was performed and the results are shown in Table 5 and Figure 6.
| Time (h) | Drug suspension (%)a | HP- SLNs (%)a* |
|---|---|---|
| 0.5 | 42.36±3.42 | 12.76±4.96 |
| 1 | 70.32±7.73 | 17.18±3.42 |
| 2 | 85.84±3.62 | 28.93±3.41 |
| 3 | 99.72±6.92 | 34.85±2.27 |
| 4 | 98.81±4.39 | 37.92±3.59 |
| 5 | - | 44.21±5.21 |
| 6 | - | 53.92±2.45 |
| 8 | - | 59.35±5.08 |
| 10 | - | 64.72±4.82 |
| 12 | - | 70.18±3.94 |
| 18 | - | 86.91±6.62 |
| 24 | - | 95.52±5.21 |
aValue are mean ± SD, n=3, *p<0.05 versus drug suspension *p<0.05 results are significant
Table 5: In- vitro release data of optimised HP-SLN formulation and drug suspension.
The dissolution profile from HP-SLNs indicated a biphasic behavior consisting of an initial burst release, followed by a phase of slow release. The initial burst release may be attributed to the presence of free drug in the external phase and drug adsorbed onto the surface of particles, while the slow release may be due to the drug encapsulated within the lipid matrix. The HP-SLNs showed initial burst release of 17.18 ± 3.42 %, whereas plain drug showed 70.32 ± 7.73 % drug release after 1 h. Thereafter, HP-SLNs showed sustained drug release with maximum value of 95.52 ± 5.21 % in 24 h, while aqueous drug suspension 98.81 ± 4.39 % drug release within 4 h. The observed results of HP-SLNs were compared with the drug suspension and it was found that a significance difference (p<0.05) was observed between drug suspension and HPSLN formulation after 4 h study.
Shelf life determination
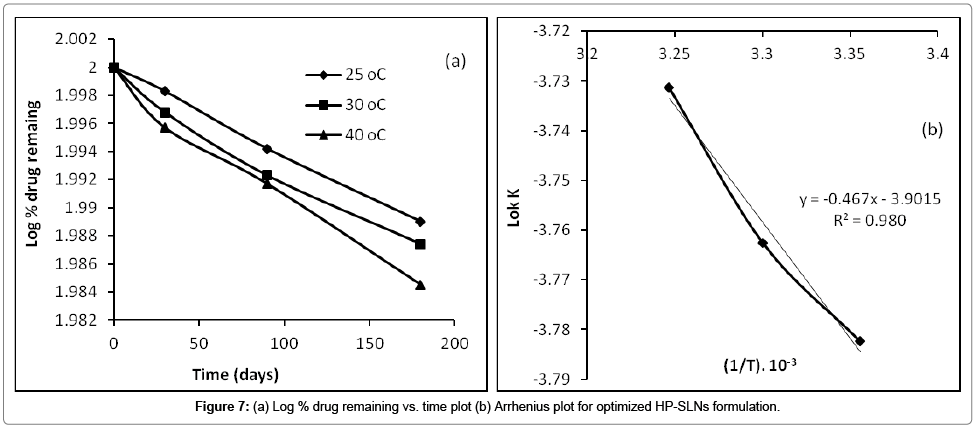
The percentage drug remaining in the optimized formulation (HP-SLNs) when stored for 180 days at elevated temperatures of 25 ± 2ºC; 30 ± 2ºC; and 40 ± 2ºC at ambient RH was determined (Table 6). Logarithm of percent drug remaining versus time (days) was plotted (Figure 7a). The degradation rate constant ‘K’ was determined from the slope of the lines at each elevated temperature using the equation (4).
| Storage condition | Sampling interval (days) | % drug remaining | Log % drug remaining |
|---|---|---|---|
| 25±2ºC | 0 | 100 | 2 |
| 30 | 99.6 | 1.9983 | |
| 90 | 98.65 | 1.9942 | |
| 180 | 97.51 | 1.989 | |
| 30±2ºC | 0 | 100 | 2 |
| 30 | 99.26 | 1.9968 | |
| 90 | 98.23 | 1.9923 | |
| 180 | 97.13 | 1.9874 | |
| 40±2ºC | 0 | 100 | 2 |
| 30 | 99.01 | 1.9957 | |
| 90 | 97.66 | 1.9917 | |
| 180 | 96.49 | 1.9845 |
Table 6: Degradation of optimized HP- SLNs kept at 25 ± 2 ºC; 30 ± 2 ºC and 40 ± 2 ºC at ambient RH.
Arrhenius plot was constructed between log K and 1/T (Table 7 and Figure 7b). From the plot, K value at 4ºC (refrigerator temperature) was determined. The value of K4 was placed in equation (4.18) and shelf life was calculated. The shelf life of haloperidol in SLNs at 4ºC was found to be 2.31 years. The degradation kinetics of haloperidol in SLNs was also determined and it was found to be first order kinetics as shown in Figure 7a.
| Temperature (ºC) |
Temperature (ºK) |
(1/ T) × 10-3 (ºK-1) |
Slope × 10-5 | K × 10-4 | Log K |
|---|---|---|---|---|---|
| 25 | 298 | 3.355704 | -7.1667 | 1.6505 | -3.78239 |
| 30 | 303 | 3.30033 | -7.5 | 1.7273 | -3.76264 |
| 40 | 308 | 3.246753 | -6.6667 | 1.5353 | -3.8138 |
| 4 | 277 | 3.610183 | --- | 1.2496 | -3.9032 |
Table 7: Various parameters for calculation of shelf life.
Conclusion
A new and simple HPLC method for haloperidol was developed and validated over the concentration range from 1-60 μg/mL. The method was successfully used for in vitro dissolution assessment of solid lipid nanoparticles. This method was also applied for quantification of haloperidol in solid lipid nanoparticles stored at different temperature and humidity conditions. For the obtained data, the shelf life of haloperidol was determined. The shelf life of haloperidol in SLNs was found to be 2.31 years.
Acknowledgements
Authors are highly thankful to Management of I.T.S College of Pharmacy, Muradnagar, Ghaziabad, India for providing e-library facility during literature survey. Authors gratefully acknowledge the Name of Prof. S. Sadish Kumar (Principal, ITS College of Pharmacy, Muradnagar, Ghaziabad, India) and Mr. D.S. Jolly for their co-operation during the preparation of this review article.
References
- Gajski G, Geric M, Garaj VV (2014) Evaluation of the in vitro cytogenotoxicity profile of antipsychotic drug haloperidol using human peripheral blood lymphocytes. Environ ToxicolPharmacol38:316-324.
- Benvegnú DM, Barcelos RC, Boufleur N, Reckziegel P, Pase CS, et al. (2011) Haloperidol-loaded polysorbate-coated polymeric nanocapsules increase its efficacy in the antipsychotic treatment in rats. Eur J Pharm Biopharm 77:332-336.
- Forsman AO (1976) Individual variability in response to haloperidol. J R Soc Med 69: 9-13.
- Yasir M, Sara UVS (2013) Preparation and optimization of haloperidol loaded solid lipid nanoparticles by Box- Behnken design. J Pharm Res 7:551-558.
- Sigrid M, Loreto P, Mario V, Carmen GG, Marta DD (2007) Quantitative determination of haloperidol in tablets by high performance thin-layer chromatography. J Sep Sci 30:772-777.
- Mojtaba S, Leila SD, Zahra T, Soheila H (2007) 19F NMR as a powerful technique for the assay of anti-psychotic drug haloperidol in human serum and pharmaceutical formulations. J Pharm Biomed Anal 43:1116-1121.
- El- Desoky HS, Ghoneim MM (2005) Assay of the anti-psychotic drug haloperidol in bulk form, pharmaceutical formulation and biological fluids using square-wave adsorptive stripping voltammetry at a mercury electrode. J Pharm Biomed Anal 38:543-550.
- Ribeiro FWP, Soares JES, Becker H, De-Souza D, Pedro- De LN (2011) Electrochemical mechanism and kinetics studies of haloperidol and its assay in commercial formulations. Electrochim Acta 56:2036-2044.
- Huang F, Peng Y, Jin G, Zhang S, Kong J (2008) Sensitive detection of haloperidol and hydroxyzine at multi-walled carbon nanotubes-modified glassy carbon electrodes. Sensors 8:1879-1889.
- European Pharmacopoeia (2002) Council of Europe. Strasbourg, pp: 1288-1289.
- JanickiCA,Ko CT (1991)Haperidol, in: Florey K, editor, Analytical profile of drug substances. Academic Press, New York 9:346-356.
- Chinese Pharmacopoeia (2005) Chinese Pharmacopoeia Commission. Chemical Industry Press: Beijing 469.
- Yasir M, Sara UVS (2014) Development and validation of UV spectrophotometric method for the estimation of haloperidol. British J Pharm Res 4:1407-1415.
- ICH Harmonized Tripartite Guideline (2005) Validation of analytical procedures: Text and Methodology Q2 (R1), International Conference on Harmonization. Geneva, Switzerland.
- Dalila, MB, Raquel CSB, Nardeli B, Camila SP, Patrícia R, et al. (2012) Haloperidol-loaded polysorbate-coated polymeric nanocapsules decrease its adverse motor side effects and oxidative stress markers in rats. NeurochemInt 61:623-631.
- Ebrahimzadeh H, Dehghani Z, Asgharinezhad AA, Shekari N, Molaei K, et al. (2013) Determination of haloperidol in biological samples using molecular imprinted polymer nanoparticles followed by HPLC-DAD detection. Int J Pharm 453: 601-609.
- Jain T, Bhandari A, Ram V, Sharma S (2011) High-performance liquid chromatographic method with diode array detection for quantification of haloperidol levels in schizophrenic patients during routine clinical practice. J Bioanal Biomed 3: 8-12.
- Wate SP, Borkar AA (2009) RP-HPLC estimation of haloperidol and trihexyphenidyl in tablets. IntJChemTech Res 1: 675-676.
- Aboul-Enein HY, Ali I, Hoenen H (2006) Rapid determination of haloperidol and its metabolites in human plasma by HPLC using monolithic silica column and solid-phase extraction. Biomed Chromato 20:760-764.
- Trabelsi H, Bouabdallah S, Bouzouita K, Safta F (2002) Determination and degradation study of haloperidol by high performance liquid chromatography. J Pharm Biomedical Anal 29: 649-657.
- Miyazaki K, Arita T, Oka I, Koyama T (1981)High performance liquid chromatographic determination of haloperidol in Plasma. J Chromat 223:449-453.
- Yasir M, Sara UVS (2014) Solid lipid nanoparticles for nose to brain delivery of haloperidol: In vitro drug release and pharmacokinetics evaluation. Acta Pharm Sinica B 4:454-463.
- Dey S, Patro S, Babu NS, Murthy PN, Panda SK (2013) Development and validation of a stability indicating RP-HPLC method for estimation of atazanavir sulphate in bulk. J Pharm Anal 5: 81-86.
- Shakeel F, BabootaS, Ahuja A, Ali J, Aqil M, et al. (2007) Nanoemulsions as vehicles for transdermal delivery of aceclofenac. APS PharmSciTechv 8:191-199.
- Jahnke S (1998) Emulsions and Nanosuspensions for the Formulation of Poorly Soluble Drugs, Stuttgart, Medpharm Scientific Publishers.
- Indian Pharmacopeia (2010) Monographs on dosage forms, Ministry of Health and Family Welfare, Government of India, Delhi, Controller of Publications.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 6041
- [From(publication date):
December-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 4941
- PDF downloads : 1100