Research Article Open Access
Development and Validation of a GC-MS with SIM Method for the Determination of Trace Levels of Methane Sulfonyl Chloride as an Impurity in Itraconazole API
Mannem Durga Babu*, Surendra Babu K and Medikondu KishoreSVRM College (Autonomous) and Research Centre, Acharya Nagarjuna University, Nagaram, Andhra Pradesh, India
- *Corresponding Author:
- Mannem Durga Babu
SVRM College, Acharya Nagarjuna University
Nagaram, Andhra Pradesh, India
Tel: +918688850113
E-mail: mannem.durgababu@gmail.com
Received: March 06, 2016 Accepted: April 01, 2016 Published: April 08, 2016
Citation: Babu MD, Babu SK, Kishore K (2016) Development and Validation of a GC-MS with SIM Method for the Determination of Trace Levels of Methane Sulfonyl Chloride as an Impurity in Itraconazole API. J Anal Bioanal Tech 7:316. doi:10.4172/2155-9872.1000316
Copyright: © 2016 Babu MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Selected-ion monitoring (SIM) mode mass selective detection was developed and validated for the trace analysis of an impurity, methane sulfonyl chloride as an impurity in Itraconazole (ICR) active pharmaceutical ingredient (API). The analytical method validation is essential for analytical method development and tested extensively for specificity, linearity, accuracy, precision, range, detection limit, quantization limit, and robustness. Accurate and precise quantitation of the impurity in drug substance was achieved with external standardization. In this research work, we present a summary of the method development and validation work performed on Methane sulfonyl chloride (MSC) in Itraconazole API by GC/MS-SIM technique. In the method development phase, the analytical procedure that is appropriate for the quantitative analysis of the MSC in ICR at ppm level was established and evaluated.
Keywords
Methane sulfonyl chloride; Itraconazole;
; Method development; Method validation
Introduction
Numerous analytical methods for the determination of pharmaceuticals and their metabolites in aqueous solutions have been described in the literature. Liquid chromatography-mass spectrometry (LC-MS) and Gas chromatography-Mass spectrometry (GC-MS) are the most widely used techniques [1]. A mass spectrometer is typically utilized in one of two ways: full scan or selected ion monitoring (SIM). The typical GC-MS instrument is capable of performing both functions either individually or concomitantly, depending on the setup of the particular instrument. The primary goal of instrument analysis is to quantify an amount of substance. This is done by comparing the relative concentrations among the atomic masses in the generated spectrum in selected ion monitoring (SIM) certain ion fragments are entered into the instrument method and only those mass fragments are detected by the mass spectrometer. The advantages of SIM are that the detection limit is lower since the instrument is only looking at a small number of fragments (e.g., three fragments) during each scan [2]. SIM mode the mass spectrometer is ‘targeting a limited mass range’; the number of scans across the peak has increased resulting in better peak shape. This is an easy solution for getting better quantitation for early eluting peaks. Inspect the ions obtained for the peak in full scan mode and use at least one of the ions in SIM to obtain a better scan rate [3].
Methane sulfonyl chloride (MSC) is an organosulfur compound with the formula CH3SO2Cl. It is a colorless liquid that dissolves in polar organic solvents but is reactive towards water, alcohols, and many amines. During the manufacturing process of Itraconazole, formation of Methane Sulfonyl Chloride is possible due to residual methanol available in the manufacturing process and may also be formed due to thermal interaction in presence of methanol.
Methane sulfonyl chloride is a potential genotoxic impurity in Itraconazole (ICR) drug substance as it was the part of synthesis process. As per the International conference on harmonization Guidelines from European medical Agency the genotoxins were to be limited to 1.5 μg/ day [4,5]. MSC is having -Chloro as a functional group with aliphatic chain, as per the guideline it is a genotoxic alerting compound. Sensitive method for the analysis of ICR as genotoxic impurity was not available. While developing method at such a low-level, interferences due to drug substance as well as other process impurities and degradation products were the major problems in achieving specificity. Hence based on published general strategies for genotoxic impurities and on the threshold of toxicological concern (TTC), MSC was evaluated in ICR drug substance.
Itraconazole (Figure 1) is a classical member of the triazole class and is an important drug in our arsenal to treat fungal infections because it exhibits broad-spectrum anti-fungal activity [6-10]. Itraconazole (+-)-ics-4[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(1H- 1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl] methoxy] phenyl]-1- piperazinyl] phenyl]-2,4-dihydro-2(1-methylpropyl)-3H-1,2,4-triazol- 3-one, is an orally active triazole antifungal agent which demonstrates broad spectrum activity against a number of fungal species including dermatophytes. It has been demonstrated that GC-MS method offers several advantages over high performance liquid chromatography (HPLC) method including better Sensitivity, specificity, and higher throughput. This paper presents a highly specific and sensitive GC-MS method for the Methane Sulfonyl Chloride in Itraconazole API as per ICH guidelines [11]. This approach eliminated the time-consuming liquid-liquid extraction used in HPLV-UV method, increased the detection limit, and greatly reduced sample processing and instrument acquisition time. Thus the paper reports an economical, simple and accurate GC-MS method for MSC in ICR.
Experimental
Chemicals and materials
Methane Sulfonyl Chloride was purchased from Sigma Aldrich, Fluka, Acros Organics. Dichloro Methane was procured from Rankem (HPLC grade). Pure sample of Itraconazole was obtained from Local research laboratory.
Preparation of standard solution
Diluent: Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents. Dichloromethane was selected as the standard and sample diluent because of its ability to dissolve a wide variety of substance.
Preparation of standard stock solution: Weighed accurately 50 mg of Methane Sulfonyl Chloride in 50 mL of volumetric flask dissolve and make up diluents (1000 ppm). Transfer 1.0 mL of above solution into a 100 mL volumetric flask make up with diluents (10 ppm).
Preparation of standard solution (1.87 ppm): Transfer 3.74 mL from Standard stock solution into a 20 mL volumetric flask and make up to the mark with the same diluents to get a standard solution (Standard solution was prepared with respect to Sample Concentration).
Preparation of sample solution: Weighed accurately 10.0 g of the Itraconazole API into 20 mL of volumetric flask, add 10 mL of Diluents mix well then make up with the same diluents.(Final concentration is 500 mg/mL of API).
Instrumentation
GC-MS analysis was carried out on GCMS-QP 2010 plus system (Shimadzu) having GC-MS Solutions software, an analytical balance (XS 205 from Mettler Toledo) and autopippette (100 μL–1000 μL from Eppendorf) were used. The GC-MS Experimental conditions for Methane Sulfonyl Chloride content in Itraconazole as shown in Table 1.
| Column | ZB-5MS, 30 m × 0.25 mm × 0.25 µm |
| Injector temperature | 150°C |
| Carrier gas | Helium |
| Carrier gas flow | 1.0 mL/min |
| Split ratio | 5:00 |
| Oven Programme | 40°C, 4.0 min |
| 20.0°C/min 200°C 13.00 min | |
| Total run time: 25.0 min | |
| Injection Volume | 1.0 µL |
| Diluent | Dichloro Methane |
| MS Parameters: | |
| Ionisation source | EI |
| Electron energy | 70 Ev |
| Source temperature | 280°C |
| Interface Temperature | 260°C |
| SIM or SIR (Selective Ion Monitoring) Parameters: | |
| m/z fragment | 79 |
| Solvent Cut time | 3.0 min |
| Detector Voltage | 0.92 KV |
| Start Time | 3.01 min |
| End time | 8.0 min |
Table 1: GCMS Experimental conditions.
Results and Discussion
Method optimization
The various Genotoxic Impurities are present in API is the foremost prerequisite for successful method development in GCMS. The successful method development should result in a fast, simple and time efficient method that is capable of being utilized in a manufacturing setting. Following were the stepwise strategies for the method development in our case.
Column selection
The primary goal of column selection was to resolve a Genotoxic Impurity which is formed during the synthesis and manufacturing of Itraconazole API. Several columns were initially investigated to finalize a single method for the separation and quantitation of solvent. Wallcoated capillary columns of various brands with a variety of phases and dimensions have been investigated, e.g., column A is ZB-624 (30 m length, 0.32 mm i.d. with a stationary phase of 6% Cynopropyl phenyl and 94% diethyl polysiloxane film of 1.8 μm) and Column B is ZB-5MS (30 m length, 0.25 mm i.d. with a stationary phase of 5% Cynopropyl phenyl and 95% diethylpolysiloxanefilm of 0.25 μm). In the above two columns, the response was found to be comparatively lower and peak shapes were found to be satisfactory in Column B. Finally column B is proved to be the best column that could fulfill all the needs of the GCMS method, i.e., higher sensitivity, shorter runtime.
Mass spectral analysis
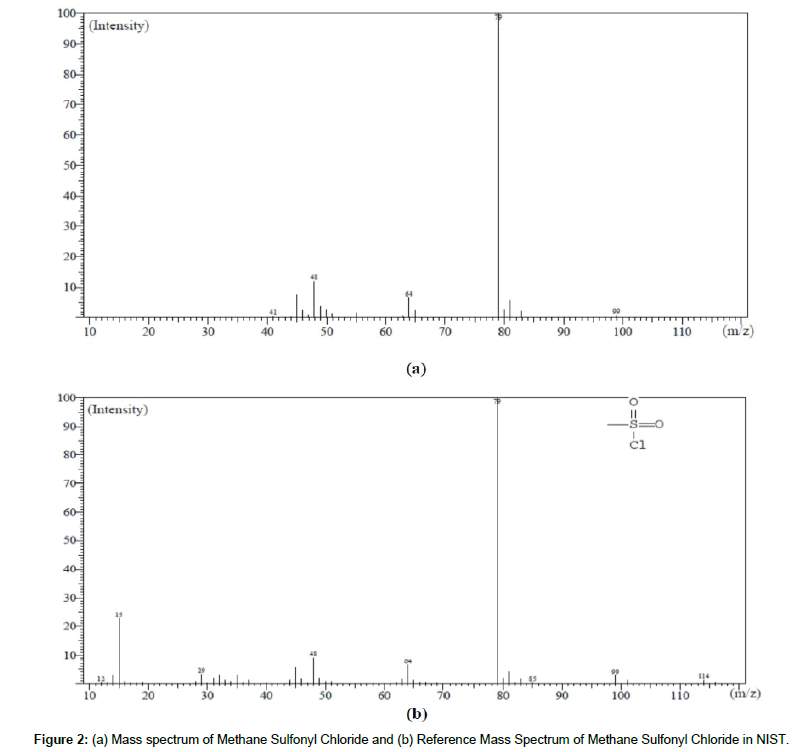
As per the analysis conducted by GC-MS and the retention times of Methane Sulfonyl Chloride was in the range 5.0 to 6.0 minutes respectively. As per the mass spectrum of Methane Sulfonyl Chloride, the fragments were observed at m/z 79. The spectrum of Methane Sulfonyl Chloride the analytes match to the reference spectrum of NIST. The Mass spectrum and reference mass spectrum of Methane Sulfonyl Chloride shown in Figure 2.
Method validation
The method validation was done by evaluating Specificity, Repeatability, linearity and range, Accuracy, Limit of Detection (LOD) and Limit of Quantitation (LOQ), LOQ- Repeatability, LOQ-Accuracy, Ruggedness and Robustness.
Specificity
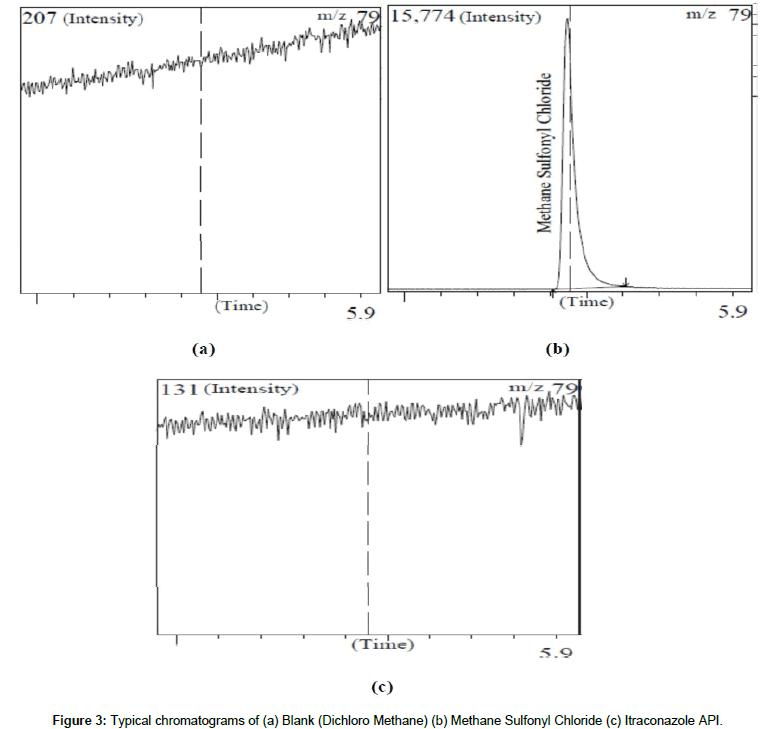
The Itraconazole API sample was spiked with Methane Sulfonyl Chloride and sample was chromatographed to examine interference, if any, of the residual solvent peaks with each other. The retention time for standard Methane Sulfonyl Chloride 5.45 min, respectively. The Chromatograms of Blank, Standard MSC and Itraconazole API were as shown in Figure 3.
Repeatability
The Methane Sulfonyl Chloride was prepared at 1.87 ppm absolute with respect to Sample concentration and injected in six replicates. The RSD (n=6) values obtained for the area of Methane Sulfonyl Chloride is 40452. The %RSD for methane Sulfonyl Chloride peak area response of Standard six injections should be not more than 15% as per USP [12]. The data of repeatability was as shown in Table 2.
| S No. | Methane Sulfonyl Chloride Area |
|---|---|
| 1 | 38014 |
| 2 | 40212 |
| 3 | 43256 |
| 4 | 40125 |
| 5 | 39851 |
| 6 | 41256 |
| Average area | 40452 |
| Standard Deviation | 1731 |
| % of RSD | 4.28 |
Table 2: Repeatability data for Methane Sulfonyl Chloride.
Linearity and range
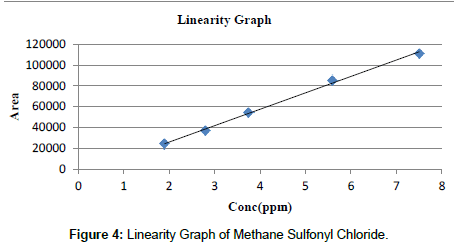
The linearity of the method was determined by making injections of Standard Methane Sulfonyl Chloride at the 1.9 ppm, 2.8 ppm, 3.75 ppm, 5.6 ppm and 7.5 ppm levels. Three replicates were performed at each level. The calibration curves were obtained with the average of peak area ratios of three replicates. The correlation coefficient (R2) value for Methane Sulfonyl Chloride was found to be higher than 0.999 and the calibration curves were linear within the range. These results revealed an excellent linearity. The linearity values for the Methane Sulfonyl Chloride as shown in Tables 3 and 4 and Linearity graph is shown in Figure 4.
| S No. | Concentration level(ppm) | Run-I Area | Run-II Area | Run-III Area | Average Area |
|---|---|---|---|---|---|
| 1 | 1.9 | 24122 | 23999 | 24911 | 24344 |
| 2 | 2.8 | 37528 | 37241 | 37002 | 37257 |
| 3 | 3.75 | 54851 | 54113 | 53989 | 54318 |
| 4 | 5.6 | 85521 | 85426 | 85422 | 85456 |
| 5 | 7.5 | 110826 | 110852 | 111001 | 110893 |
Table 3: Linearity data for Methane Sulfonyl Chloride.
| Concentration (ppm) | Area |
|---|---|
| 1.9 | 24344 |
| 2.8 | 37257 |
| 3.75 | 54318 |
| 5.6 | 85456 |
| 7.5 | 110893 |
| Correlation coefficient (R2) | 0.999 |
Table 4: Linearity Graph data for Methane Sulfonyl Chloride.
Accuracy (%Recovery)
Weighed accurately 10.0 g of the Itraconazole API into three different 20 mL of volumetric flasks and spiked with 1.9 ppm, 3.75 ppm and 5.6 ppm standard solutions of Methane Sulfonyl Chloride, add 10 mL of diluents mix well then make up with the same diluents. Inject three levels in triplicate. From accuracy data, the % recovery of Methane Sulfonyl Chloride was found within the limits (100 ± 15%). Results indicates that the method has an acceptable level of accuracy. The results are presented in below Table 5.
| S No. | Sample+1.9ppm Area |
Sample+3.75ppm Area |
Sample+5.6ppm Area |
|---|---|---|---|
| 1 | 21230 | 42452 | 61638 |
| 2 | 21290 | 42136 | 61851 |
| 3 | 21546 | 42546 | 61251 |
| Average area | 21355 | 42378 | 61580 |
| %Recovery | 105.58 | 104.76 | 101.49 |
| Standard Average Area: 40452 | |||
Table 5: Accuracy data for Methane Sulfonyl Chloride.
Limit of detection (LOD) and quantitation (LOQ)
The LOD and LOQ were calculated by instrumental and statistical methods. For the instrumental method, LOD is determined as the lowest amount to detect, and LOQ is the lowest amount to quantify, by the detector. The LOD and LOQ of Methane Sulfonyl Chloride in Itraconazole API were determined based on Linearity. Prepare the Standard Methane Sulfonyl Chloride solution at LOD (0.44 ppm) and LOQ (1.32 ppm) concentrations. The area of Methane Sulfonyl Chloride at LOD Concentration is 3985 and LOQ concentration 13134. The linearity also passed at LOQ Concentration. The data of LOD and LOQ as shown in Table 6.
| S No. | Conc. (ppm) | Area |
|---|---|---|
| 1 | 1.9 | 24344 |
| 2 | 2.8 | 37257 |
| 3 | 3.75 | 54318 |
| 4 | 5.6 | 85456 |
| 5 | 7.5 | 110893 |
| Correlation Coefficient(R2) | 0.999 | |
| Slope | 15742 | |
| STEYX | 2078 | |
| LOD | 0.44 ppm | |
| LOQ | 1.32 ppm | |
Table 6: Linearity Graph data for Methane Sulfonyl Chloride at LOQ Concentration.
Repeatability at LOQ concentration
Prepare the Standard Methane Sulfonyl Chloride solution at LOQ concentration (1.32 ppm) and injected in six replicates. The RSD (n=6) values obtained for the area of Methane Sulfonyl Chloride is 13134. The acceptance criteria of %RSD for Methane Sulfonyl Chloride is more than 15%. The LOQ Repeatability data and Chromatograms of LOD and LOQ as shown in Table 7 and Figure 5.
| S No. | Methane Sulfonyl Chloride Area |
|---|---|
| 1 | 12024 |
| 2 | 13884 |
| 3 | 12076 |
| 4 | 15470 |
| 5 | 11292 |
| 6 | 14058 |
| Average Area | 13134 |
| Standard Deviation | 1589 |
| % of RSD | 12.10 |
Table 7: Repeatability data for Methane Sulfonyl Chloride at LOQ Concentration.
Accuracy at LOQ concentration
Weighed accurately 10.0 g of the Itraconazole API into three different 20 mL of volumetric flasks and spiked with LOQ level (1.32 ppm) standard solution of Methane Sulfonyl Chloride, add 10 mL of diluents mix well then make up with the same diluents and inject in triplicate. From accuracy data at LOQ level, the % recovery of Methane Sulfonyl Chloride was found within the limits (100 ± 15%). The data of LOQ-Accuracy was as shown Table 8.
| SNo. | Sample+LOQ Level Area |
|---|---|
| 1 | 14064 |
| 2 | 13697 |
| 3 | 14686 |
| Average area | 14149 |
| Standard Average Area | 13134 |
| %Recovery | 107.73% |
Table 8: LOQ Accuracy data for Methane Sulfonyl Chloride.
Ruggedness
Ruggedness of the method was evaluated by performing the sample analysis in six replicates using different analyst on different days. The %RSD values of less than 15.0% for Methane sulfonyl chloride content indicate that the method adopted is rugged. The data of Ruggedness was shown in Table 9.
| SST Parameter | Day-1 | Day-2 | Analyst 1 | Analyst 2 |
||||
| Analyst 1 |
Analyst 2 |
Analyst 1&2 |
Analyst 1 |
Analyst 2 |
Analyst 1&2 |
Day 1&2 |
Day 1&2 |
|
| %RSD | 6.09 | 5.01 | 5.32 | 4.84 | 4.91 | 4.69 | 5.44 | 4.77 |
Table 9: Ruggedness data for Methane Sulfonyl Chloride.
Robustness
This study was performed by making small but deliberate variations in the method parameters. The effect of variations in flow rate of carrier gas and Column Oven temperature was studied. Under all the variations, system suitability requirement is found to be within the acceptance criteria and hence the proposed method is robust. The relative standard deviation of area counts for Methane Sulfonyl Chloride peak obtained from six replicate injections of standard solution should be not more than 15.0%. The data of Robustness was as shown in Table 10 and Table 11.
| System Suitability Parameter |
0.5mL/min (Flow Minus) |
1.0mL/min (Control) | 1.5mL/min (Flow Plus) |
| % RSD | 5.69 | 5.28 | 5.58 |
Table 10: Methane Sulfonyl Chloride Robustness (Flow variation).
| System Suitability Parameter |
195°C (Temperature Minus) |
200°C (Control) | 205°C (Temperature Plus) |
|---|---|---|---|
| % RSD | 4.83 | 4.71 | 4.39 |
Table 11: Methane Sulfonyl Chloride Robustness (Column Oven Temperature).
Conclusion
A simple high throughput GC-MS method has been developed and fully validated for determination of Methane sulfonyl chloride in Itraconazole API. This method is specific, sensitive, and reproducible and has been successfully to monitor and control impurity level. The residue Methane Sulfonyl Chloride was determined in ppm levels also. The method well suits for the intended purpose.
Acknowledgements
SVRM College and Management are acknowledged for financial support of this research project.
References
- Verenitch SS,Lowe CJ, Mazumder A (2006) J Chromatogr A 1116: 193-203.
- Wikipedia,Gas chromatography-mass spectrometry [https://en.wikipedia.org/wiki/Gas_chromatography%E2%80%93mass_spectrometry]Wikipedia.
- SGE, How to Improve Your GC-MS Analysis [http://www.sge.com/uploads/55/6a/556ae4e55c042c95832191d30557f017/TA-0061-C.pdf] SGE.
- The European Agency for the Evaluation of Medicinal Products (1998)ICH Topic S1B, Note for Guidance on Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals.
- European Medicines Agency for the Evaluation of Medicines for Human Use (2006) Guideline on the Limits Of Genotoxic Impurities.
- MamidalaDKAnalysis of Genotoxic Impurities in API’s in Compliance With ICH Guidelines Using Gas Chromatography Mass Spectrometry.Centre of Excellence for Analytical Sciences, PerkinElmer (India) Pvt Ltd.
- Zhao L, Quimby B, Analysis of Drugs by using the Ultra Inert Inlet Liners with Wool. Agilent Technologies, Inc, USA.
- Sanjay BB,Kadam BR, Jaiswal YS, Shirkhedkar AA (2007) Significance in Active Ingredient. Eurasian Journal of Analytical Chemistry 2: 33-53.
- Veenaeesh P, Manikumar G, Manjusha. P, Padmasri A (2014)Determination of Methyl Methane sulfonate, Ethyl Methane sulfonate and Isopropyl Methane sulfonate Impurities in Lopinavir API by GC/MS/MS using Electron Ionization. International Journal of Pharma Research & Review3: 11-16.
- Kashif UL HAQ, KumarN(2014) Validation of LC-MS/MS Method for the Simultaneous Estimation of Itraconazole and its Metabolite hydroxyl Itraconazole in Plasma. Asian J Pharm Clin Res 7: 131-136.
- WHO (2001) The Impact of Implementation of ICH Guidelines in Non ICH Countries. Report of a WHO Meeting, Geneva.
- USP(2006) United States Pharmacopoeia pp: 1577-1578.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16773
- [From(publication date):
June-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 15498
- PDF downloads : 1275