Research Article Open Access
Development and Evaluation of Sustained Release Microspheres of Glibenclamide by Emulsion Solvent Evaporation Method
| Rashmi R Kokardekar, Yogesh S Chaudhari, Suresh D Kumavat and Harshal A Pawar* | |
| Dr. L. H. Hiranandani College of Pharmacy, C.H.M Campus, Ulhasnagar, Maharashtra, India | |
| Corresponding Author : | Harshal Ashok Pawar Assistant Professor and Head of Department (Quality Assurance) Dr. L. H. Hiranandani College of Pharmacy Smt. CHM Campus, Opp. Ulhasnagar Railway Station Ulhasnagar-421003,Maharashtra, India Tel: +91-8097148638 E-mail: hapkmk@rediffmail.com |
| Received November 18, 2014; Accepted November 28, 2014; Published December 2, 2014 | |
| Citation: Kokardekar RR, Chaudhari YS, Kumavat SD Pawar HA (2014) Development and Evaluation of Sustained Release Microspheres of Glibenclamide by Emulsion Solvent Evaporation Method. Clin Pharmacol Biopharm 3:127. doi:10.4172/2167-065X.1000127 | |
| Copyright: © 2014 Kokardekar RR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Oral administration of Glibenclamide appears to lower the blood glucose acutely by stimulating the release of insulin from the pancreas. Gastro intestinal absorption of Glibenclamide in man is uniform, rapid and essentially complete having peak plasma concentration 1-3 hours after single oral dose and half-life of elimination three hours in normal subjects. The objective of the present investigation was to formulate and evaluate microspheres of Glibenclamide by emulsion – solvent evaporation method. Microspheres were prepared using Ethyl Cellulose N100 and characterized for their micromeritic properties, particle size and encapsulation efficiency. The in-vitro release studies were performed using pH 1.2 (0.1N HCL) buffer revealed that the drug release was sustained up to 24 hours. SEM studies showed that the microspheres were spherical and porous in nature. In-vivo studies were performed in healthy rabbits to analyze the floating efficiency of microspheres. Microspheres of glibenclamide were prepared successfully and could help to manage better the complications involved in Type II diabetes.
| Keywords |
| Microspheres; Type II Diabetes; In vitro release; In vivo floating studies |
| Introduction |
| Glibenclamide in oral conventional dosage form has the dosage regime of three times a day due to short elimination half-life of 3 hours [1,2]. The extended release single unit dosage form has the demerits of all and nothing effect, person to person variability and non-uniform drug release [2,3]. These complains certainly can be overcome by the sustained release multiunit dosage form like microspheres. The principal aim of the investigation undertaken was to develop a Multi-Particulate Drug Delivery System for non-insulin dependent diabetes mellitus drug, Glibenclamide. In the present investigation, microspheres were prepared using emulsion-solvent evaporation technique and Ethyl Cellulose N100 was used as a rate retardant polymer. Ethyl Cellulose N100 is a water insoluble polymer which is widely used as a wall material for controlled release microparticles. This is due to its biocompatibility, good stability, easy fabrication and low cost. |
| Materials and Methods |
| Materials |
| Glibenclamide was obtained as gift sample from Inga Laboratories, India. Ethyl Cellulose N100 was obtained from Hercules Incorporated, Aqualon division, India. Tween 80 was obtained from Suyojit Chemicals Works Private Limited, India. Other chemicals used were of analytical reagent grade. |
| Preformulation study |
| The presence of incompatibilities between an active drug substance and different excipients forms an important part of the preformulation. The drug excipient interaction was studied by Differential Scanning Calorimeter (DSC). DSC measures heat flows coupled with transitions in materials as a function of time and temperature in a controlled atmosphere. This provides qualitative and quantitative information about physical and chemical changes that involve endothermic or exothermic reactions. |
| Preparation of microspheres |
| Emulsion Solvent evaporation method was used for the preparation of Glibenclamide microspheres [4-6]. Glibenclamide was dissolved in organic solvent containing polymer by stirring with manual stirring with glass rod till it dissolved completely. This solution was the poured slowly into the continuous aqueous phase containing emulsifying agent. This resulted in formation of o/w emulsion. This emulsion was stirred at a constant speed till the organic solvent evaporated which lead to the precipitation of polymer, thus encapsulating the drug and hence formation of rigid microspheres. After removal of organic solvent, the microspheres formed were collected by filtration, washed 3-4 times with distilled water. Microspheres were allowed to dry at room temperature for 24 hours. Nine different batches of microspheres were prepared by changing drug to polymer ratio and stirring speed. The composition of formulated batches (Code: EC1-EC9) of Glibenclamide microspheres is shown in Table 1. |
| Evaluation of microspheres |
| The prepared batches of microspheres were characterized for their micromeritic properties (Particle size, angle of repose, bulk density, tapped density and Carrs index) and encapsulation efficiency as per the standard procedures reported in literature [1,3]. |
| The in vitro dissolution of batch EC5 was carried out in 900 ml of pH 1.2 Buffer as the dissolution medium using USP Type II apparatus (TDT -08L, Electrolab) apparatus at 75 rpm as per the dissolution conditions recommended by US-FDA OGD. The temperature was maintained at 37 ± 0.5°C. The dissolution was carried out for 24 hours. The sampling volume was 5 ml. The time points included were 0.5 hr, 1 hr, 2 hr, 3 hr, 4 hr, 5 hr, 6 hr, 7 hr, 8 hr, 9 hr, 10 hr, 11 hr, 12 hr, 22 hr, 23 hr and 24 hrs. The absorbance’s of the sample at different time intervals were carried out using UV visible spectrophotometer (UV 1800, Shimadzu) at λmax of 300 nm. The drug release was calculated using calibration curve subjected to various kinetic models such as zero order, first order, Higuchi model, Hixson crowell model and Korsmeyer Peppa model to study the mechanism of release of drug from microspheres. |
| In-vivo floating efficiency of microspheres was studied in healthy rabbits [7]. Scanning electron microscopy (SEM) was performed on optimized batch of microspheres to study the surface morphology of microspheres. |
| Results and Discussion |
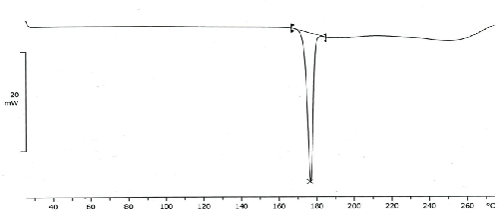
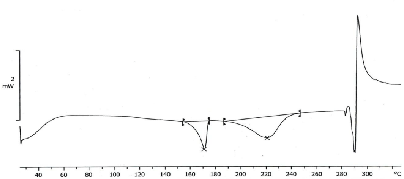
| The thermograph of DSC of glibenclamide pure drug (Figure 1) showed a sharp endothermic peak around 175°C whereas no such peaks were observed in the thermograph of the formulation (Figure 2) indicating the drug has been dispersed thoroughly at molecular level in the formulation. Therefore, it may be concluded that the drug is in intact form within the formulated microspheres. |
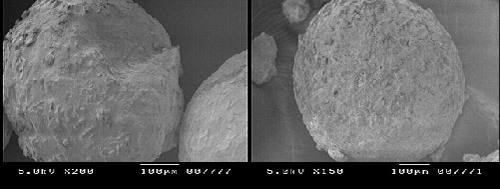
| Thermal behavior of glibenclamide microspheres with Ethyl cellulose N100 by DSC shows no peak indicating no drug polymer interaction. But its mixture with Ethyl cellulose N100 reduce the number of peaks and peak heights which suggest that the crystalline form got converted to amorphous form and it is in good agreement with enhanced solubility. Scanning electron microscopy images demonstrated spherical shaped microspheres. |
| The prepared glibenclamide microspheres by solvent evaporation technique were discrete, spherical and free flowing having good drug entrapment. The results of micromeritic properties of microspheres and percentage drug entrapment (Entrapment efficiency) of various batches of microsphere are summarized in Table 2. The results indicated that all the prepared microspheres possess good flow property with particle size ranging from 111.13 μm to 140.12 μm. The batch EC5 showed highest drug entrapment of about 82.33%. |
| The in vitro release data indicated that batch no. EC5 shows satisfactory sustained release through microspheres for 24 hrs and hence was considered as optimized batch. The in-vitro drug release data of optimized batch is represented in Table 3. |
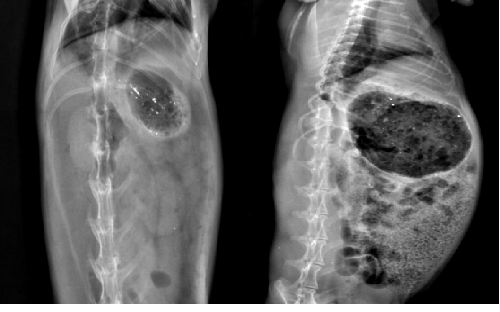
| The in vivo floating behavior of ethyl cellulose microspheres loaded with barium sulphate was investigated by radiographic images (X-ray photographs) of rabbit’s stomach at specific periods. The amount of X-ray opaque material in these hollow was sufficient to ensure visibility by X-ray but at same time the amount of barium sulphate (15 mg) was low enough to enable the hollow microspheres to float. The hollow microspheres did not adhere to the gastric mucous and floating on the gastric fluid for more the 12 h. This was evident by the X-ray photographs taken at 12 h & 24 h (Figure 3) [8,9]. |
| The in-vivo study carried out in rabbits confirmed that the microspheres remained in the floating state till 24 hours. |
| In order to assess the kinetic release of the formulation, it was subjected to various kinetic release models as summarized in Table 4. |
| It could be seen that the formulation followed Korsmeyer-Peppas model with highest regression coefficient value of 0.991. |
| Korsmeyer and Peppas (1983) equation i.e Qt / Q∞ = KKt n, where Qt / Q∞ is the fraction released by the drug at time t, KK is a constant incorporating structural and geometric characteristic and n is the release exponent characteristic for the drug transport mechanism. Table 5 summarizes the release exponent ‘n’ value for different release kinetics reported in literature [10]. |
| The value of n as estimated by linear regression of log Qt / Q∞ vis log t of formulation EC5 was 0.964 indicated that drug release from microspheres followed Anomalous (non-fickian) Diffusion. |
| The SEM photomicrographs of optimized batch no. EC5 depicted in Figure 4. Photomicrographs show that the microspheres are white, spherical with smooth surface. |
| Conclusion |
| Based on the results obtained for in-vitro drug release, it was concluded that microspheres formulated with glibenclamide to ethyl cellulose ratio 1:2 and keeping as stirring speed 800 RPM shows satisfactory drug release up to 24 hrs. These microspheres were also evaluated for in-vivo floating ability in the upper part of the GIT (gastric fluid), so that it would be retained in the upper part of GIT and subsequently sustain the release of drug for a period of as long as 24 h. The in-vivo floating ability of microspheres was captured at different time intervals with the help of X-Ray machine and it was concluded that the microspheres withstood the motility of the stomach and were successfully retained in the GIT. Microspheres of glibenclamide were prepared successfully and could help to manage better the complications involved in Type II diabetes. |
| Acknowledgements |
| Authors are very much thankful to Dr. P.S. Gide, Principal of Hyderabad Sindh National Collegiate Boards (HSNCB’s) Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar for his continuous support, guidance and encouragement. |
| References |
References
- Tashtoush BM, Al-Qashi ZS, Najib NM (2004) In vitro and in vivo evaluation of glibenclamide in solid dispersion systems. Drug Dev Ind Pharm 30: 601-607.
- Mudgal SS, Pancholi SS (2012) Formulation of Glibenclamide Solid Dispersions by Solvent Evaporation Technique. Journal of Chemical and Pharmaceutical Research 4: 353-359.
- Dash SK, Khan AS, Das SR, Padhan A, Rout D, et.al. (2012) Formulation and In-Vitro Evaluation of Sustained Released Glibenclamide Microspheres. International Journal of Pharmaceutical Sciences and Research 3: 1433-1443.
- Prasanth VV, Moy AC, Mathew ST, Mathapan R (2011) Microspheres - An Overview. International Journal of Research in Pharmaceutical and Biomedical Sciences 2: 332-338.
- Madhav NVS, Kala S (2011) Review on Microparticulate Drug Delivery System. International Journal of PharmTech Research 3: 1242-1254.
- Ashvini V, Kavitha K, Mehaboob Y (2011) Design and Evaluation of Ketoprofen Loaded Albumin Microspheres. Pharma Science Monitor: An International Journal of Pharmaceutical Sciences 2: 189-203.
- Dutta P, Sruti J, Niranajan Patra Ch, Bhanoji Rao ME (2011) Floating Micropsheres: Recent Trends in the Development of Gastroretentive Floating Drug Delivery System. International Journal of Pharmaceutical Sciences and Nanotechnology 4: 1296-1306.
- Singh BN, Kim KH (2000) Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 63: 235-259.
- Srivastava AK, Ridhurkar DN, Wadhwa S (2005) Floating microspheres of cimetidine: formulation, characterization and in vitro evaluation. Acta Pharm 55: 277-285.
- Chandra D, Kumar A, Singh R , Raj T, Maurya JK, et al. (2013) Formulation and Evaluation of Proniosomal Gel of Flurbiprofen. American Journal of PharmTech Research 3: 622-637.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 14774
- [From(publication date):
February-2015 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 10067
- PDF downloads : 4707
