Development and Evaluation of Mucoadhesive Patch Using a Natural Polysaccharide Isolated from Cordia dichotoma Fruit
Received: 14-Oct-2014 / Accepted Date: 17-Nov-2014 / Published Date: 22-Nov-2012 DOI: 10.4172/2329-9053.1000120
Abstract
Mucoadhesive formulations have been developed for oral, buccal, nasal, ocular, rectal and vaginal routes for either systemic or local effects. This drug delivery system is suitable for drugs undergoing high first pass metabolism. It improves bioavailability, reduces dosing frequency and thereby minimizes the side effects and also makes the dosage forms more cost effective. In the present study, polysaccharide derived from the fruits of Cordia dichotoma (Family-Boraginaceae) was investigated as a mucoadhesive polymer in oral (Buccal or sublingual) patch formulation. Ramipril, an antihypertensive drug, was selected as a model drug. The patches of Ramipril were prepared separately using different concentrations (2.5%, 5%, 7.5% and 10%) of isolated Cordia dichotoma fruit polysaccharide (Natural) by solvent casting method. The prepared patches were evaluated for various parameters. The drug excipient interactions were characterized by FTIR studies. The formulation F4 showed good swelling index with excellent mucoadhesive strength and convenient residence time as well as slow and sustained drug release.
Keywords: Mucoadhesive Patch; Cordia dichotoma; Solvent Casting; Boraginaceae
1627Introduction
Buccoadhesive (either sublingual or buccal) route of drug delivery provides direct access to the systemic circulation through the internal jugular vein bypassing the first pass metabolism leading to high bioavailability [1]. Other advantages such as excellent convenience, low enzymatic activity, painless administration, easy drug withdrawal, capability to include permeation enhancer, enzyme inhibitor or pH modifier in the formulation, flexibility in designing as multidirectional or unidirectional release systems for local or systemic actions make mucoadhesive drug delivery system as promising option for continued research [2].
In the present research work Cordia dichotoma polysaccharide was used as base matrix polymer for development of mucoadhesive bilayer patch of Ramipril. Because of the properties such as hydrophobicity, low water permeability, drug impermeability, and moderate flexibility, ethyl cellulose was used as a backing layer polymer [3,4].
Cordia dichotoma Forst. Fruits are 1.3-2.5 cm long globose or ovoid glossy, yellowish brown, pinkish or nearly black when ripe, usually single seed surrounded by a transparent, sticky and sweet edible pulp. Conventionally Cordia dichotoma fruits were used as vegetables, for making pickles and glues [5]. The other parts of the plant like leaves and bark have been reported for various pharmacological activities such as analgesic, anti-inflammatory, antioxidant, antitumour etc. [6]. Qualitative analysis for the presence of plant constituents such as carbohydrates, alkaloids, glycosides, flavonoids, tannins and saponins have been reported in literature [7]. Chemicals screening of both the leaves and the fruits showed the presence of pyrrolizidine alkaloids, coumarins, flavonoids, saponins, terpenes and sterols [8]. The fruit contains about 70% pulp. The pulp contains 6 gm water, 35 gm protein, 37 gm fat, and 18 gm carbohydrate per 100 gm. The seed contains per 100 gm: water 32 gm, fat 46 gm, the principle fatty acids are: palmitic acid, stearic acid and linoleic acid [9-11].
Ramipril was selected as a model drug in the present study. Ramipril is (1S, 5S, 7S)-8-[(2S)-2-[[(1S)-1-Ethoxycarbonyl-3-/phenyl-propyl] amino] propanoyl] azabicyclo [3.3.0] octane-7-carboxylic acid. It is used to treated high blood pressure and heart failure. It is administered orally, in doses of 2.5, 5, 7.5 or 10 mg/d [12]. It has low oral bioavailability (28%) due to high first pass metabolism which makes oral treatment unsatisfactory. Ramipril is a prodrug. It is used as a drug for treatment of hypertension and related cardiovascular diseases [13]. It acts on the renin–angiotensin aldosterone system. It inhibits the conversion of the inactive angiotensin-I to the highly potent vasoconstrictor, angiotensin-II, and also reduces the degradation of bradykinin [14]. The extent of absorption is 50-60% and is not significantly influenced by the presence of food in the GI tract. Protein binding of Ramipril is about 73%. It is a highly lipophilic and poorly water soluble drug, with absolute bioavailability of 28-35% [14]. This justifies a need to develop an effective formulation, which permits the drug to directly enter into the systemic circulation, bypassing the first-pass metabolism, thereby increasing bioavailability of Ramipril. Sublingual and buccal route of administration is one such alternative.
In the present study, oral mucoadhesive patch of Ramipril for sublingual and buccal administration was developed and optimized. The efforts were made to improve sublingual and buccal penetration of the drug. Bilayer design of the patch was selected to obtain unidirectional release of the drug, greater surface area of contact, and administer the bitter drug without the taste masking.
Materials And Methods
Ramipril was received as a gift sample from Lupin pharmaceuticals, Mumbai, India. Toluene and ethanol were purchased from Merk, India. Ethyl cellulose was obtained from Colorcon whereas Poly Ethylene Glycol 400 was received as a gift sample from Alkem Laboratories, Mumbai.
Collection and authentication of plant material
Cordia dichotoma fruits were collected from Maharashtra region (India) in the month of June. Plant was authenticated by Dr. Rajendra D. Shinde, Associate Professor, Blatter Herbarium; St. Xavier’s College, Mumbai and was identified as Cordia dichotoma G. Forst (Herbarium Specimen no. 1702 of S.M. Almeida.). The specimen sample of the plant is preserved with department of Quality Assurance, Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar.
Isolation of polysaccharide from cordia dichotoma fruits
The polysaccharide was isolated from Cordia dichotoma fruit using solvent precipitation method [10]. Ripe fruits of Cordia dichotoma were extracted with water (1:2) by stirring for 3h. The viscous solution obtained was filtered through Muslin cloth. Ethanol (95%) was added with continuous stirring to the viscous solution obtained in the ratio 1:1 to precipitate out polysaccharide present in the fruit. The precipitated polysaccharide was transferred to an evaporating dish and treated consecutively with ethanol to make it free from impurities. The polysaccharide obtained was dried in oven at temperature 40-45°C. The dried polysaccharide was then size reduced by passing through sieve no. 60 and stored in airtight container.
Fourier transmission infrared (FTIR) spectroscopy
The compatibility study was carried out using FTIR (Shimadzu IR affinity1). The compatibility testing was carried out by using Potassium bromide (KBr) pellet method. KBr was mixed with the sample in a mortar pestle. The sample was compacted using a pellet press. The pellet was placed in the IR sample holder. A background scan of KBr was taken followed by the IR scan of the sample [15].
IR spectrum of samples (Drug, polysaccharide and other excipients) were recorded using potassium bromide pellet over range 4000-500 cm-1 and standard peaks were measured using Shimadzu IR affinity1.
Preparations of mucoadhesive patch
Mucoadhesive bilayered patches were prepared by using solvent casting method. The patches were prepared using following two steps:
Preparation of backing membrane: 4 gm of ethyl cellulose was soaked in 20 ml of alcohol: toluene mixture (1:4) and kept for 1 h. To this solution remaining quantity (80 ml) of above solvent and glycerol 10-20% w/v of polymer concentration added as a plasticizer and mixed using mechanical stirrer till finely dispersed thick solution was obtained. Plasticized polymeric solution then poured on plain glass mould and dried immediately in oven at 40° C [16].
Preparations of core layer: Soaked polymer into half quantity of solvent for 2 h. Plasticizer and remaining quantity of solvent added into it and stirred till get homogenous solution. Calculated amount of drug equivalent to 2.5 mg of drug/cm2 was added to the solution and mixed. The prepared solution was poured in to mould and dried at room temperature. An inverted funnel was placed over the mould to prevent fast evaporation of the solvent. The dried patches were packed in aluminium foil and stored in desiccators to maintain the integrity and elasticity of the patches until further use [16]. The composition of various mucoadhesive patch formulations prepared is shown in (Table 1)
| Formulation Code | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Ramipril (mg) Cordia dichotomapolysaccharide (%) Sodium CMC(mg) Propylene Glycol (ml) Distilled Water (ml) |
2.5 2.5 100 0.5 10 |
2.5 5 100 0.5 10 |
2.5 7.5 100 0.5 10 |
2.5 10 100 0.5 10 |
Table 1: Composition of Mucoadhesive Patch.
Evaluation of mucoadhesive patch
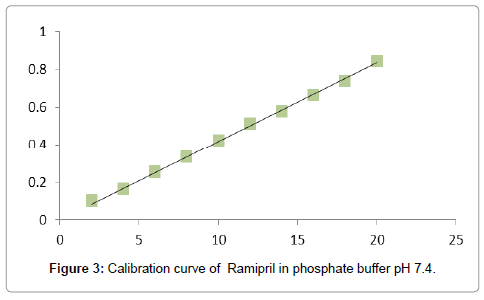
Preparation of standard calibration curve of ramipril: Calibration curve of Ramipril was made by using phosphate buffer pH 7.4 [17]. The stock solution (100 ppm) of Ramipril was prepared by dissolving 10 mg of drug initially in 10 ml of methanol and then followed by dilution up to 100 ml with phosphate buffer pH 7.4 From above stock solution dilutions were made in phosphate buffer pH 7.4 to obtain 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 μgm/ ml concentration of drug. The absorbances of the solutions of different concentration were taken at 210 nm.
Physical appearance: All prepared patches were visually inspected for its color, clarity, flexibility and smoothness [11].
Weight uniformity: Five patches of size 1 × 1 cm2 were weighed individually and average value of all patches was determined [17,18].
Thickness uniformity: It was measured for five samples using a screw gauge and average thickness was calculated [18].
Folding endurance: Patch was repeatedly folded at same place till it broke. Number of times patch folded at same place without breaking gives value of folding endurance [11].
Determination of moister content and moister absorption
a) Moister content: Individual Patches were weighed accurately and kept in desiccators containing anhydrous
calcium chloride. After three days, the patches were taken out and weight. The percent moister content was determine by calculating % moister loss using the following formula [17]

b) Moister absorption: Individual patches were weighted accurately and placed in desiccator containing 100 ml saturated solution of aluminum chloride, which maintains 80% and 90% relative humidity. After three days, the patches were taken out and weight. The % moister absorption was calculated using following formula [11,17].
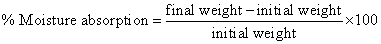
Water vapor transmission rate: Glass vials of 5 ml capacity were washed thoroughly and dried to constant weight in an oven. About 1 gm of fused calcium chloride was taken in vials and the polymer films of 2.25 cm2 were fixed over the brim with help of an adhesive tape. Then the vials were weighed and stored in humidity chamber of 80-90% RH condition for a period of 24 h. the vials were removed and weighed at 24 h time interval to note down the weight again [11].

Measurement of % elongation breaks: The initial length of patch was measured on scale and applying the force on patch unit until the patch was broken and calculated the % elongation of patch by using following formula [18].
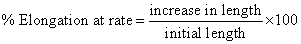
Drug content: The patches (1 cm2) were cut and added to 100 ml of phosphate buffered saline of pH 7.4. The medium was stirred with magnetic bead. The contents were filtered using whatman filter paper and the filtrate was examined for the drug content against the phosphate buffered saline of pH 7.4 at 210 nm using UV - Spectrophotometer. Three such trials were carried out [11,18].
In vitro drug release studies: These studies were performed by using a modified diffusion cell with a receptor compartment capacity of 16 ml. The synthetic cellophane membrane was mounted between the donor and receptor compartment of the diffusion cell. The formulated patches were cut into size of 1 cm2 and placed over the drug release membrane and the receptor compartment of the diffusion cell was filled with phosphate buffer pH 7.4. The whole assembly was fixed on a magnetic stirrer, and the solution in the receptor compartment was constantly and continuously stirred using magnetic beads at 50 rpm. The temperature was maintained at 37 ± 0.5°C. The amount of drug permeated was determined by removing aliquot of 1 ml sample at appropriate time interval and analyses for drug content was performed spectrophotometrically at 210 nm against blank. The receptor phase was replaced with an equal volume of phosphate buffer pH 7.4 at each time of sample withdrawal [17]. The obtained in vitro release data was fitted to various kinetic model such as zero order, first order, Higuchi model, Hixon-Crowell and Korsmeyer Peppas model.
Surface pH study: Patch was allowed to swell by keeping it in contact with 2% agar gel plate for 2 h at room temperature. The pH was measured by bringing electrode in contact with surface of patch and allowing it to equilibrate for 1 min [18].
Ex-vivo mucoadhesive residence time: Measured (n = 3) after application of patches onto freshly cut goat buccal mucosa. The fresh buccal mucosa was fixed in inner side beaker, above 2.5 cm from bottom, with cyanoacrylate glue. One side of each patch was wetted with one drop of isotonic phosphate buffer pH 6.8 and pasted to goat buccal mucosa by applying the small force with fingertip for 30 s. The beaker was filled with 500 ml of isotonic phosphate buffer pH 6.8 and was kept at 37 ± 1°C after two min. Stirring rate of 50 rpm was applied to stimulate the buccal cavity environment. The time required for patch to detach from goat buccal mucosa was recorded as mucoadhesive time [18].
Swelling study
a) Weight increase due to swelling: Drug loaded patches of 1× 1 cm2 were weighted individually (W1) and placed separately in 2% agar gel plates with core facing the gel surface and incubated at 37 ± 1°. At regular intervals (1, 2, 3, 4, 5, and 6 h) the patches were removed from petri dishes and excess water removed carefully using filter paper. The swollen patch was then reweighted (W2) and swelling index (SI) were calculated using the formula [17].
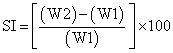
b) Area increase due to swelling: Drug loaded patch size 1×1 cm2 was cut and placed in petridish. A graph paper was placed in petridish (beneath) to measure the increase in the area. 50 ml of phosphate buffer pH 6.8 was poured into petridish. An increase in length and breadth of patch was noted at five min intervals for 60 min. and area was calculated. The% swelling (% S) was calculated using formula
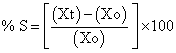
Where;
Xt = weight or area of swollen patch after time t
X0 = original patch weight or area at time=0
Drug release from backing layer: For determination of drug release from the backing layer, Franz diffusion cell was used. A bilayered buccal patch was placed between donor and receptor compartment. The complete unit was maintained at 37°C, donor compartment (3 ml) was filled with simulated saliva pH 6.8 (sodium chloride 4.5 gm, potassium chloride 0.3 gm, sodium sulfate 0.3 gm, ammonium acetate 0.4 gm, urea 0.2 gm, lactic acid 3 gm and distilled water up to 1,000 ml, adjusting pH of solution to 6.8 by 1 M NaOH solution) and receptor compartment (21 ml) contained phosphate buffer pH 7.4 with synchronous stirring. At predetermined interval 2 ml sample was removed from donor compartment and analyzed at 210 nm by UV spectrophotometric analysis to check release of drug from the backing layer of the patch [19].
Results And Discussion
The polysaccharide isolated from Cordia dichotoma fruits was a light brown colored powder. Ramipril patch formulations were prepared using various concentrations of isolated Cordia dichotoma polysaccharide with an objective to control release of the drug for better patient compliance and improved bioavailability. Cordia dichotoma has good mucoadhesive and swelling properties [20]. The excipients were selected based on preformulation study and the information available from various literatures [3,4]. Sodium carboxy methyl cellulose has good swelling and viscosity property. Therefore, Sodium carboxy methyl cellulose was used as film forming agent. Propylene glycol was used as plasticizer since it reduces the glass transition temperature of polymers. Because of the properties such as hydrophobicity, low water permeability, drug impermeability, and moderate flexibility, ethyl cellulose was used as polymer for preparation of backing membrane by using solvent mixture of toluene and alcohol to confirm unidirectional release of drug from formulations [4].
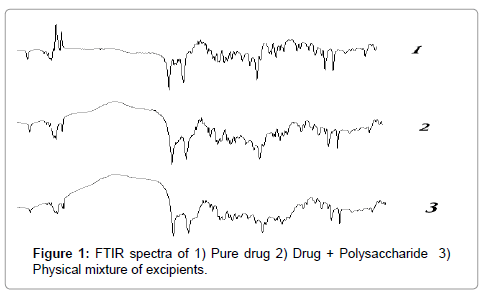
The FTIR spectra of drug, polymer and the amalgamation of drug and other excipients showed no significant interaction between drug and polymer / excipients of the prepared formulations as revealed in (Figure 1).
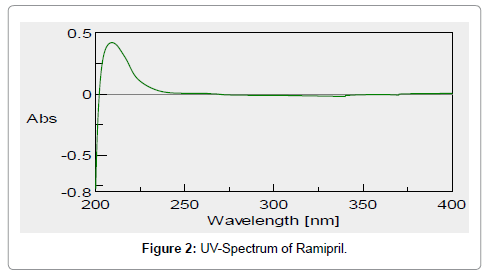
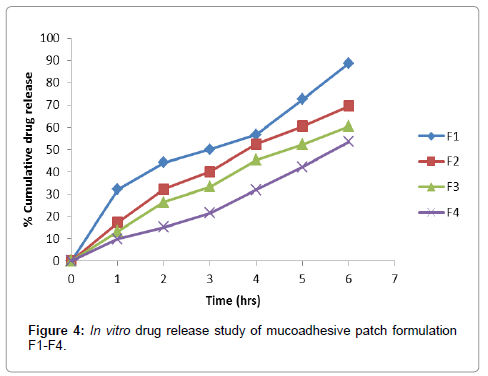
A UV maximum (λ max) of Ramipril was found to be 210 nm (Figure 2). Calibration curve of drug was found to be linear in the range of 2 μg/ml to 20 μg/ml. The regression coefficient (R2) obtained was 0.9987. Calibration curve of drug (Figure 3). In-vitro drug release data of various formulations (F1-F4) is depicted in Figure 4
The characterization parameters of the prepared patches are summarized in (Table 2). All formulated patches were visually inspected for their color and clarity. All patches were transparent in nature, smooth in appearance, and flexible in nature.
| Evaluation parameters | Formulation Code | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Weight uniformity (mg)* Thickness (mm)* Folding endurance % MC* % MA* % WVTR* % E. St. (Kg) % Drug Content In-vitro % CDR at 6th hr Surface pH MRT (Hrs.) % Weight increase % Area increase RBM (%) |
0.115 ± 0.03 0.08 ± 0.03 290 4.55 ± 1.42 5.85 ± 1.52 0.0045 ± 0.0001 7.3 97.53 88.82 6.25 3.25 260.54 56.72 0.10 |
0.118 ± 0.02 0.09 ± 0.02 298 3.85 ± 1.62 6.57 ± 2.43 0.0036 ± 0.0005 11.4 98.43 69.57 6.98 4.30 345.21 78.42 0.00 |
0.120 ± 0.05 0.10 ± 0.03 302 4.83 ± 1.54 7.25 ± 3.64 0.0042 ± 0.003 18.5 96.86 60.51 7.29 6.35 578.32 92.46 0.00 |
0.123 ± 0.03 0.13 ± 0.02 315 4.82 ± 1.26 7.45 ± 2.13 0.0037 ± 0.0005 22.15 97.14 53.50 7.15 7.15 698.49 106.34 0.10 |
Table 2: Characterization of Mucoadhesive Patch.
Average weights of all the prepared batches (F1-F4) were within limit and all the formulated batches passes weight variation criteria prescribed by USP. Thickness value was measured and found more or less uniformity in the range from 0.08 to 0.13 mm which is acceptable for buccal/sublingual administration. Folding endurance test indicates that the patch would retain the integrity with mucosa when applied. It was found that folding endurance values of patches were increased with concentration of Cordia dichotoma polysaccharide. The folding endurance was found in between 290 to 315 times for the prepared patches. This makes the system acceptable for movement of mouth, indicating good strength and elasticity.
The patches were evaluated for Percent Moisture Absorption and Percent Moisture Content to examination the physical stability of the patch at high humid circumstances and reliability of the patch in dry circumstances. Percent Moisture absorption was found between 5.85 to 7.45%. Percent Moisture Content was found in the range from 3.85 to 4.83%. Water vapor transmission studies indicate that all the patches were permeable to water vapor. The water vapor transmission values were found to be in the range of 0.0036 to 0.0045%. Percent elongation of patches was found to be in the range of 7.3–22.15 kg.
Drug content was found to be in range of 96.86% to 98.43%. The weight uniformity was satisfactory for all the formulations. The observed results of drug content uniformity indicate that the drug was uniformly dispersed, with minimum intra-batch variability.
The acidic or alkaline pH may cause irritation to the buccal and sublingual mucosa and influence the rate of hydration of the polymers. Hence, the surface pH of the mucoadhesive patch was determined. The pH was lies within the range of salivary pH, i.e. 6.5–6.8. The apparent surface pH of the preparations was found to be in the range of 6.85–7.29. The outcomes demonstrating that there is no substantial transformation in the surface pH of all the formulations, thus not causing irritation in the site of administration.
All the formulations showed good mucoadhesive strength. Among the formulations F4 showed maximum mucoadhesive strength while formulation F1 showed less mucoadhesive strength. All formulations were showed satisfactory mucoadhesive time. Formulation F4 showed maximum mucoadhesive time while formulation F1 showed less mucoadhesive time. Mucoadhesive residence time of patches was found from 3.25 h to 7.15 h. The adhesion occurs shortly after swelling but the bond formed is not very strong. The adhesion increases as the degree of hydration till the point of separating at the polymer tissue surface, which leads to sudden descent in adhesive strength due to over hydration. The formulation F4 showed highest swelling percentage among the formulated preparations. Incorporation of hydrophilic polysaccharide increases the swelling index. Patches prepared with polysaccharide concentration 10% showed highest degree of due to absorption and retention of more amounts of water and thus increasing weight and swelling index. It was observed that the percentage swelling indices of various formulations were in order of F4>F3>F2>F1. Percentage swelling of formulation F4 is the highest because it contains highest amount of hydrophilic polysaccharide as compared to other formulations. Formulation F1 showed less swelling index values due to lower polysaccharide content. Increase in weight of patch was from 260.54 mg to 698.49 mg and increase in area was from 56.72% to 106.34%.
The effectiveness of the buccal or sublingual absorption of Ramipril administration was determined by in vitro drug release studies. The in vitro drug release a study was performed using cellophane membrane. In vitro drug releases from the patches were found to be in range of 53.50% to 88.82%. In-vitro drug release data of various formulations (F1-F4) is depicted in Figure 4. The release rate from different formulations through cellophane membrane showed that, release of drug from these patches exhibited two phases. There is a initial burst effect is followed by the completion of a stable gel layer which in turn, controls the release of drug from the delivery system. The formulation F1 and F2 yielded a faster initial burst effect while the formulation F3 and F4 showed sustained release. This indicates that where there is increase in the viscosity of formulation, there is a decrease in the release rate of Ramipril. Release of drug from backing membrane was carried out to confirm unidirectional release of drug from patches. Drug release from backing membrane was found to be less than 1%.
The kinetic release data of formulation F1 to F4 is summarized (Table 3).The zero-order describes the drug release rate is independent of its concentration. The first-order describes the release of the drug from the systems is concentration dependent. Higuchi’s model describes the drug release from an insoluble matrix as a square root of a time-dependent process based on Fickian diffusion. The Hixson-Crowell cube root law model was used to understand the change in patch surface area and diameter with the progressive dissolution as a function of time [21].
| Formulation code | Zero order release | First order release | Higuchi model | Korsmeyer -Peppas model | Hixson Crowell | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | n | R2 | R2 | |
| F1 | 0.965 | 0.842 | 0.927 | 0.35 | 0.952 | 0.922 |
| F2 | 0.968 | 0.989 | 0.988 | 0.575 | 0.968 | 0.985 |
| F3 | 0.969 | 0.989 | 0.988 | 0.63 | 0.967 | 0.983 |
| F4 | 0.993 | 0.971 | 0.955 | 0.678 | 0.982 | 0.985 |
Table 3: Kinetic release data.
The obtained kinetic data explains that batch F1 follows zero order release kinetics with highest regression coefficient (R2) value 0.965. Batch F2 follows first order release kinetics with R2 vlue 0.989. Batch F3 follows higuchi release kinetics with R2 0.988. Batch F4 follows zero order drug release kinetics with R2 0.993.
The magnitude of the release exponent ‘n’ in Korsmeyer-Peppas’s model indicates that the drug release mechanism from the patch is either through Fickian diffusion, or anomalous transport as shown in Table 4 [22].
| Diffusion exponent (n) | Overall solute diffusion mechanism |
|---|---|
| 0.45 | Fickian diffusion |
| 0.45<n<0.89 | Anomalous (Non-Fickian) diffusion |
| 0.89 | Case-II transport |
| n>0.89 | Super Case-II transport |
Table 4: Diffusion exponent and solute release mechanism.
Conclusion
Cordia dichotoma polysaccharide has good film forming as well as mucoadhesive property and can be used in the sublingual/buccal patch formulation. Since Cordia dichotoma polysaccharide is inexpensive, non-toxic, compatible and easy to manufacture, it can be used in place of commercially available synthetic polymer. A percentage cumulative drug release of 53.50 at the end of 6th hr indicated that release of drug from the polymeric matrix is more prolonged at a concentration of 10% of Cordia dichotoma polysaccharide. The preliminary data obtained in the present study explains the utility of Cordia dichotoma polysaccharide as a mucoadhesive retardant polymer in formulation of sustained release dosage form. Ramipril sublingual/buccal patches could be formulated by using solvent evaporation technique using Cordia dichotoma polysaccharide. The formulation F4 showed good swelling index with excellent mucoadhesive strength and convenient residence time as well as slow and sustained drug release. Further detailed in vitro drug release study, stability studies and in vivo clinical studies are required to be carried out for the F4 formulation using a suitable animal/human model.
Acknowledgements
Authors are very much thankful to Dr. P. S. Gide, Principal, Hyderabad (Sindh) National Collegiate Board’s Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar for his continuous support, guidance and encouragement.
References
- Shojaei AH (1998) Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci 1: 15-30.
- Hoogstraate AJ, Wertz PW (1998) Drug delivery via the buccal mucosa. Pharmaceutical Science & Tech Today 7: 309–316.
- Nafee NA, Ismail FA, Boraie NA, Mortada LM (2003) Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm 264: 1-14.
- Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ (1998) Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release 55: 143-152.
- Vidyasagar G, Jadhav AG, Bendale AR, Sachin B (2011) Isolation of Cordia Mucilage and its comparative evaluation as a binding agent with standard binder. Der Pharmacia Sinica 2: 201-207.
- Anand SD, Mallikarjun SC, Arvind MB, Muralikrishna KS (2011) Cordia Dichotoma Polysaccharide : A Functional Polysaccharide For pharmaceutical applications. International Journal of Current Pharmaceutical Research 4: 10- 13.
- Parmar NS, Parmar S (1998) Anti-ulcer potential of flavonoids. Indian J Physiol Pharmacol 42: 343-351.
- Martin DL, AlurconMJ, Motilva UJ (1994) Antiulcerogenic activity of flavonoids and gastric protection. Ethnopharmacol 42: 161-170.
- Srivastava SK, Srivastava SD (1979) Taxifollin 3,5-dirhamnoside from the seeds of Cordia dichotoma. Phytochemistry 18: 205-208.
- Pawar HA, Jadhav P (2014) Int. J. Biol. Macromol. DOI: 10.1016/j.ijbiomac.2014.10.048 (Article in Press)
- Senthilkumar SK, Bharath N, Tamizhmani T (2011) Design and evaluation of Transdermal films of Ramipril. Int journal of pharmaceutical sciences Letters 2: 44-48.
- Warner GT, Perry CM (2002) Ramipril: a review of its use in the prevention of cardiovascular outcomes. Drugs 62: 1381-1405.
- Tiwary AK, Sapra B, Jain S (2007) Innovations in transdermal drug delivery: formulations and techniques. Pat Drug Deliv Formul 1: 23-36.
- American society of Health system Pharmacists (2004) Drug Information, 7272 Wisconsin Avenue, Bethseda, , MD 20814, Inc. AHFS 1869-75 .
- Priya S, Rathnanand M (2011) Preparation and Evaluation of Buccal Mucoadhesive Patch of Betamethasone Sodium Phosphate for the Treatment of Oral Submucous Fibrosis. Journal of Chemical and Pharmaceutical Research 6: 56-65.
- Anjankumar PB (2011) Design and evaluation of Buccal patches of valsartan. International journal of pharmaceutics and cosmetology 2: 51-55.
- Samyaktha RB (2013) Formulation and in-vitro characterization of venlafaxine HCL mucoadhesive buccal patches. International journal of inventions in pharmaceutical sciences 1:15-21.
- Amit K, Parridhi J, Dheeraj B, Dinesh J (2009) Development of mucoadhesive buccal patch cointaing Aceclofenac: in vitro evaluations. International journal of Pharm Tech Research 4: 978-981.
- Woolfson AD, McCafferty DF, McCarron PA, Price JH (1995) A Mucoadhesive patch Cervical drug delivery system for theadministration of 5-fluorouracil to cervical tissue. J Control release 35: 49–58.
- Uma Shankar MS, Satheesh Madhav NV (2008) A novel smart mucoadhesive biomaterial from Cordia dichotoma fruit pulpâ€. International Journal Pharmbit1.
- Costa P, Sousa Lobo JM (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13: 123-133.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA, et al. (1983). Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15: 25–35.
Citation: Pawar AH, Surekha MJ, Pravin TJ, Rachel G (2014) Development and Evaluation of Mucoadhesive Patch Using a Natural Polysaccharide Isolated from Cordia dichotoma Fruit. J Mol Pharm Org Process Res 2: 120. DOI: 10.4172/2329-9053.1000120
Copyright: ©2014 Pawar AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15890
- [From(publication date): 12-2014 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 11189
- PDF downloads: 4701