Development and Evaluation of Mouth Dissolving Film of Ondansetron Hydrochloride Using Hpmc E 5 in Combination with Taro Gum and Other Commercially Available Gums
Received: 02-Oct-2017 / Accepted Date: 28-Oct-2017 / Published Date: 02-Nov-2017 DOI: 10.4172/2329-9053.1000138
Abstract
Objective: Ondansetron hydrochloride belongs to the class of 5HT3 antagonists. It is approved by United States Food and Drug Administration (US FDA) to control chemotherapyinduced nausea and vomiting. The oral bioavailability of ondansetron is approximately 60%, indicating first pass metabolism. The present research work was carried out with an objective to increase the bioavailability of the drug ondansetron hydrochloride by developing its mouth dissolving film using taro gum in combination with HPMC E5.
Method: Solvent precipitation method was used for extraction of polysaccharide. Polysaccharide (Taro gum) was extracted from tubers of Colocassia esculenta which was used as a polymer in film formulation. Films were formulated using solvent casting method. Xanthan gum, guar gum, sodium alginate, gum ghati and taro gum were used as polymer in film formulation. Concentration for taro gum was optimized. At this optimized concentration, films were formulated using other commercially available natural gums. These films were evaluated for various parameters like tensile strength, disintegration time, In vitro release and stability.
Result: All the formulated films showed disintegration time less than 60 s. The film formulated using taro gum showed better release profile, disintegration time and tensile strength than the films prepared using other commercially available gums. Taro film showed 90.64% release at the end of 5 min. Disintegration time of films prepared using taro gum was comparable to that of marketed film. Also, the film prepared using taro gum was more stable as compared to other films.
Conclusion: Taro gum being extracted from edible tubers is non-toxic in nature. It can be extracted easily and successfully used in the development of mouth dissolving film of Ondansetron hydrochloride.
Keywords: Mouth dissolving film; Natural polymers; Ondansetron hydrochloride
Introduction
Fast-dissolving drug delivery systems like fast dissolving films are rapidly gaining interest in the pharmaceutical industry. These systems are either dissolve or disintegrate generally within a minute, without needing water or chewing [1].
Mouth Dissolving Films can be produced by either solvent cast methods or hot-melt extrusion technology. The solvent casting method suffers from several disadvantages over the hot melt extrusion method due to the solvent residues within the film and the environmental risks in the case of organic solvents [2].
Mouth dissolving films (MDF) disintegrate or dissolve within the oral cavity and are emerged as a convenient way of dosing medications, not only to special population/groups with swallowing difficulties such as children and the elderly, but also to normal people. MDF are prepared using hydrophilic polymers that rapidly dissolve on the tongue or buccal cavity, delivering the drug to the systemic circulation via dissolution on contact with saliva. MDF are typically designed for oral administration, with the user placing the strip on or under the tongue (sublingual) or along the inside of the cheek (buccal). As the strip dissolves, the drug can enter the blood stream primarily buccally and sublingually [3].
Owing to large surface area of the film formulation, there is greater disintegration and dissolution in the oral cavity. As the drug is absorbed through buccal mucosa, first pass metabolism is avoided thus enhancing the bioavailability. Films prove to be advantageous in case of dysphagic patient. As compared to orally disintegrating tablets the films are less fragile and hence provide ease of transportation [4].
Ondansetron hydrochloride was selected as model drug. It is a 5- HT3 receptor blocker with a bioavailability of 60% due to first pass metabolism. The present work was carried out to increase the bioavailabilty of the drug ondansetron hydrochloride by formulation of mouth dissolving film. Natural polysaccharide extracted from tubers of Colocassia esculenta (also known as taro gum) was used a polymer for film formulation along with semisynthetic polymer HPMC E5 (Hydroxy propyl methyl cellulose) [5]. Also, the films using established natural polymers namely xanthan gum, guar gum, gum ghati and sodium alginate were formulated in combination with HPMC E5. The natural polymers are comparatively cheaper with desired properties like abundantly available, non-irritating and non-toxic in nature. The use of natural polysaccharides contributes to changes in different properties like disintegration time, tensile strength, and In vitro drug release [6]. All the films formulated using different natural polymers by solvent casting method were evaluated for different parameters and on the basis of the results obtained one film formulation was considered to be better over the others. The films were also compared to the marketed film of Ondansetron hydrochloride.
Materials and Methods
Materials
Ondansetron hydrochloride was obtained from InventIa Health Care Pvt. Ltd., Mumbai, India. HPMC E5 was obtained from Colorcon, Goa, India. Xanthan gum EP, guar gum LR, sodium alginate IP and gum ghati EP were obtained from Molychem, Mumbai, India. Aspartame was obtained from C P Kelco, Mumbai, India. All the chemicals and reagents used were of analytical grade.
Isolation and purification of polysaccaride from Colocasia esculenta tubers
Fresh tubers of Colocasia esculenta were washed, peeled and diced into small pieces. It was then blended in a blender with little purified water for 30 min at room temperature. This was then passed through muslin cloth to get a slimy solution. Acetone was added twice the amount of solution in order to precipitate the polysaccharide. The polysaccharide was separated using muslin cloth and dried overnight at 40°C. The dried polysaccharide was pulverized in mortar pestle. The powder obtained was passed through 100 number sieve [7]. The isolated polysaccharide was purified by dissolving it in water and reprecipitating with acetone. This procedure was repeated thrice. The isolated polysaccharide was evaluated for its physicochemical properties as follows:
Solubility
Solubility of the polysaccaride was determined as given in Indian Pharmacopoeia 1996 in different solvents [8].
Powder characteristics
Following characteristics were studied using procedures reported in literature [9].
Bulk density- In a 25 ml dry measuring cylinder, 5 g of gum was taken and initial bulk volume was noted. Measurements were done in triplicate.
Bulk density=mass/bulk volume
Tapped density- The above cylinder was tapped 100 times to get tapped volume. Reading was taken in triplicate.
Tapped density=mass/tapped volume
Carr’s index= [(tapped density-bulk density)/tapped density]*100
Hausner’s ratio=tapped density/bulk density
Angle of repose- 5 g of gum was taken and passed through a short stem funnel on a paper. The diameter and height of the powder heap was noted. The distance between the surface and the tip of funnel was kept less than 2 cm. Readings were taken in triplicate.
Angle of repose=tan-1[height/radius]
pH
1% (w/v) gum dispersion was made in water and its pH was checked after stirring for 5 min using a digital pH meter whose electrode was set at neutral before immersing the electrode into the dispersion. Readings were taken thrice.
Loss on drying (LOD)
One gram gum sample was heated in a hot air oven at 105°C till constant weight was observed. The loss in moisture content was then calculated by the following equation.
%LOD=(weight of water in sample/weight of dry sample)*100
Specific gravity
Specific gravity of the gum was determined using Skoog and West’s method (1963) using a pycnometer [10]. One percent solution of gum was prepared and poured into a dry calibrated pycnometer. It was capped and weighed at room temperature. Similarly, weight of pycnometer with water was noted at room temperature. Specific gravity was obtained by following equation.
Specific gravity=weight of water/weight of gum solution
Swelling index
Swelling index procedure was followed as per British Pharmacopoeia [11]. One gram of gum was finely grounded and introduced into a 25 ml measuring cylinder. Water was added up to 25 ml. The cylinder was shaken intermittently every 10 min for 1 h. It was then allowed stand for 3 h at room temperature. The volume occupied by the gum, including any sticky mucilaginous portion was measured. Swelling index was calculated as per following formula.
Swelling index (SI) = [(Xt-X)/X]*100
Where X is initial height of the powder and Xt is the height occupied by the swelled gum after 3 h.
Preformulation studies
Preformulation studies were carried out for the drug using UV spectrophotometer and FTIR. Caliberation curve was plotted for different concentration for the drug in 0.1N HCl using Jasco UV spectrophotometer at 266 nm. The drug excipient compatability was checked using Fourier Transform Infrared (FTIR) spectroscopy. KBr pellets of the drug, drug excipient mixtures were made. IR spectrum obtained in scanning range of 500-4000 cm-1.
Formulation development of mouth dissolving film
MDFs were prepared by solvent casting method using the extracted polymer (Taro gum) and xanthan gum, guar gum, gum ghati and sodium alginate [12].
The plasticizer, glycerine, was added to 5 ml of distilled water and kept for stirring on a magnetic stirrer. To this solution, the weighed quantity of polymer was added and stirred till it gets dissolved completely. Drug was solubilized in 5 ml of distilled water. The drug solution was then added to the polymer solution with continuous stirring to get homogenous solution. Weighed quantity of excipients like the flavor, sweetener and saliva stimulating agent were then added to the solution. In case of air entrapment, the air bubbles were removed by sonication (Oscar sonicator). The homogenous solution was then casted on a petri plate and dried at 40°C in hot air oven for 20 h. The composition of various film formulations prepared is summarized in Tables 1 and 2.
| Ingredients | F0 | F1 | F2 | F3 | F4 | F5 |
| Ondansetron Hydrochloride | 0.106 | 0.106 | 0.106 | 0.106 | 0.106 | 0.106 |
| HPMC E5 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Taro | -- | 0.01 | 0.03 | 0.05 | 0.07 | 0.09 |
| Glycerine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Citric Acid | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Aspartame | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| Flavor | 0.022 | 0.022 | 0.022 | 0.022 | 0.022 | 0.022 |
| Water | 10ml | 10ml | 10ml | 10ml | 10ml | 10ml |
Table 1: Composition of films (F0-F5) prepared using taro gum (quantities in grams).
| Ingredients | F6 | F7 | F8 | F9 |
| Ondansetron Hydrochloride | 0.106 | 0.106 | 0.106 | 0.106 |
| HPMC E5 | 0.15 | 0.15 | 0.15 | 0.15 |
| Xanthan Gum | 0.01 | -- | -- | -- |
| Guar Gum | -- | 0.01 | -- | -- |
| Sodium Alginate | -- | -- | 0.01 | -- |
| Gum Ghati | -- | -- | -- | 0.01 |
| Glycerine | 0.1 | 0.1 | 0.1 | 0.1 |
| Citric Acid | 0.01 | 0.01 | 0.01 | 0.01 |
| Aspartame | 0.04 | 0.04 | 0.04 | 0.04 |
| Flavor | 0.022 | 0.022 | 0.022 | 0.022 |
| Water | 10ml | 10ml | 10ml | 10ml |
Table 2: Composition of films (F6-F9) prepared using commercially available gums (quantities in grams).
Calculation of amount of drug per batch
Dose of drug per film=8 mg
Area of one film=3.24 cm2
Area of petri plate=42.986 cm2
Drug to be added in one batch=(Dose of drug per film × Area of petri plate)/Area of one film
=(8 × 42.986)/3.24=0.106 g.
Evaluation
All the prepared batches were evaluated for following parameters as per the standard procedure reported in the literature.
Physical properties
Thickness: Thickness of the optimized batch formulation was carried out by using micrometer screw gauge. The thickness of each film was calculated at three different places and standard deviation was also calculated [13].
Tensile strength: Tensile strength is the maximum stress applied to a point at which the film specimen breaks. Tensile strength of the optimized batch formulation was evaluated by a digital tensile strength tester. The test was carried out in triplicates and the average value was noted [14].
Folding flexibility: Folding flexibility/Folding endurance is the number of times the film is folded at the same place without breaking. The test was carried out thrice [15].
Disintegration time (DT)
10 ml of distilled water was placed in a Petri dish and one film was added on the surface of the water and the time measured until the oral film was dissolved completely. The measurements were carried out for three different films and the range was reported [16].
Weight variation
Individual weights of film were measured using Shimadzu electronic weighing balance. The average weight and standard deviation were then calculated [17].
Percent moisture content
The amount of moisture affects the brittleness and friability of films. The amount of moisture present in the film was determined by weighing method [18].
Surface pH
Film was soaked in 2 ml of distilled water for 15 min. Surface pH of films was determined using pH paper [19].
Drug content
The formulated film was dissolved in 100 ml volumetric flask containing 0.1N HCl. One milliliter of this stock solution was further diluted to 10 ml with 0.1N HCl. The absorbance of this solution was noted. The caliberation curve was constructed for different concentration of ondansetron hydrochloride in 0.1N HCl. Drug content of the batches were determined by using UV-VIS Spectroscopy [20]. Average of three determinations was reported.
In vitro drug release
The In vitro drug release of the formulated films was determined in paddle type dissolution apparatus (Electrolabs) using 900 ml of 0.1N HCl at 50 rpm. The study was conducted at 37 ± 0.5°C. Aliquots were taken at different time intervals. The absorbance was obtained using UV–Vis spectrophotometer [21]. The In vitro release data was subjected to zero order, first order, Higuchi and Korsemeyer-Peppas mathematical model in order to establish the drug release mechanism and kinetics of drug release from the polymeric matrix of optimized film formulation. The In vitro release of drug from different film formulations was evaluated by fitting the dissolution data obtained to the following equations.
Zero order equation:
Where Ct is the amount of the drug released at time t, C0 is the initial amount of drug in the tablet and K0 is the zero-order release rate constant.
First order equation:
Where Ct is the amount of drug remaining as a solid state at time t, C0 is the total amount of drug in the matrix and K1 is the first-order release rate constant.
Higuchi model equation:
Where Co is the initial drug concentration, t is time of release, Q is the amount of drug released/unit area and D is the diffusion coefficient and it was calculated according to the following equation:
The preference of a certain mechanism was based on the correlation coefficient (r) for the parameters studied, where the highest correlation coefficient is preferred for the selection of mechanism of release. To confirm the mechanism of release of drug from film, the obtained data of In vitro drug release were fitted to Korsmeyer and Peppas (1983) equation i.e Mt/M∞=K·tn, where Mt/M∞ is the fraction released by the drug at time t, K is a constant incorporating structural and geometric characteristic and n is the release exponent characteristic for the drug transport mechanism. When n=0.5 fickian diffusion is observed and the release rate in dependent on t, while 0.5<n
Surface morphology
Morphology of the prepared films was observed under a scanning electron microscope (SEM) (ESEM TMP with EDAX, Philips, Holland). The sample was attached to the slab surface with double sided adhesive tapes and the scanning electron photomicrograph was taken at magnification. The morphology was studied for the final optimized film formulation.
Stability study
The samples were kept for stability studies in aluminum foil packaging at different temperature and humidity conditions namely room temperature(deep freezer(4-8°C), refrigeration (20°C) and stability chamber(40°C/75% RH).
Results and Discussion
Preformulation study
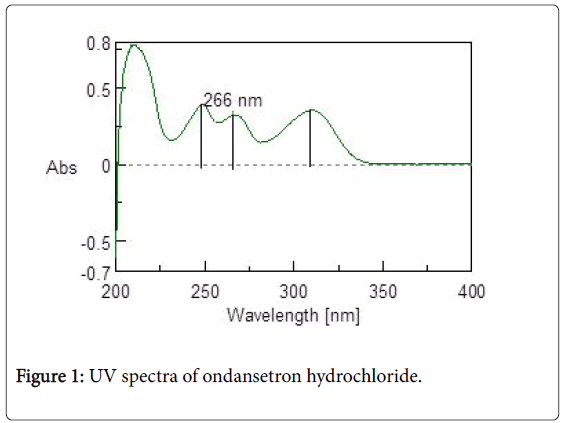
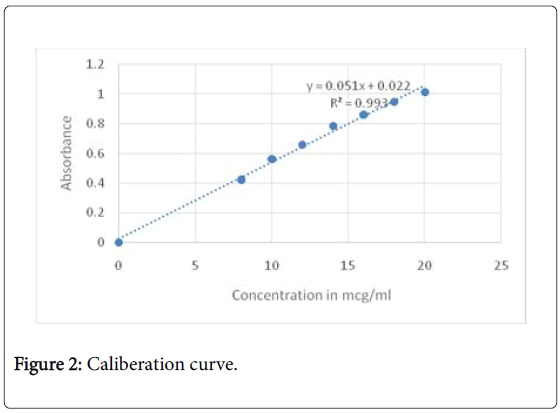
Calibration curve of ondansetron hydrochloride: UV spectra of 10 mcg/ml of ondansetron hydrochloride in 0.1N HCl was taken in range of 200-400 cm-1. Three maximas were observed for ondansetron hydrochloride as depicted in Figure 1, out of which 266 nm was selected as analytical wavelength because at this wavelength placebo interference was minimal. The calibration curve (Figure 2) obtained for different concentration ranging from 10 mcg/ml to 40 mcg/ml was linear with R2 value of 0.9968.
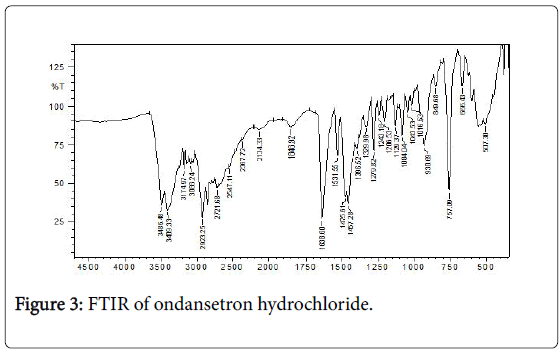
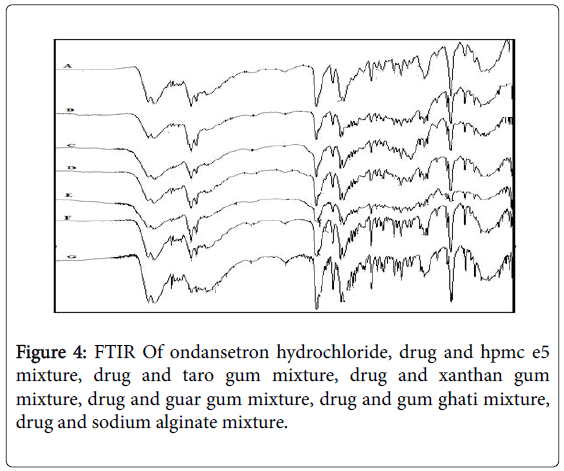
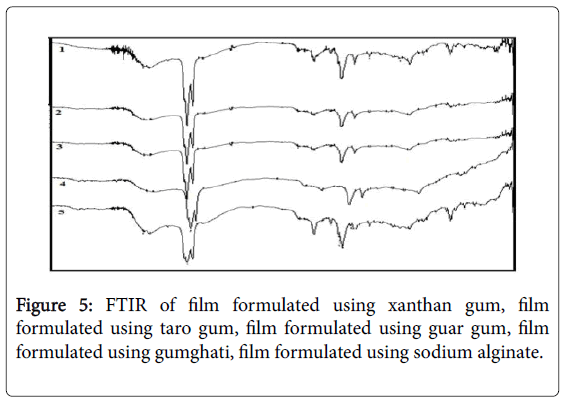
Fourier Transform Infrared (FTIR) spectra of drug and polymer: The FTIR spec of ondansetron hydrochloride is depicted in Figure 3 with the values in Table 3. The results matched the literature [22]. Excipients were selected based upon preformulation studies. FTIR studies revealed that there was no physicochemical interaction between ondansetron hydrochloride and other excipients used in preparation of MDF (Figure 4). Fourier Transform Infrared Spectra of various formulated films are depicted in Figure 5. FTIR spectra for the formulated films indicated that there is no interaction between the drug and excipients used in the formulation.
| IR Values (cm-1) | Functional Group |
| 758 | Ortho- disubstituted benzene ring |
| 1280 | Cyano group vibration (-CN) |
| 1459 | Methyl group (-CH3) |
| 1531 | Aromatic ring double bond (-C=C-) |
| 1638 | Cyano and keto group in 6 membered ring (-C=O,-CN) |
| 3487 | Hydroxyl group (-OH) |
| 3410 |
Table 3: FTIR of ondansetron hydrochloride.
Evaluation of extracted polymer
The results of all the evaluation parameters of the extracted polysaccharide are summarized in Table 4. The results indicated that the polymer has good flow properties. The pH being neutral there is no risk of irritation to the mucosal surface.
| Parameters | Results | |
| Solubility | Forms viscous colloidal solution in hot water, insoluble in acetone, ethanol, methanol, DMSO and ether. | |
| Powder characteristics | Bulk density | 0.5455 g/cc |
| Tapped density | 0.7692 g/cc | |
| Angle of repose | 16.19% | |
| Carr’s index | 29.08% | |
| Hausner’s ratio | 1.41 | |
| pH | 6.88 | |
| Loss on drying | 15.6% w/w | |
| Specific gravity (1% w/v solution) | 0.9937 g/ml | |
| Swelling capacity | 10.67 | |
| Viscosity (0.1% w/v, in water) | 0.93 cps | |
Table 4: Results of evaluation of extracted polysaccharide.
It has been reported that swelling is one of the mechanism for disintegration property. Owing to good swelling index, taro gum can show good disintegrating properties [23].
Evaluation of formulation
The films formulated were clear in appearance, easily peelable and smooth in texture (Figure 6). The results of all the formulated films are mentioned in Tables 5 and 6.
| Batch | Wt Variation | Thickness (mm) | Disintegration time (sec) | % Dissolution at the end of 5 min | Drug Content | Surface pH | Folding Endurance | Tensile Strength N/mm2 |
|---|---|---|---|---|---|---|---|---|
| F1 | 27 ± 4 mg | 0.052 ± 0.021 | 11-May | 90.64 | 99.22% | 7-Jun | 233 | 1.5 |
| F2 | 30 ± 3 mg | 0.06 ± 0.028 | 13-18 | 89.79 | 96.40% | 7-Jun | 212 | 1.3 |
| F3 | 32 ± 2 mg | 0.063 ± 0.021 | 15-28 | 88.55 | 95.80% | 7-Jun | 223 | 1.1 |
| F4 | 35 ± 2 mg | 0.067 ± 0.023 | 16-30 | 84.39 | 95.92% | 7-Jun | 230 | 0.95 |
| F5 | 36 ± 3 mg | 0.073 ± 0.025 | 28-50 | 82.44 | 95.05% | 7-Jun | 226 | 1.1 |
| FM | 41 ± 1 mg | 0.13 ± 0.004 | 20-25 | 95.63 | 98.20% | 7-Jun | 320 | 2.3 |
Table 5: Results of evaluation of developed films (F1-F5).
| Batch | Weight Variation | Thickness (mm) | Disintegration time (sec) | Dissolution | Drug Content | Surface pH | Folding Endurance | Tensile Strength N/mm2 |
|---|---|---|---|---|---|---|---|---|
| F6 | 23 ± 2 mg | 0.035 ± 0.004 | 13-18 | 63 | 95.60% | 7-Jun | 209 | 1.8 |
| F7 | 23 ± 3mg | 0.032 ± 0.01 | 19-25 | 59.76 | 94.90% | 7-Jun | 215 | 0.95 |
| F8 | 35 ± 3mg | 0.052 ± 0.01 | 15-28 | 80.42 | 96.20% | 7-Jun | 235 | 0.8 |
| F9 | 24 ± 2 mg | 0.032 ± 0.008 | 13-23 | 68.68 | 95.43% | 7-Jun | 223 | 0.98 |
Table 6: Results of evaluation of developed films (F6-F9).
The films formulated using natural polysaccharide taro without HPMC E5 polymer gave higher values of disintegration time values than that required for MDF. HPMC E5 was selected on the basis of extensive literature survey [24]. Concentration for HPMC E5 was optimized on trial and error basis. HPMC E5 was used in combination with varying concentration of taro gum. The film formulated using taro in combination with HPMC E5 when compared to film containing only HPMC E5 showed better disintegration time, In vitro release and tensile strength results. Taro at concentration greater than 0.1 g gave brittle films which were not so easily peelable. As shown in Table 1, the concentration of 0.01 g (F1-F5) of taro gum was found to give least disintegration time with better In vitro release (Table 5). Hence, concentration of 0.01 g was considered optimum.
Films were developed using different commercially available polysaccharides namely xanthan gum, guar gum, sodium alginate and gum ghati at optimized concentration of taro (0.01%) for comparison. Xanthan gum is a glucomannan which is widely used in pharmaceutical field [25]. It is used as a film modifier. Guar gum, a galactomannan gives a viscous solution and is used as adjuant to polymer in film formulation [26]. Gum ghati is an arabinoglucan which is used for different purposes in pharmaceutical industry [27]. Sodium alginate consists chiefly of the sodium salt of alginic acid, which is a mixture of polyuronic acids composed of residues of Dmannuronic acid and L-guluronic acid [28]. Glycerin (Propane-1, 2, 3- triol) was used as a plasticizer in film formulation [29].
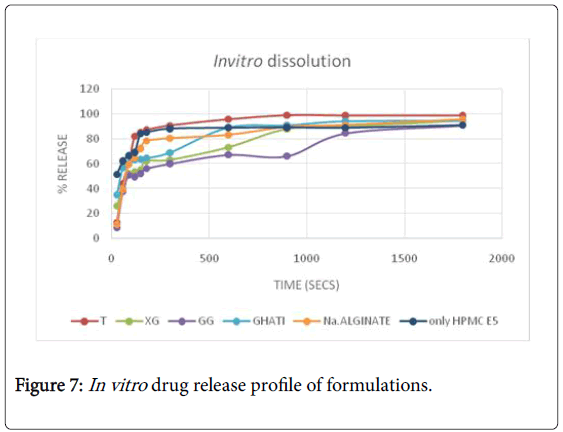
All the films prepared were transparent and easily peelable. All the individual films of every batch prepared gave acceptable results for weight variation and thickness. Also, the moisture content of all the formulated films was within acceptable range. Surface pH of all the films evaluated was found to be neutral which ensured no irritation to the mucosa. Disintegration time for formulation F1 was found to be less as compared to other film formulations. On comparison with the marketed formulation, the disintegration time for F1 was less. The In vitro drug release pattern for F1 and F6 were comparable to that of marketed formulation (Figure 7). The folding endurance for all the films formulated were more than 200 indicating mechanical strength. Also the tensile strength results for F1 and F6 were comparable to that of marketed film. The tensile strength results for film formulation F7, F8 and F9 were not satisfactory. On the basis of all the evaluation parameters, taro film and xanthan film were better than others and also comparable to that of marketed film formulation. The In vitro release of taro gum was better than other formulated films. Taro film showed 90.64% release at the end of 5 min. The results of kinetic study are tabulated in Table 7. The best fit kinetic model was Korsmeyer peppa with highest correlation coefficient value of 0.968. The n value of 0.5<n
| Batch code | Regression coefficient (R2) | ||||
|---|---|---|---|---|---|
| Zero order | First order | Higuchi | Korsmeyerpeppas | Value of n | |
| F13 | 0.413 | 0.921 | 0.582 | 0.968 | 0.82 |
Table 7: Data of kinetic release study.
Taro film showed comparable results to that of marketed film. On considering the disintegration time, taro film proves to be better than xanthan gum film. In formulation F1, less amount of excipients used lead to less weight of film along with less disintegration time value. Hence, the formulation is cost effective as compared to marketed film.
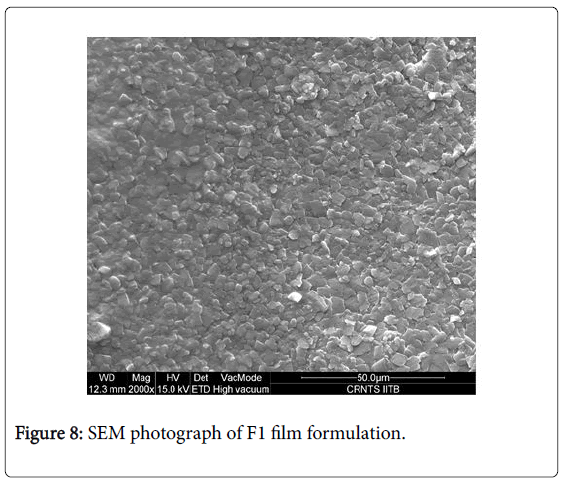
The surface morphology of this film was studied using Scanning Electron Microscopy (SEM). Macroscopically, the prepared films were clear and colorless (Figure 8), thus indicating uniform distribution of Ondansetron hydrochloride.
The results for stability studies are mentioned in Table 8. The formulated films were stored at room temperature, 4-8°C, 20°C and 40°C for stability studies for period of 3 months. It was noted that the drug precipitated out at higher temperature conditions i.e. at room temperature and 40°C/75% RH and the films became brittle with no tensile strength. Whereas the films stored at 4-8°C and 20°C were found to be stable. The films were evaluated for disintegration time and the tensile strength as shown in Table 6. There was not much difference in the disintegration time for all the prepared films.
| Batches | 4-8°C | 20°C | ||||||
|---|---|---|---|---|---|---|---|---|
| DT (sec) | Tensile strength (N/mm2) |
Appearance | Drug content | DT (sec) | Tensile strength (N/mm2) |
Appearance | Drug content | |
| F1 | 11-Aug | 1 | Transparent film | 96.93% | 13-May | 1.1 | Transparent film | 96.20% |
| F6 | 14-20 | 1.5 | Transparent film | 95.20% | 13-22 | 1.4 | Transparent film | 95.01% |
| F7 | 20-25 | 0.6 | Transparent film | 101.20% | 22-25 | 0.5 | Transparent film | 101.50% |
| F8 | 15-23 | 0.7 | Transparent film | 98.22% | 15-20 | 0.7 | Transparent film | 97.56% |
| F9 | 18-25 | 0.5 | Transparent film | 99.22% | 20-28 | 0.5 | Transparent film | 96.30% |
Table 8: Results of stability study.
On the basis of obtained results the film formulated using taro gum was considered to be comparable to the marketed film and also better than other formulated films in terms of disintegration time and tensile strength.
Conclusion
Natural polymers are biocompatible and can be used widely in pharmaceutical field. They are easily available and are nontoxic in nature. Natural polymers along with other drug delivery systems can be efficiently used in film formulation as well. Novel extracted polymer Taro was found to enhance the properties such as the tensile strength, In vitro disintegration time and the In vitro release of the film when used in combination with the semisynthetic polymer like HPMC E5. Also, it provided film formulation which was comparable to the marketed formulation of the drug Ondansetron Hydrochloride and also to the formulations made using other established natural polymer.
All the films formulated using the natural polymer and HPMC E5 with glycerin by solvent cating method showed disintegration time less than 60 sec. Ondansetron hydrochloride mouth dissolving films were successfully formulated using natural polymers to enhance the bioavailability of the drug and also to provide rapid onset of action.
Conflict of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
Acknowledgement
Author is very much thankful to Dr. Parag Gide; Principal of Hyderabad Sindh National Collegiate boards (HSNCB’s) Dr. L. H. Hiranandani College of Pharmacy, Ulhasnagar for his continuous support and encouragement.
References
- Aggarwal J, Singh G, Saini S, Rana AC (2011) Fast dissolving films: A novel approach to oral drug delivery. IRJP 2: 69-74.
- Kunte S, Tandale P (2010) Fast dissolving strips: A novel approach for the delivery of verapamil. J Pharm Bioallied Sci 2: 325–328.
- Kulkarni PK, Dixit M, Gunashekara K, Kulkarni A (2011) Formulation and Evaluation of Mouth dissolving film containing Rofecoxib. IRJP 2: 273-278.
- Upreti K, Kumar L, Pathak S, Chawla V (2014) Formulation And Evaluation Of Mouth Dissolving Films Of Paracetamol. IJPPS 6: 200-202.
- Lin H, Huang AS (1993) Chemical composition and some physical properties of a water-soluble gum in taro (Colocasia esculenta.). Food Chemistry 48: 403–409.
- Nagar P, Chauhan I, Yasir M (2011) Insights into Polymers: Film Formers in Mouth Dissolving Films. Drug Invent Today 3: 280-289.
- Vidyasagar G, Jadhav AG, Bendale AR, Narkhede SB (2011) Isolation of Cordiamucilage and its comparative evaluation as a binding agent with standard binder. Der Pharmacia Sinica 2: 201-207.
- Indian Pharmacopoeia (1996) Ministry of Health and Family Welfare, Government of India, Controller of Publication, New Delhi 556: A100–A111.
- Alalor CA, Avbunudiogba JA, Augustine K (2014) Isolation And Characterization Of Mucilage Obtained From Colocasia esculenta. IJPBS 4: 25-29.
- Skoog DA, West DM (1963) Fundamentals of Analytical Chemistry, 4th ed., Holt-Rinehart and Winston, Philadelphia, New York. 880.
- British Pharmacopoeia, vol. IV (2008) The Department Of Health, Social Services And Public Safety, Council Of Europe, Appendix XI E A273.
- Dhere PM, Patwekar SL (2011) Review on preparation and evaluation of oral disintegrating films. IJPT 3: 1572-1585.
- Gavaskar B, Kumar SV, SharanG (2010) Overview on Fast Dissolving films. IJPPS 3: 29-33.
- Nafee NA, Boraie NA, Ismail FA, Mortada LM (2003) Design and characterization of mucoadhesivebuccal patches containing Cetylpyridinium chloride. Acta Pharm 53: 199-212.
- Anand V, Kataria M, Kukkar V, Saharan V, Choudhury PK (2007) The latest trends in the taste assessment of pharmaceuticals. Drug Discov Today 12: 257-265.
- Doaa AE,Nevine SA (2010) Formulation of a Novel Tianeptine sodium orodispersible film. AAPS 11: 1018-24.
- Mahesh A, Shastri N ,Sadanandam M (2010) Development of Tastes Masked Fast Disintegrating film of Levocetirizine Dihydrochloride for Oral Use. Curr Drug Deliv 1: 21-27.
- Mukherjee B,Mahapatra S, Gupta R (2005) A comparison between povidone-ethylcellulose and povidone-eudragit transdermal dexamethasone matrix patches based on In vitro skin permeation. Eur J Pharm Biopharm 59: 475-483.
- Bootenberg P, Cleymaet R, de Muynck C, Remon JP, Coomans D, et al. (1991) Development and testing of bioadhesive slow-release tablets for oral use. J Pharm Pharmacol43:457-464.
- Murata Y, Isobe T, Kofuji K, Nishida N, Kamaguchi R (2010) Preparation of Fast Dissolving Films for Oral Dosage from Natural Polysaccharides.Mat 3:4291-4299.
- Choudhary DG, Patel VA, Chhalotiya UK, Patel HV, Kundawala AJ (2012) Natural polysaccharides as film formers: A feasibility study for development of rapid dissolving films of Ondansetron Hydrochloride. IJPPS 4: 78-85.
- Mohanachandran PS, Sindhumol PG, Kiran TS (2011)Superdisintegrants: an overview. IJPSRR 6: 105-109.
- Galgatte UC, Khanchandani SS, Jadhav YG, Chaudhari PD (2013) Investigation Of Different Polymers, Plasticizers And Superdisintegrating Agents Alone And In Combination For Use In The Formulation Of Fast Dissolving Oral Films. Int J Pharm Tech Research 5: 1465-1472.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn (2009) In: Handbook of Pharmaceutical Excipients.(6th edn). Published by American Pharmacists Association: Washington. 782-785.
- Raymond C Rowe, Paul J Sheskey, Marian E Quinn (2009) In: Handbook of Pharmaceutical Excipients.(6th edn). Published by American Pharmacists Association: Washington. 298-300.
- Jani GK, Shah DP, Prajapati VD, Jain VC (2009) Gums and mucilages-versatile excipients for pharmaceutical formulations. AJPSR 4: 308-322.
- Rowe RC, Sheskey PJ, Owen SC (2006) Sodium alginate, Pharmaceutical Excipients. Pharmaceutical Press and the American Pharmacists Association. 466-470.
- RC Rowe, PJ Sheskey, SC Owen (2006) Glycerine, Pharmaceutical Excipients. Pharmaceutical Press and the American Pharmacists Association. 466-471.
Citation: Pawar HA, Kamat SR (2017) Development and Evaluation of Mouth Dissolving Film of Ondansetron Hydrochloride Using Hpmc E 5 in Combination with Taro Gum and Other Commercially Available Gums. J Mol Pharm Org Process Res 5: 138. DOI: 10.4172/2329-9053.1000138
Copyright: ©2017 Pawar HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7561
- [From(publication date): 0-2017 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 6453
- PDF downloads: 1108