Development and Evaluation of Curcumin Emulgel with Cumin Oil for Topical Application
Received: 18-Aug-2021 / Accepted Date: 06-Sep-2021 / Published Date: 13-Sep-2021 DOI: 10.4172/2167-065X.1000233
Abstract
In this research, Emulgel of curcumin was developed by using Carbopol 940 as a gelling agent. Curcumin is a hydrophobic natural origin drug and Emulgel can suitably deliver hydrophobic drugs. Therefore this is a challenging task for the administration of curcumin through the skin. During the development of Emulgel, curcumin was rarely solubilized in the water phase, so firstly we dissolve them in ethanol and dispersed them in the water phase. Curcumin is the natural origin drug obtained from Curcuma longa, they have a broad number of activities like antifungal, antibacterial, anti-inflammatory, anticancer, etc. Curcumin is a well-identified herbal-origin drug by traditional medicine scriptures. It is well established for the cure of various ailments including cancer, fungal infection, and bacterial infection. However, the natural origin drug imparts a bioavailability problem. To overcome this obstacle the emulgel was developed. Emulgel was developed by adding the oil phase into the aqueous phase by continuous stirring. Therefore the gel phase was developed by dissolving Carbopol 940 in purified distilled water followed by gentle heat on a magnetic stirrer, finally developed emulsion and gel was mixed (1:1 ratio) properly by the propellant mixer. In the Preformulation study solubility, PH, color, FTIR of curcumin were analyzed properly. Further, all the developed formulations were evaluated for their PH, physical characteristics, spreadability, phase separation, in-vitro drug release, drug content, and viscosity, etc. After a study of all obtained results, it was founded that formulation F2 and F4 best in all prospects. The viscosity of F2 and F4 was 12600 Centipoise and 11400 Centipoise respectively. The % release of the drug from Emulgel formulation was 80-95%. Finally, F2 and F4 were decided as an optimized formulation for the final formulation. The drug content of all developed formulations was founded to be 83-90%. Also after 3 months, the drug content should maintain the formulation. From this study we say that Emulgel is an appropriate topical drug delivery system as compared to another already available topical dosage form, Emulgel has the best spreadability and adhesive property therefore it is suitable for dermal application. To know the detailed antibacterial activity and irritability property of curcumin Emulgel there is a need for in vivo study in the future.
Keywords: Emulgel; Cumin oil; Ternary phase; Gelling agent
Introduction
From ancient times, topical and transdermal dosage forms were used broadly. At that time the formulation was used by the Greek physician containing salt, vinegar, honey, and resins to treat various skin problems like lesions and ulcers. The dosage form which has the consistency between solid and liquid dosage form is identified as a 'semi-solid dosage form' having trait rheological properties. Semisolid dosage form comfortably applied on the biological membrane which can be held on to the site of application for a long period. The ingredients in semisolid dosage form either dissolved or suspended or in a solubilized state and are intended to apply externally to the skin or on to the surface of eyes, vaginally, nasally, or rectally to achieve the local or systemic effect [1].
The topical drug delivery system has followed the 'bypass first-pass metabolism', therefore the chance of degradation of medicaments from the GI tract and the bother of intravenous therapy is avoided. The gels have various positive parameters but they have a major limitation, they unable to deliver the hydrophobic drug. So to avoid this type of problem, firstly the medicament is incorporated into an emulsion and then conveniently incorporated into the gel to get the best results. The collective formulation of emulsion and gel is known as 'Emulgel’. The Emulgel is an emulsion either; they are gelified by the use of gelling chemicals that are appropriate [2,3].
EG is the semisolid dosage form, in which the formed emulsion system is incorporated into the gelling agent. EG has followed the 'bypass first-pass metabolism', therefore the chance of degradation of medicaments from the GI tract and the bother of intravenous therapy is avoided. Therefore the bioavailability of a drug should enhance [4,5]. Emulgel can be categorized into two categories-
• water- in- oil type Emulgel (O/W type)
• oil- in- water type Emulgel (W/O type)
Emulsion gel formulation has been showing fruitful importance in the pharmaceutical field under the category of semisolid dosage form from the past of the mid-1980s. Emulgel is acquiring importance due to its many positive prospects. They justify the best application property against some semisolid formulations such as cream ointment and gels. They show the faster release of medicament from the vehicle to the dermal system of the skin. Emulgel is easily applied due to a lack of grease and application residue. They have both oleaginous and aqueous components; therefore the badly water-dissolve drug can conveniently be delivered by this formulation [6].
In skin diseases, superficial fungal infections are a very common disease, which affects millions of people all over the world. They can affect both healthy and immune-compromised people. The responsible species of fungal infection such as dermatophytes, yeasts, and nondermatophytes molds. Specifically, epidermophyton, trichophyton, and Microsporumspiceases are the most common dermatophytes which cause the fungal infection, and some yeasts are also responsible for the infection such as Candida albicans, etc. the candida species are the most common yeasts which contribute the 10-15% of total nosocomial infection cases [7].
Curcumin (CUR) (Figure 1) is a polyphenolic compound extracted from C.longa (turmeric) rhizomes. In 1815, this compound was first isolated by two scientists, namely, Pelletier and Vogel. Following this discovery, there was a growing research interest regarding CUR, which led to the identification of the numerous health benefits of CUR. This polyphenolic compound is also familiar with diferuloylmethane. Its molecular weight is 368.38 and its chemical formula is C21H20O6. CUR has shown its activity against multiple chronic diseases including neurodegenerative disorders, obesity, liver disease, metabolic syndrome, arthritis, inflammation, and multiple cancers [8].
Curcumin antimicrobial properties are well-established and they perform the antimicrobial mechanism by interfering with the FtsZ protein which is responsible for the cellular division of microorganisms. Nearly all the prokaryotic species have the FtsZ protein, and FtsZ is also required for the mitochondria and chloroplast division in some eukaryotes. The various immunolocalization studies have shown that bacteria develop a Z-ring. Therefore it is confirmed, FtsZ is a cytoskeleton protein, revealing cytoskeleton protein functional homology eukaryote and tubulin [9]. Based on one previous study, the development of FtsZ 168 was potentially inhibited by curcumin and also there are no changes in the segregation and organization of the molecule [10,11].
Materials and Methods
The API Curcumin was obtained from Himedia Pvt. Ltd., Mumbai India. The Carbopol-940 used in this study as a polymer was fulfilled by laboratory Goel institute of pharmacy and sciences, Lucknow Uttar Pradesh. Cumin oil was used as an oil phase, which was purchased from Devinez Pvt. Ltd. fulfilled by Amazon. All other chemical used in this study was appropriate grades supplied by justified vendors.
The equipment used in this study during work was electronic balance, UV/Vis spectrophotometer, Micropipette, autoclave, water bath, magnetic stirrer, incubator, homogenizer, Brookfield viscometer, digital PH meter, Vortex shaker, FTIR spectrophotometer, melting point apparatus, and thermometers, etc. For experimenting the required apparatus such as beaker, volumetric flask, measuring cylinder, glass rod RD bottles, etc. was used, the all requirements were fulfilled by GIPS, Lucknow.
Method for preformulation studies
Preformulation study comprises the identifications of rudimental properties (physical and chemical) of the drug and excipients which are used in formulation development by engaging different methods and techniques.
UV – spectroscopy: The UV- spectroscopic test of curcumin was performed to identify their authenticity. The curcumin was dissolved in ethanol in ten ml of the flask and diluted up to 10 folds by using the dilution method. The λ max of curcumin in ethanol was determined by UV- spectroscopy. And the standard curve of curcumin in ethanol was plotted.
Preparation of standard graph of curcumin in ethanol:Stock I: 100 mg curcumin was weigh accurately into a 100 ml of volumetric flask and dissolve in ethanol and make up the volume by using ethanol.
Stock II: From the stock solution first, 100 ml solution is taken into a 10 ml of volumetric flask and makes up the volume up to 100 ml, the concentration of the solution is to be 100 μg/ml.
Stock III: from second stock solution, different concentration solution was prepared such as 2, 4, 6, 8, and 10 μg/ml, and their corresponding absorbance was measured at 429 nm in a UV visible spectroscopy [6,13].
Liquefaction point: The liquefaction point of the drug was detected by the help melting test apparatus. In this, the curcumin was filled into a capillary tube hand placed in the apparatus. Then visually observed the temperature in the temperature scale at which the curcumin was melt.
Curcumin solubility testing: Solubility of Curcumin was checked in oil & surfactant and co-surfactants. In 5 mL surfactant or oil, an excess quantity of curcumin was added. Each formed liquid was shaken back and forth at 25°C to 24 hrs. After that at 13500 RPM, the liquid was whirled for about 10 minutes. Therefore obtained resilience was obtained in addition to concentration of medicament was estimated using UV- spectroscopy after 10-time dilution by ethanol. Triplicate analysis was performed [14].
FTIR Analysis: The possible physicochemical interaction between drug and other used excipients was analyzed by FTIR (Agilent Cary 630 FTIR spectrometer) range 4000-450 cm-1 at CSIR- CDRI [13].
Development of an Emulgel
Method of preparation of Carbopol 940 gel: 1g of Carbopol-940 was dissolved in 100 ml of DW and placed on a magnetic stirrer for 20 min at 300°C. Then adjust the PH of the carbopl-940 solution by using triethanolamine (TEA), and left the gel for 24 h at room temperature.
Method of preparation of Emulsion: The given amount of oil was taken in a beaker and placed on a water bath which was adjusted at 65- 700°C temperature (Table 1). The curcumin was dissolved in surfactant (Tween 80) followed by ethanol and added to the aqueous phase which contained co-surfactant (PEG & PG), then aqueous phase was placed on water which having the same temperature as the oil phase. Both phases were heated on a water bath till the temperature of both phases was reached 750C. Then aqueous phase and oil phase were mixed by uninterrupted stirring by glass rod and magnetic stirrer. The coarse emulsion was formed.
| Sr. No. |
Ingredients | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1. | Carbopol 940 |
1 | 1 | 1 | 1 | 1 | 1 |
| 2. | Curcumin | 500 µg/ml | 500 µg/ml | 500 µg/ml | 500 µg/ml | 500µg/ml | 500 µg/ml |
| 3. | Cumin oil | 6 | 6.4 | 6 | 4.6 | 5.2 | 5 |
| 4. | Tween 80 | 2 | 2.3 | 1.3 | 3 | 1.5 | 2.8 |
| 5. | PEG | 2 | 2.3 | 1.3 | - | - | - |
| 6. | Propylene glycol |
- | - | - | 3 | 1.5 | 2.8 |
| 7. | Ethanol | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 8. | Purified distilled water |
20 (q.s.) | 20 (q.s.) | 20 (q.s.) | 20 (q.s.) | 20 (q.s.) | 20 (q.s.) |
Preparation of Emulgel: After the development of emulsion, the emulsion was incorporated into the gel phase having a ratio of 1:1 (Emulsion: Gel) for the production of an Emulgel (Figure 1). After that, they are filled in a suitable container and stored at room temperature [4,15,16].
Characterization of Emulgel
Physical examination: The developed formulation (Emulgel) was examined visually for its physical appearance, color, and consistency [17,18].
pH of Emulgel: The pH of the finally developed formulation was checked by calibrated pH meter and pH strip. The 1 gm. of Emulgel was diluted with 10 ml purified DW, therefore pH was measured at ambient temperature [19].
Viscosity: The Brookfield viscometer was used to measure the viscosity. The viscosity was measured at room temperature at 250°C.
The Emulgel formulation was filled in a beaker and the spindle was permitted to completely travel within the Emulgel, and the result was recorded. Experiments were performed in triplicate for each formulation by operating the speed of the spindle from low to high and high to low [20,21].
Drug content Determination: The Amount of drug of the improved formulations was determined by sonicating a predetermined amount of emulgel in ethanol (solvent) and filtering the resultant solutions using Whatman filter paper. Using a UV/VIS spectrophotometer, absorbance was determined at 429 nm following appropriate dilution. Finally, the drug content was determined [22].
Spreadability study: In the spreadability study, the apparatus used is given by Burkiet al18. The process depended on the slip and drag method. In these utensils, there are 2 wooden blocks present. The block was attached with a pan, and a glass slide was attached with the pan end. The second ground wooden block consisted of another glass slide. Therefore approximately 2 g of Emulgel was stacked between the upper and ground slides. Then 40gm. Weight was put on the pan which moves and drags the upper slide over the lower slide. The time which was required (in seconds) by the upper slide to carry a distance of 6-cm was noted. Spreadability data of all batches were measured and calculated with the help of Eq.-
S= M x L/T (1)
There is, M is the weight which was kept at the top movable slide, L is slide length, and T is the time which is grasping by slides to slip off [20].
Bioadhesive strength measurement: There is the altered process was implemented to the determination of Bioadhesive capacity. The utensil comprises 2 arms balances. Both ends of the arm balance were tied with glass slides by employing string. One 1side consist of 2 glass slides, while another glass slide was present. Both side arms were measured with putting more emphasis on left glass slides. The balance was left for about 5 minutes in the same positions. Then 1g prepared formulation was put amidst these two glass slides having hair-free fresh rat dermis pieces and sandwiched them by removing the weight from slides. The balance was left for 5 minutes in this position. Therefore, on the left-hand side, the Weight was gradually increased at a rate of 200 mg/min till the two glass slides were parted from one another. The weight which was required to separate the Emulgel from the slide surface was noted [21]. Finally, the Bioadhesive strength was calculated by applying the formula:
Bioadhesive strength = weight required (gm) / Area (cm2) (2)
Centrifugation test
The centrifugal test was used to evaluate the stability of Emulgel. In this test about 5-6 g of finally optimized Emulgel formulation was taken in a centrifuge tube and centrifuged at 5000 rpm for about 10 min at 250 °C. Therefore the Emulgel was observed visually for any indication of phase separation or creaming [20,21].
In-vitro release study
In-vitro medication release tests were performed by utilizing customized Franz diffusion (FD) cell. Between donors and receptors compartments of the FD cell, a dialysis membrane was installed, and approximately. 2 g of optimized Emulgel formulation was put on the dialysis membrane. The used dissolution media was phosphate buffer PH 5.4. A circulating water jacket was assembled with the system to maintain the temperature of the cell at 37°C. The whole designed equipment was placed on a magnetic stirrer, and continuously the dissolution media was stirred with the help of magnetic beads. After that on suitable time intervals sample (5ml) was withdrawn from the receptor compartment and an equal volume of the same fresh media was placed into the receptor compartment. The cumulative % drug release was calculated by spectrophotometric analysis of samples at 429 nm by using a double beam spectrophotometer [23].
In-vitro Antibacterial and antifungal activity study
Preparation of culture media: The Staphylococcus aureus, Escherichia coli, and Aspergillus niger were used in the antimicrobial testing of Curcumin and curcumin-loaded Emulgel formulations. These microorganisms were obtained from the Biotech department of Goel Institute of technology and management, Lucknow, India. Before the good diffusion process of experiments, bacteria and fungi were inoculated by using Nutrient broth and PDA broth respectively and incubated at 37 °C for 24 h. after that check visually to observe turbidity to confirm the suitable growth of microbes. Therefore the number of colonies were picked and suspended on the nutrient agar and PDA agar plate. For the inoculation purpose, the streaking method was used under the aseptic medium (Laminar Air Flow). The inoculated NA plate and PDA plates were placed into the incubator at 37°C for 24 h to the proper growth of colonies of microorganisms [24,25].
Determination of zone of inhibition: For the determination of anti-bacterial and anti-fungal properties against test strains on NA and Potato Dextrose Agar (PDA) plate, the good diffusion method was used. Already inoculated (Containing colonies of microbes) NA plate and PDA plate was taken and wells were formed by using well borer under the aseptic environment. The wells were filled by the standard curcumin solution was filled in the wells having conc. 500μg/ ml as standard and optimized Emulgel having conc. Of curcumin 500 μg/g. then the plates were incubated for 24 h at 37°C by using a wellworking incubator. Finally, the antimicrobial and antifungal activity was determined by estimating ZOI [24].
Stability analysis: For the stability research, the optimum Emulgel formulation was chosen. A sufficient amount of Emulgel formulation was packed in a 5 g collapsible tube in triplicate and submitted to stability tests for three months at room temperature. The samples were tested for pH, drug content, and phase separation at predefined periods [22].
Result
UV- spectroscopy
The λ max of curcumin in ethanol was found to be at 429 nm which shows that the purity of curcumin, based on the obtained result we can say that the obtained sample was authentic.
Calibration curve
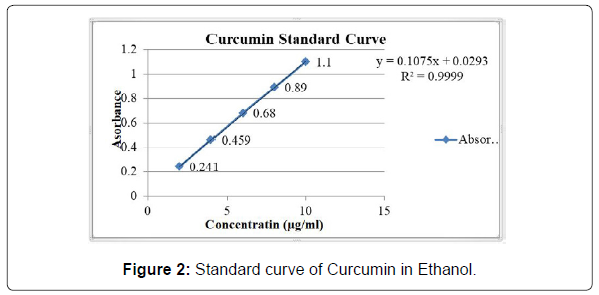
Ethanol was used for the preparation of the calibration curve. The absorbance of different concentrations of solution of curcumin was measured at λ max 429 nm which was given in Table 2, and a calibration curve was shown in Figure 2.
| Sr. No. | Concentration (µg/ml) | Absorbance |
|---|---|---|
| 1 | 2 | 0.241 |
| 2 | 4 | 0.459 |
| 3 | 6 | 0.680 |
| 4 | 8 | 0.890 |
| 5 | 10 | 1.10 |
Liquefaction point
The result was founded to be 181. The average value was taken from triplicate readings. The reference melting point is about 179 - 182.
Solubility
Solubility of curcumin in various solvents was performed by the given method in the methodology section. The obtained data are as follows (Table 3).
| Sr. No. | Oil and excipients | solubility |
|---|---|---|
| 1. | Cumin oil | 1.175+0.135 |
| 2. | Tween 80 | 3.189+0.546 |
| 3. | Ethanol | 21.451+0.509 |
| 4. | Water | 0.003+0.0002 |
FTIR analysis
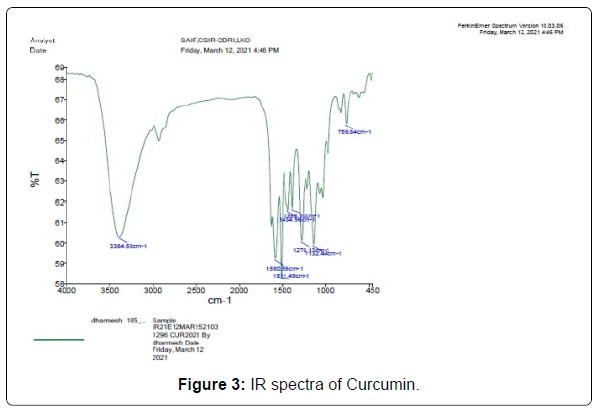
The Infrared spectroscopy of curcumin shows stretch vibration at 1628 cm-1 owing largely to the overlapped stretch vibration of alkenes (C=C) and carbonyl (C=O) characteristics. Stretch vibrations at 3384- 3500 cm-1 owing to O-H groups, C=C aromatic stretch vibrations at 1434 cm-1, as well as high-intensity band 1511 cm-1 apportioned to blended vibration such as stretch carbonyl bond vibration (C=O), in-plane bending vibrations all over aliphatic CC-C, CC=O, and in bending vibration around aromatic CC-H of keto and enol setups. All obtained IR data and their graph is given in Table 4 and Figure 3.
| Curcumin | |||
|---|---|---|---|
| Reported peak value (cm-1) | Observed peak value(cm-1) | Bonds | Functional groups |
| 3785-3500 | 3384.58 | O-H Stretch | Free hydroxyl-alcohol and phenols |
| 1600-1400 | 1580.58 | C=C(aromatic) | Alkenes |
| 1550-1500 | 1511.49 | N-O stretching | Nitro compound |
| 1390-1310 | 1383.10 | O-H bending | Phenol |
| 1440-1395 | 1434.96 | O-H bending | Carboxylic acid |
| 1150-1085 | 1132.44 | C-O stretching | Aliphatic ether |
Characterization of optimized Emulgel formulation
Physical examination: The final formulation batches were examined for their physical appearance and the founded data was given in Table 5.
| Sr. No. | Parameters | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1. | Color | Yellow | yellow | Yellow | Yellow | Yellow | Yellow |
| 2. | Odor | Cumin like |
Cumin like |
Cumin like |
Cumin like |
Cumin like |
Cumin like |
| 3. | Consistency | Good | Better | Good | Better | Good | Good |
| 4. | Phase separation |
No | No | No | No | No | No |
pH of Emulgel: In this study, the PH of all prepared batches was checked by a digital PH meter. The obtained data are shown in Table 6.
| Sr. No. | Parameters | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1 | pH | 6.6 | 5.76 | 6.5 | 6.2 | 6.8 | 6.2 |
Viscosity: Obtained viscosity of Emulgel was appropriate. From obtained data of viscosity, we found that formulation F2 F4 was best for topical application (Table 7).
| Viscosity (in centipoise) | |||||||
|---|---|---|---|---|---|---|---|
| Sr. No. | RPM | F1 | F2 | F3 | F4 | F5 | F6 |
| 1. | 2.5 | 68000 | 88000 | 77000 | 48000 | 62000 | 59000 |
| 2. | 50 | 12200 | 12600 | 12400 | 11400 | 11900 | 11600 |
Spreadability study: The spreadability of all Emulgel batches was founded to be in the range of 3.57-16.66 g.cm/sec which was shown in Table 8. From the study, we found that the formulation with code F2 has more spreadability as compare to other batches.
| Sr. No. | Parameter | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1 | Spreadability (g.cm/sec) | 13.56 | 16.66 | 13.57 | 14.47 | 15.25 | 16.25 |
Drug content analysis
The drug content of the formulation was resolute by linear regression analysis of the calibration curve. The drug content of all Emulgel formulations is given in Table 9.
| Sr No | Formulation | % of drug content |
|---|---|---|
| 1 | F1 | 86.42 |
| 2 | F2 | 89.49 |
| 3 | F3 | 83.17 |
| 4 | F4 | 90.24 |
| 5 | F5 | 88.56 |
| 6 | F6 | 83.36 |
Bioadhesive strength measurement
The obtained Bioadhesive data are given in Table 10. From the obtained data we found that the F2 and F4 were shown appropriate Bioadhesive properties.
| Sr. No. | Parameter | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1. | Bioadhesive strength (Kg/cm2) | 2.5 | 5.1 | 3 | 4.9 | 3.5 | 3.9 |
Centrifugation test
Centrifugation test data of all formulation batches were performed identified visually. After this test, we found that all batches were stable there is no phase separation occur.
In-vitro release study: The release study was conducted by the method the same as given in the methodology section. in this study we found that the drug release from its Emulgel formulations can be categorized in the successive descending order: F2 > F4 > F1 > F5 > F6 > F3, the release of drug from the formulations after 360 min were 95.33%, 93.53%, 82.41%, 81.79%, 80.25%, & 80.10% respectively (Table 11).
| Sr. No. |
Time (Min) |
F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|---|
| 1. | 00 | 00.00 ± 00.00 | 00.00 ± 00.00 | 00.00 ± 00.00 | 00.00 ± 00.00 | 00.00 ± 00.00 | 00.00 ± 00.00 |
| 2. | 30 | 11.19 ± 0.01 | 14.04 ± 03.25 | 14.66 ± 02.44 | 11.01 ± 02.21 | 11.26 ± 05.12 | 11.07 ± 02.32 |
| 3. | 60 | 15.99 ± 01.1 | 19.23 ± 05.21 | 15.28 ± 04.62 | 14.19 ± 03.25 | 11.38 ± 02.14 | 19.01 ± 05.12 |
| 4. | 90 | 20.00 ± 02.21 | 25.61 ± 06.24 | 20.99 ± 03.01 | 27.50 ± 08.21 | 11.32 ± 08.21 | 25.01 ± 04.12 |
| 5. | 120 | 25.30 ± 00.25 | 30.34 ± 03.54 | 23.81 ± 05.28 | 39.81 ± 01.02 | 11.13 ± 06.21 | 31.32 ± 09.45 |
| 6. | 150 | 31.88 ± 05.15 | 35.71 ± 02.25 | 29.66 ± 07.25 | 45.65 ± 04.85 | 11.38 ± 04.58 | 39.07 ± 10.15 |
| 7. | 180 | 36.56 ± 03.25 | 40.55 ± 04.65 | 35.43 ± 01.00 | 50.02 ± 06.10 | 11.13 ± 01.85 | 43.13 ± 07.25 |
| 8. | 210 | 39.57 ± 01.25 | 52.42 ± 03.54 | 38.99 ± 08.01 | 68.05 ± 05.21 | 11.07 ± 04.25 | 50.07 ± 08.98 |
| 9. | 240 | 41.26 ± 05.71 | 65.40 ± 12.21 | 45.33 ± 06.67 | 70.49 ± 04.21 | 11.01 ± 10.20 | 56.01 ± 12.68 |
| 10. | 270 | 49.07 ± 01.85 | 75.18 ± 04.85 | 58.56 ± 05.85 | 75.34 ± 06.45 | 11.07 ± 09.15 | 62.07 ± 08.91 |
| 11. | 300 | 60.49 ± 04.25 | 80.03 ± 10.25 | 68.32 ± 04.2 | 80.75 ± 07.54 | 23.46 ± 06.21 | 68.01 ± 13.79 |
| 12. | 330 | 74.50 ± 07.15 | 86.73 ± 16.25 | 70.88 ± 17.25 | 90.68 ± 06.51 | 24.21 ± 02.95 | 76.01 ± 15.12 |
| 13. | 360 | 82.41 ± 08.12 | 95.33 ± 06.21 | 80.10 ± 16.12 | 93.53 ± 09.12 | 81.79 ± 10.25 | 80.25 ± 19.25 |
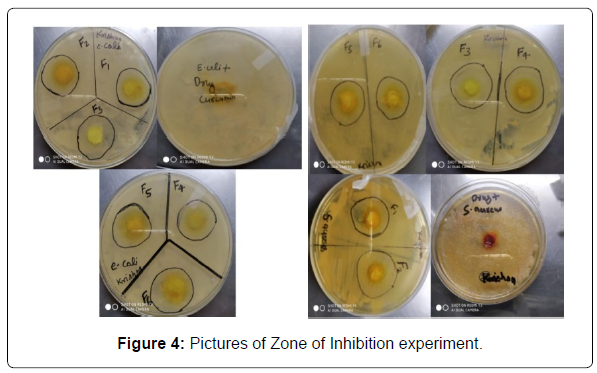
Antifungal and antibacterial activity: The determined ZOI data of all the batches are given in the table based on the obtained result we assumed that the formulation F2 and F4 show the best activity against both the bacteria and fungi (Table 12 and Figure 4).
| Sr No | Organism | ZOI of Curcumin (mm) | ZOI of Emulgel (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |||
| 1 | S. aureus | 17 | 21 | 32 | 23 | 29 | 19 | 20 |
| 2 | E. coli | 29 | 14 | 34 | 19 | 32 | 25 | 18 |
| 3 | A. Niger | 19 | 15 | 25 | 18 | 24 | 17 | 15 |
Stability study: All the developed curcuminemulgel were found to be stable after 3 months at room temperature. No, an interchange was founded in parameters like drug content, pH, visual appearance, and spreadability. Syneresis is the main problem with gel; also, there was no syneresis effect. Obtained data of stability analysis is given in Table 13.
| Sr No | Formulation | % Drug content | pH | Visual appearance | Spreadability | |
|---|---|---|---|---|---|---|
| Color | Phase separation | |||||
| 1 | F1 | 86.33 | 6.6 | Yellow | No | 13.56 |
| 2 | F2 | 89.40 | 5.7 | Yellow | No | 16.66 |
| 3 | F3 | 82.98 | 6.5 | Yellow | No | 13.57 |
| 4 | F4 | 90.14 | 6.2 | Yellow | No | 14.47 |
| 5 | F5 | 88.38 | 6.8 | Yellow | No | 15.25 |
| 6 | F6 | 83.17 | 6.2 | Yellow | No | 16.25 |
Discussion
The curcumin shows low solubility which tends to lower oral bioavailability and challenging to developed suspension formulation due to its lower wettability and its lower aqueous solubility. So we have developed a suitable dosage form to short out the bioavailability and administration hurdles. Emulgel is one of the best options to enhance the bioavailability of drug and their suitability during application on the skin.
So we have chosen the best and appropriate excipients (oil, surfactant, and co-surfactant) in which curcumin shows more solubility and compatibility to achieve high efficacy and activity. The solubility of curcumin founded to be 21.451 in ethanol (co-surfactant) and 3.189 in tween 80 (Surfactant).
The physical appearance of curcuminEmulgel formulation was founded to be appropriate which has the PH of approx. 7 which was observed safely in use due to its neutral PH which is similar to skin ph. The color of the formulation was yellow due to the coloring matter present in the curcumin.
The viscosity of the formulation increases during the testing, this shows that the increase in the concentration of surfactant causes the formulation to become thicker; therefore it has a smaller spread because the viscosity increases so that the spreadability of the formulation is more challenging to spread. The adhesive property of the formulation was not affected by an increase in the concentration of surfactants. On centrifugation, the study formulation doesn't show any phase separation.
The adhesion property of Emulgel has a sharp decrease after 90 days of storage. Emulgel which has more concentration of oil and fat becomes more liquid while the increase in temperature therefore the adhesion property of developed formulation becomes smaller. The spreading power of the Emulgel was relatively stable, statistical data proved that there were no significant differences after storage.
The drug release study of formulation shows that all formulation shows 80-95 % of drug release after 360 minutes. Batch F2 and F4 show 95 % and 93% drug release respectively after 360 minutes, which was assumed as the best formulation in respect to drug release.
The antibacterial and antifungal study of developed formulations shows that all the developed batches were shown the best activity against tested microbes and fungi. F2 & F4 has the more effective against the microbes and fungi, which confirms that the synergistic effect of cumin oil and curcumin was found.
Stability test on storage exhibits that the developed Emulgel doesn’t alter its shape, odor, or color after storage of 90 days. Therefore it is proved that the purified curcumin Emulgel was organoleptically stable. The viscosity of Emulgel changes a little bit due to an increase in temperature.
Conclusion
The result of the physical property of formulation (Emulgel) liable to fulfill the requirements but at storage, it causes the stickiness’ and viscosity of all formulations to decrease significantly. We suggest for further research to put storage preferably at lower temperature and protected from light, then it is possible to be more stable for a long time. Finally from all the studies, it can be gathered that the Emulgel formulation to be an effective novel drug delivery system for topical usage, which is stable and can prove to be more potent at lower concentrations of curcumin. In the future, the in-vitro study must be performed to determine the potential of the developed formulation.
Conflicts of Interests
The authors declare no conflict of interest.
Acknowledgments
We acknowledge HiMedia Lab. (Mumbai) for providing Curcumin sample.
References
- Sabri HS, Ali WK, Abdullahb BH, Al-Ani WMK (2016) Formulation Design and Evaluation of Anti-Microbial Activity of Emulgel Containing Essential Oil of Myrtus communis L. Int J Pharm Sci Rev Res 40:271-277.
- Pakhare AV, Deshmane SV, Deshmane SS, Biyani KR (2017) Design and Development of Emulgel Preparation Containing Diclofenac Potassium. Asian J Pharma 11:712.
- Minal PK (2021) Formulation and evaluation of emulgel containing Piper nigrum and Curcuma longa extract for vitiligo. Int J Global Trends Pharm Sci 12:9283-9293.
- Reddy R, Priya S, Akula G, Santhosh S, Jaswanth A (2021) Formulation and evaluation of Naproxen Emulgel for Topical Delivery. Research J Pharm and Tech 14:1961-1965.
- Shid SS, Dhadde G, Gidde ND, Vakhariya RR, Raut ID, et al. (2021) Formulation and Characterization of Ciclopirox Olamine Emulgel. PENSEE 51:337-349.
- Nayak A, Mandal SK, Ramadan M, Rath SK (2021) Formulation, Development and Physicochemical Characterization of Diclofenac Topical Emulgel. Egypt J Chem 64:1563-1573.
- Yadav S, Wairkar S, Invally M, Ranade S (2017) Topical emulgel of tolnaflate with penetration enhancer: development, characterization and Antifungal activity. Indian J Med Res Pharma Sci 4:28-35.
- Kabir T, Rahman H, Akter R, Behl T, Kaushik D, et al. (2021) Potential Role of Curcumin and its Nanoformulations to treat Various Types of Cancers. MDPI Biomolecules 11.
- Kaur S, Modi NH, Panda D, Roy N (2010) Probing the binding site of curcumin in Escherichia Coli and Bacillus subtilis FtsZ- A structural insight to unveil antibacterial activity of curcumin. Eur J Med Chem 45:4209- 4214.
- Rai D, Singh JK, Roy N, Panda D (2008) Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J 410:147-155.
- Javed M, Shoaib M, Iqbal Z, Khan MA, Hussain S, et al (2020) Phytochemical and Biological Studies on Curcuma longa L. in Pattoki (Kasur), Pakistan. B Life and Environment Sciences 57:59-66.
- Karimi A, Majlesi M, Rafieian-Kopaei M (2015) Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol 4:27-30.
- Jahanshiri Z, Shams-Ghahfarokhi M, Allameh A, Razzaghi-Abyaneh M (2012) Effect of Curcumin on Aspergillus parasiticus Growth and Expression of Major Genes Involved in the Early and Late stages of Aflatoxin Biosynthesis. Iran J Public Health 41:72-79.
- Bergonzi MC, Hamdouch R, Mazzacuva F, Isacchi B, Bilia AR (2014) Optimization, Characterization and in vitro evaluation of curcumin microemulsions. LWT- Food Science and Technology (Elsevier) 59:148-155.
- Khullar R, Kumar D, Seth N, Saini S (2012) Formulation and evaluation of mefenamic acid Emulgel for topical delivery. Saudi Pharm J 20:63-67.
- Shahin M, Hady SA, Hammad M, Mortada N (2011) Novel Jojoba Oil-Based Emulsion Gel Formulations for Clotrimazole Delivery. AAPS Pharm SciTech 12:439-447.
- Kaushik D, Verma R (2020) Design and optimization of candesartan loaded self-nano emulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Deliv 27:756-771.
- Kocaadam B, Sanlier N (2017) Curcumin, an active component of turmeric (Curcuma longa), and its effect on health. Crit Rev Food Sci Nutr 57:2889-2895.
- Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, et al. (2019) A novel self-nano emulsifying drug delivery system for curcumin used in the treatment of wound healing and inflammation. 3 Biotech 9:360.
- Burki IK, Khan MK, Khan BA, Uzair B, Braga VA, et al. (2020) Formulation Development, Characterization, and Evaluation of a Novel Dexibuprofen-Capsaicin Skin Emulgel with Improved In Vivo Anti-inflammatory and Analgesic Effects. AAPS PharmaSciTech 21:211.
- Mahajan VR, Basarkar GD (2019) Formulation design, development and characterization of dexibuprofen emulgel for topical delivery: In- vitro and In-vivo evaluation. J Drug Deliv Therap 9:330-342.
- Banyal M (2020) Formulation and evaluation of Fluconazole Emulgel By using different Polymers. WJPR 9:2084-2098.
- Ngwabebhoh FA, Erdagi SI, Yildiz U (2018) Pickering emulsion stabilized nano cellulosic-based nanoparticles for coumarin and curcumin nanoencapsulation: In vitro release, anticancer and antimicrobial activities. Carbohydrate Polymers J 201:317-328.
- Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N (2011) Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J Agric Food Chem 59:2056-2061.
- Natalia M, Mahdi J (2014) Physical stability and Antibacterial activity of Black cumin oil (Nigella sativa L.) Nanoemulsion Gel. Int J Pharm Tech Res 6:1162-1169.
Citation: Kumar K, Kumar N, Singh A, Gupta A (2021) Development and Evaluation of Curcumin Emulgelwith Cumin Oil for Topical Application. Clin Pharmacol Biopharm, 10: 233. DOI: 10.4172/2167-065X.1000233
Copyright: © 2021 Kumar K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4102
- [From(publication date): 0-2021 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3275
- PDF downloads: 827