Determining the In Vitro Effects of Aqueous Elephant Dung and Fadogia ancylantha Leaf Extracts on Isolated Rat Pregnant Uterus Horns
Received: 26-Feb-2020 / Accepted Date: 17-Mar-2020 / Published Date: 24-Mar-2020 DOI: 10.4172/2573-4555.1000282
Abstract
Background: In Zimbabwe, elephant dung and Fadogia ancylantha are widely used in the third trimester of pregnancy. They are believed to facilitate childbirth because they open the birth canal during delivery. However, the effects of these medicines in pregnancy have not been investigated. This study aimed to determine the effects of elephant dung and F. ancylantha leaf extracts on isolated pregnant and non-pregnant uterine horns.
Materials and Methods: Elephant dung and Fadogia ancylantha leaves were air-dried, macerated in water, filtered, evaporated and freeze dried. Uterine horns from 72 non-pregnant and 72 pregnant female Sprague-Dawley rats were used. The ‘test’ strips were separately exposed to elephant dung (20-640 mg/ml) or F. ancylantha (25-800 mg/ml) while the 'controls' received distilled water only. Effects of oxytocin on tissues exposed to these extracts were measured.
Results: Elephant dung aqueous extract contracted the uterus in a dose-dependent manner whilst Fadogia ancylantha relaxed uterine tissue in a dose-dependent manner. The effects of both the Elephant dung and Fadogia ancylantha extracts were higher in pregnant uteri versus non-pregnant uteri. Oxytocin potentiated elephant dunginduced contraction, but only slightly shifted baseline upwards in tissues exposed to Fadogia ancylantha.
Conclusion: Elephant dung possessed oxytoxic effects and contracted uteri in a dose-dependent manner. Fadogia ancylantha relaxed the uterus in a dose-dependent manner, suggesting that it might slow down labour progression since uterine contraction will not be occurring at a sufficient enough pace to facilitate labour and delivery. Pregnant women and health care workers need education to understand the possible effects of using these traditional medicines in pregnancy.
Keywords: Elephant dung; Fadogia ancylantha ; Pregnancy; Uterus; Oxytoxic
Abbreviations
GD: Gestation day, ANOVA: Analysis of variance, IP3: Inositol-1,4,5-triphosphate, FAE: Fadogia ancylantha Extract
Introduction
Traditional medicines are used to treat and manage a number of health conditions in many African countries, including Zimbabwe. This has been attributed to the belief that the medicinal effects help to normalize physiological function and correct the underlying cause of the disorder [1]. Different plants and animal matter have also been used as medicine during pregnancy, birth, and for postpartum care in many areas around the world for generations [2]. Pregnant women use these medicines to prevent miscarriages, ease morning sickness, reduce swelling of the legs and ankles, and treat abdominal pains [3]. They are also used during childbirth to speed up labour contractions, prevent post-partum haemorrhage, cleanse the womb and encourage lactation [4].
In Zimbabwe, local women use a range of traditional medicines during pregnancy including Pouzolzia mixta (Soap nettle/ Munhanzva ), Abelmoschus esculuntus (Okra/Derere ), and Dicerocaryum zanguebarium (Devil thorn/Ruredzo) [5,6]. These plants are mainly used in the third trimester to induce labour, avoid perineal tearing and ensure safe delivery [5-7]. Soil from a burrowing mole and holy water, to mention a few, are also used to precipitate or facilitate labour and protect the unborn child, respectively [5]. Zimbabwean women also use elephant dung (Ndove yenzou) and Fadogia ancylantha leaves (Makoni tea) during pregnancy, mainly to widen the birth canal and to prevent perineal tearing [6].
Despite the widespread use of such traditional medicines, there is insufficient scientific data to justify its use in pregnancy. The bodies of pregnant women are extremely vulnerable to the effects of drugs due to the physiological changes that occur in pregnancy [8], with some drugs being harmful to both the mother and foetus. In addition, understanding the pharmacological effects of some of these traditional medicines might eventually lead to the development of novel drugs. This study was thus conducted to determine the in vitro effects of two traditional medicines that are commonly used by Zimbabwean women during pregnancy: elephant dung and Fadogia ancylantha.
Materials and Methods
This was an experimental in vitro study.
Ethical consideration
This study was approved by the Joint Research and Ethics Committee of the University of Zimbabwe and Parirenyatwa group of hospitals and the Medical Research Council of Zimbabwe. The study conformed to the “Handbook of laboratory animal management and welfare” [9].
Materials
Elephant dung was identified and collected by a game park ranger from Hwange Game Park which is located 19.1241°S, 26.5926°E, in December 2017. A voucher specimen of the Elephant dung was stored in the Clinical Pharmacology Department (TM/ED-01/17). Fadogia ancylantha was collected from Domboshava in January 2018 (17.6118°S, 31.1432°E) after it had been identified by a botanist from the Harare Botanical gardens. A voucher specimen was stored in the Clinical Pharmacology department (TM/FD-01/18).
Preparation of the elephant dung aqueous extract and Fadogia ancylantha leaf aqueous extract
One kilogram of fresh Elephant dung and one kilogram of Fadogia ancylantha leaves were separately air-dried at room temperature (26 ± 1°C) for one week. The dried products were milled (using a mortar and pestle) into a fine powder. The powders were separately macerated in 2.5 liters of distilled water for 48 hours at room temperature (26 ± 1°C). The mixtures were filtered and the supernatants were collected. A rotary evaporator was then used to concentrate the aqueous extracts by drying them at 60 ± 1°C. Freeze-drying gave 107.4 g (10.74% yield) of a dark green, powdery, crude extract of the Elephant dung, and 92.3 g (9.23% yield) of a dark brown powdery extract of the Fadogia ancylantha. Aliquot portions of the two extract residues were weighed and dissolved in distilled water (at room temperature) for use on each day of the experiments.
Animals
12 healthy young male and 154 young healthy female Sprague- Dawley rats weighing between 160-200 g were purchased from the University of Zimbabwe college of Health Sciences Animal House. The animals were housed in the Department of Clinical Pharmacology under standard laboratory conditions, with free access to standard pellets for food and drinking tap water. Male animals were housed in a separate cage whist the females were divided into two groups i.e. Elephant dung and Fadogia ancylantha treatment groups containing 72 females each. Of the 72 animals in a group, 36 were used in the non-pregnant state, whilst 36 rats were allowed to mate with the males to ensure pregnancy. The pregnant animals used in the experiments were between 15-18 days pregnant. Prior to the experiment, animals were fasted for 16 hours, but they had access to drinking water.
Mating
To ensure pregnancy, 12 male and 36 female animals were allowed to mate. 3 female rats were put into a cage and a single male was introduced into each cage at a ratio of 3:1. The animals were left to mate over a six-day period. Female rats were then checked for a copulation plug daily and when a plug was observed the females were separated from the males. The day the plug was observed was recorded as gestation day zero (GD0) and the female rats ’ weights were recorded. Animals with no plug were left in the cages with the male for mating to continue and were removed only when a plug was observed. To confirm pregnancy, the female rats were weighed every morning and their weights were recorded (pregnant rats gain more than 1.75 g by gestation day 8).
Determining the pharmacological effects of elephant dung and Fadogia ancylantha aqueous leaf extracts
Pregnant and non-pregnant rats were quickly excised after being euthanized via CO2 inhalation just before the uteri were removed. Segments of 2 cm in length were dissected rapidly and adhering connective tissues and fat were removed. Uteri segments were mounted in 25 ml Iworx tissue organ-baths containing Tyrode ’ s physiological solution of the following composition NaCl2 g, KCL 10% 0.5 ml, CaCl2 0.5 ml, MgCl2 0.25 ml, NaHCO3 0.25 g, and NaH2 PO4 0.125 ml. Organ bath temperature was maintained at 37°C. The lower ends of the uterine tissues were attached using silk sutures to tissue holders and the upper ends to force transducers connected to Iworx- IWX214. Two tissues from the same animal were set up i.e. the ‘test’ and the ‘control’ segments. The uterine segments were given time to equilibrate in Tyrode’s solution for 30 minutes with the bath solution being changed every 15 minutes.
After the equilibration period, graded concentrations of Elephant dung (20, 40, 80, 160, 320, 640 mg/ml) or Fadogia ancylantha (25, 50,100,200,400,800 mg/ml) (n=6) were added to the bath-fluid at different times. (These doses were selected based on reported doses that the women used during pregnancy). In addition, dose response effects were determined by incubating tissues with oxytocin (2 μU/ml). After exposing the tissues to the Elephant dung or Fadogia ancylantha for about 5 minutes, bath-applied concentrations were washed out 4-5 times, and the tissue was allowed to rest for 5-10 minutes, or until its basal tone returned to baseline point. The ‘control’ smooth muscle strips were treated with distilled water (0.1-2 ml only equivalent to the volume of bath applied Elephant dung or Fadogia ancylantha ). Each of the ‘test’ smooth muscle preparations were used for one concentrationresponse curve only, with extract-induced responses on the smooth muscle strips being recorded using LabScribe ® software.
Data analysis
The contraction/relaxation produced by each concentration of either elephant dung extract or Fadogia ancylantha was measured and expressed as a percentage of contraction/relaxation of the maximum tension (100%). Experimental data were expressed as mean ± standard error of the mean. Statistical comparison was performed using Graphpad Prism® Version 7 using Student ’ s t-test and one way ANOVA between ‘ tests ’ data and ‘ control ’ data. When ANOVA exhibited a statistical difference, Dunnets post hoc tests were applied. P-values of <0.05 were considered to be statistically significant.
Results
Effect of elephant dung on the uterus
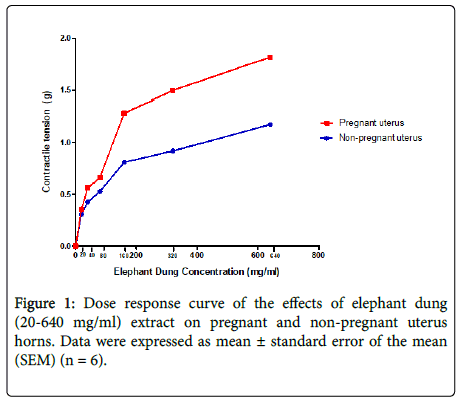
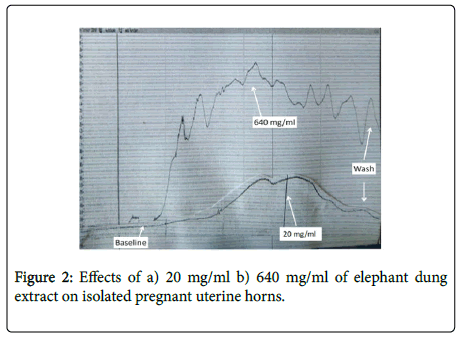
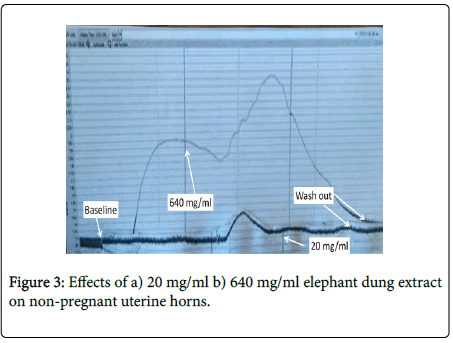
Elephant dung extract significantly contracted both pregnant and non-pregnant uteri in a dose-dependent manner (Figure 1). The effects of the extract were 0.68 g (59%) greater in the pregnant uterus as opposed to the non-pregnant one. Significant (p<0.05-0.001) dose-dependent increases in amplitude and frequency of contractions were observed after the extract was added to pregnant uteri, with an upward shift in baseline contractions (Figure 2). Although an upward shift in baseline and an increase in contractile amplitude were also observed in the non-pregnant uterus, elephant dung extract had no effect on the frequency of contractions (Figure 3). Oxytocin significantly (P<0.05) potentiated the contractile effects of elephant dung extract on pregnant uteri (Table 1).
| Elephant dung extract alone | Elephant dung extract+Oxytocin 2 µU/ml | |||
|---|---|---|---|---|
| Concentration mg/ml | Contractile tension % | N | Contractile tension % | N |
| 20 | 32.9 | 6 | 44.9 | 6 |
| 40 | 33.9 | 6 | 63.6 | 6 |
| 80 | 39.5 | 6 | 72.2 | 6 |
| 160 | 44.3 | 6 | 81.5 | 6 |
| 320 | 48.1 | 6 | 89.9 | 6 |
| 640 | 50.1 | 6 | 92.6 | 6 |
Table 1: Effect on pregnant uterus of elephant dung extract alone and elephant dung+Oxytocin.
Oxytocin potentiated elephant dung-induced contractions in a dose-dependent manner.
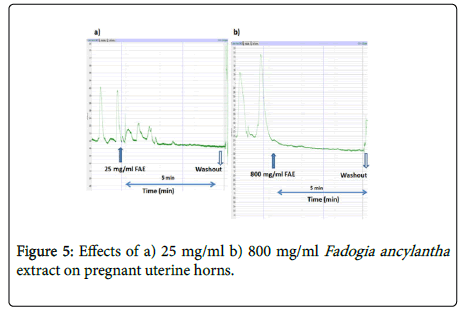
Effect of Fadogia ancylantha extract on the uterus
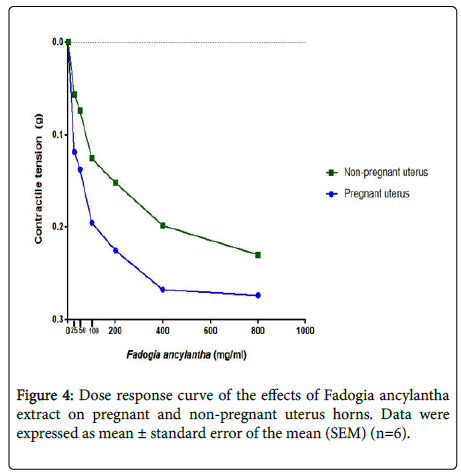
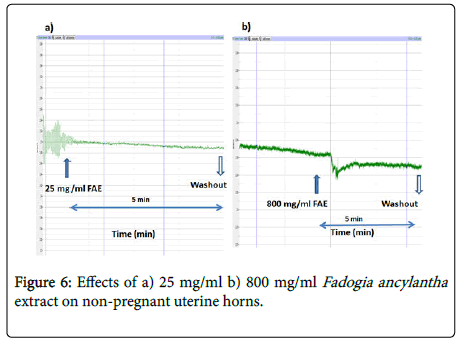
Fadogia ancylantha aqueous leaf extracts (25-800 mg/ml) relaxed uterine tissues in a dose-dependent manner (Figure 4). The extract significantly (p<0.05-0.001) reduced the frequency and amplitude of contractions in pregnant uterus with a downward shift in baseline (Figure 5). This effect was pronounced in the pregnant uterus. In nonpregnant uteri Fadogia ancylantha aqueous extracts significantly shifted baseline downwards (Figure 6). Adding oxytocin to pregnant tissues pre-incubated with Fadogia ancylantha slightly shifted baseline upwards, although no changes in frequency or amplitude of contraction were recorded (Table 2).
| Fadogia ancylantha extract alone | Fadogia ancylantha extract+Oxytocin | |||||
|---|---|---|---|---|---|---|
| Concentration mg/ml | Mean relaxatory tension % | SEM | N | Mean relaxatory tension % | SEM | N |
| 25 | 8.1 | 0.5 | 6 | 5.7 | 1.2 | 6 |
| 50 | 13.5 | 1.1 | 6 | 9.1 | 2.1 | 6 |
| 100 | 32.7 | 2.4 | 6 | 26.7 | 2.7 | 6 |
| 200 | 54.4 | 2.6 | 6 | 48.8 | 2.5 | 6 |
| 400 | 82.2 | 2.1 | 6 | 73.5 | 1.4 | 6 |
| 800 | 84.3 | 2.9 | 6 | 76.9 | 2.8 | 6 |
Table 2: Effect on pregnant uterus of Fadogia ancylantha extract alone and Fadogia ancylantha extract+Oxytocin.
Oxytocin slightly shifted the baseline upwards in pregnant tissues that were pre-incubated with Fadogia ancylantha . Data were expressed as mean ± standard error of the mean (SEM) (n=6).
Discussion
We found that elephant dung extract (20-640 mg/ml) contracted the uterus in a dose dependent manner in both pregnant and non-pregnant uteri. The contractile effects of the extract were more pronounced in the pregnant uteri as opposed to the non-pregnant ones. Fadogia ancylantha aqueous leaf extract relaxed the uterus in a dose-dependent manner with the effects also being more prevalent in pregnant versus non-pregnant uteri. Oxytocin potentiated the contractile effects of elephant dung extracts whilst it reduced the relaxatory effects of F. ancylantha.
Elephant dung aqueous extract
The uterine changes associated with pregnancy could account for the differences observed between the pregnant and non-pregnant uterine horns after exposure to the elephant dung extract [10]. Under normal circumstances, phasic contractions of the uterus occur due to spontaneous changes in electrical activity within the myometrial cells [11]. This results in action potential generation and depolarization. Depolarization causes the opening of voltage-gated, L-type calcium channels, rapid calcium entry and an elevation in intracellular calcium. During pregnancy however, uterine muscle fibres undergo muscular hypertrophy and multiplication due to the effects of both oestrogen and progesterone. These increases in hormone levels and their receptors increase the contractility of the uterus.
In the presence of elephant dung extract, oxytocin potentiated the contractile effects of the extract in a dose dependent manner. It has been reported that during pregnancy the concentration of oxytocin in the blood is slightly elevated and the number of oxytocin receptors also increases [12]. When oxytocin or oxytocin agonists bind to the receptors, contraction of the uterus occurs via the release of inositol-1,4,5-triphosphate (IP3) from the hydrolysis of phosphoinositide. IP3 binds to and opens ligand gated ion channel that allow Ca2+ to move into the cytosol, where it activates various cellular processes such as: mobilization of Ca2+ from storage organelles, release of prostaglandins, and regulation of cell proliferation. These processes all increase contractile tension as they also increase the sensitivity of tissues to calcium [13], which could account for the potentiation that was observed when elephant dung aqueous extract was mixed with oxytocin.
The in vitro effects of elephant dung could also be attributed to the different foods that are eaten by the elephants. Numerous studies analysing the feeding patterns of elephants showed that elephants consume over 307 food and non-food items, with 70% of their food coming from trees [14]. Some of the food trees consumed by elephants include Acacia xanthophloea, Afzelia bipindensis, Coloncoba welwitschii, Bridelia ferruginea and Terminalia superba [15]. A number of these trees contain anthraquinones, flavonoids, saponins, terpenoids and condensed tannins, which are known to facilitate uterine contraction. The phytochemicals present in the trees could thus be responsible for the observed effects [16].
Fadogia ancylantha aqueous leaf extract
Traditionally Fadogia ancylantha is used to treat a variety of ailments including abdominal and chest pain, asthma, toning muscles and diabetes [17]. Some of these conditions arise as a result of smooth muscle contraction. The ability of the plant to treat these illnesses suggests that F. ancylantha extracts treat disease by relaxing the smooth muscle, consistent with the results observed in this study.
Different authors have attributed the effects of the plant to its phenolic components [18,19]. These include phytochemicals such as acetylchalcone, glucosidic chalcone, hydrocoumarin, glucosidic coumarin, saponins and triterpenoids [18,19]. Saponins and polyphenolic compounds present in F. ancylantha act as free radicals scavengers, whilst tritepenoids inhibit various enzymes involved in tissue contraction [17,20,21]. The combined effect of these phytochemical products could thus be promoting tissue relaxation, which could explain the study results.
In addition, as pregnancy progresses myometrial response to various agonists and antagonists is enhanced [22]. Tocolytic agents however, can stop the ability of the uterus to contract thus temporarily delaying delivery. These agents are mainly calcium receptor/channel antagonists, -adrenergic agents, oxytocin antagonists, prostaglandin antagonists, and magnesium sulphate [23]. In addition, nitric oxide donors can also decrease uterine contractility in pregnant rats [23]. Study results therefore suggest that Fadogia ancylantha might be acting as a tocolytic agent at different receptors, since contractile tension decreased significantly after the extract was added, and no significant increases in tension were recorded when oxytocin was added.
Although these traditional medicines are commonly used in pregnancy, we found varying effects on pregnancy and child birth. The contractile effects of elephant dung could facilitate childbirth when used during labour, but could also induce premature labour if used earlier in pregnancy. The relaxation effects of Fadogia ancylantha could slow down labour during childbirth, which could result in complications to both the mother and the unborn baby. Women therefore need to be educated during antenatal care visits on the effects of using these medicines during pregnancy. Similarly, healthcare workers need to be sensitized on these results so as to ensure that they ask relevant questions when women register for antenatal care.
Limitations
The observed effects were mainly in vitro effects. In vivo effects might produce different results as the two extracts might be converted into different products when ingested orally. Research is needed to determine the effects of these extracts in vivo. In addition, phytochemical analysis of the extracts was not conducted. Understanding the phytochemistry of these two crude extracts could have helped to explain the observed effects.
Conclusion
In our study, we found that elephant dung possessed oxytoxic effects and contracted the uterus in a dose dependent manner, whilst Fadogia ancylantha relaxed the uterus in a dose dependent manner. Using these remedies could either delay or speed up natural labour progression and complicate the childbirth process. Pregnant women and healthcare workers should therefore be educated and informed on the possible complications that can arise from using these traditional medicines in pregnancy.
Conflict of Interest
The authors declare there is no conflict of interest.
Acknowledgements
The authors would like to thank the University of Zimbabwe PERFECT program (grant number: 1D43TW010137), National Institutes of Health and Fogarty international for funding the project. We would also want to thank Sister N. Chifamba for her assistance throughout the study and Dr. M Cornell for her assistance with writing this paper.
References
- Murray MT, Pizzorno JE (1999) Textbook of Natural Medicine. Churchill Living, China.
- De Boer H, Lamxay V (2009) Plants used during pregnancy, childbirth and postpartum healthcare in Lao PDR: A comparative study of the Brou, Saek and Kry ethnic groups. JÂ Ethnobiol Ethnomed 5:25.
- Gruber CW, O'Brien M (2011) Uterotonic plants and their bioactive constituents. Planta Medica 77(3):207-220.
- Ticktin T, Dalle S (2005) Medicinal plant use in the practice of midwifery in rural Honduras. Journal of Ethnopharmacol 96(1-2):233-249.
- Mureyi DD, Monera TG, Maponga CC (2012) Prevalence and patterns of prenatal use of traditional medicine among women at selected Harare clinics: a cross-sectional study. BMC Complement Med Ther 12:164-170.
- Mawoza T, Magwali T, Nhachi C (2019) Prevalence of Traditional Medicine Use during Pregnancy, at Labour and for Postpartum Care in a Rural Area in Zimbabwe. Clinics in mother and child health 16(2):321.
- Glover GD, Amonkar M, Rybeck BF, Tracy TS (2003) Prescription, over-the-counter and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol 188(4):1039-1045.
- Maroyi A (2013) Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J Ethnobiol Ethnomed  9:31.
- Wolfensohn S, Lloyd Mc (2007) Handbook of laboratory animal and management and welfare. Oxford University Press: New York, USA.
- Cruz YP, Selwood L (1997) Histological differences between gravid and non-gravid uteri in the dasyurid marsupial, Sminthopsis macroura (Spencer). J Reprod Fertil Suppl 111(2):319-325.
- Arrowsmith S, Kendrick A, Wray S (2010) Drugs acting on the pregnant uterus. Obstet Gynaecol Reprod Med 20(8):241-247.
- Prevost M, Zelkowitz P, Tulandi T, Hayton B, Feeley N (2014) Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front Public Health 2:1.
- Somlyo AP, Somlyo AV (1998) From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta. Physiologica Scandanavica 164(4): 437-48.
- Tchamba MN, Seme PM (1993) Diet and feeding behaviour of the forest Elephant in the Santchou Reserve, Cameroon. Afr J Ecol 31(2):165-171.
- Codron J, Lee-Thorp JA, Sponheimer M, Codron D, Grant RC (2006) Elephant (Loxodonta africana) Diets in Kruger National Park, South Africa: Spatial and Landscape Differences. J Mammal 87(1):27-34.
- Nundkumar N, Ojewole JAO (2002) Studies on the antiplasmodial properties of some South African medicinal plants used as antimalarial remedies in Zulu folk medicine. Methods Find Exp Clin Pharmacol 24(7):397-402.
- Muchuweti M, Chirongo M, Ndhalala AR, Mupure CH, Chitindingu K (2008) Recent progress in medicinal plants 19:307-317.
- Nyirenda KK, Kalenga-Saka JD (2014) Antidiabetic and antioxidant activities of phytoconstituents from Fadogia ancylantha: Abstracts of Pharma Nutrition 2013. PharmaNutrition 2:75-119.
- Mecherini T, Picerno P, Gaudio PD, Festa M, Capasso A, et al. (2010) Saponin and polyphenols from Fadogia ancylantha (Makoni Tea). J Nat Prod. 2010; 7:247-251.
- Feng Z, Wu P, Li W, Guo T, Liu Q (2015) Concise synthesis and antidiabetic effect of three natural triterpenoid saponins isolated from Fadogia ancylantha (Makoni Tea). Helvetica Chimica Acta 98:1254-1266.
- Bhebhe M, Chipurura B, Muchuweti M (2015) Determination and comparison of phenolic compound content and anti-oxidant activity of selected local Zimbabwean herbal teas with exotic Aspalathus linearis. South African Journal of Botany 100:213-218.
- Mackler AM, Ducsay CA, Veldhuis JD, Yellon SM (1999) Maturation of Spontaneous and Agonist-Induced Uterine Contractions in the Peripartum Mouse Uterus. Biol Reprod 61(4):873-878.
- De Heus R, Mulder EJH, Derks JB (2008) Acute tocolysis for uterine reductions in term labour; a review. Obstet Gynecol Surv 63:633-638.
Citation: Mawoza T, Magwali T, Nhachi C (2020) Determining the In Vitro Effects of Aqueous Elephant Dung and Fadogia ancylantha Leaf Extracts on Isolated Rat Pregnant Uterus Horns. J Tradit Med Clin Natur 9: 282. DOI: 10.4172/2573-4555.1000282
Copyright: © 2020 Mawoza T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7466
- [From(publication date): 0-2020 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 6740
- PDF downloads: 726