Determining the Health Risks of Heavy Metals on Freshwater Dolphin (Finless Porpoise) in Poyang Lake
Received: 18-Jul-2022 / Manuscript No. JMSRD-22-69408 / Editor assigned: 20-Jul-2022 / PreQC No. JMSRD-22-69408 (PQ) / Reviewed: 03-Aug-2022 / QC No. JMSRD-22-69408 / Revised: 09-Jan-2023 / Manuscript No. JMSRD-22-69408 (R) / Published Date: 18-Jan-2023
Abstract
Globally, heavy metal pollution in the lake ecosystem is a major concern, especially in developing countries. Water is a significant portion of the ecosystem and has ecological, social and economic importance, providing habitat and nutritional resources for many aquatic species. These lake ecosystems have, however, been rapidly destroyed by anthropogenic activities. Industrial runoff, agriculture, transportation, atmospheric deposition, etc. contribute thousands of known and unknown hazardous contaminants to rivers, estuaries and lakes. In recent years, there has been an increase in concern over heavy metal accumulating in China. Dolphins are well-known and highly respected residents of Chinese seas, where they play a vital part in the marine ecology. Humans have had a huge influence on dolphins and their surroundings over the years. The impact of heavy metals is particularly concerning due to the possible harmful consequences on marine life as well as the overall health and productivity of the ecosystem. Dolphins are at the top of the food chain, making them vulnerable to contaminants like heavy metals that may accumulate throughout the food chain.
Heavy metals, which may have accumulated through the food chain, are a particular concern because dolphins are long lived and at the top of the food chain and are therefore susceptible to such pollutants. The effects of heavy metals on marine life may be acute or long-term and may have a significant impact on the health and productivity of the entire ecosystem.
In this study, Poyang Lake, a largest river connected lake and China's largest freshwater lake, in the middle reaches of the Yangtze River was chosen as the research field. We investigated the heavy metals coming from four the sites; Kangshan, Benghu, Xingzi and Duchang to the Poyang Lake. The water of poyang lake was tested in the year 2018 where the amounts of arsenic, selenium and zinc were present at higher levels. The Poyang lake water was then tested in 2020 which showed high level of heavy metals added up in the coming years which were mercury, chromium, selenium and arsenic this was a matter of concern as heavy metals load has increased therefore in order to identify the sources that might be adding the potential load, Kangshan, Benghu, Xingzi and Duchang were selected.
Water transparency decreased in the months December>April>September>March>August>October, November>June, July>May. The transparency of water was being affected by the presence of algal blooms which result due to the excessive amounts of nitrogen and phosphorous in the water. The presence of algal structures was identified by measuring chlorophyll-a, which was highest in July>August>May>October>September> December>November>March>June>April i.e., (0.0071>0.00688>0.00641>0.00617>0.00604>0.00578>0.00400> 0.00348>0.00301>0.00276).
Temperature of a waterway is significant because it affects the amount of dissolved oxygen in the water. As the water temperature increases, the chemical oxygen demand increases. Chemical Oxygen Demand (COD) are the organics and inorganics in the water. This means that the periods of extreme rainfalls i.e. April-June receive a large amount of chemicals through runoffs of agriculture and industrial sites thus leading to the accumulation of heavy metals and production of algal blooms. Chemical oxygen demand was observed the lowest in the months of April and October followed by September and May, March and July, November and December i.e. (13.00, 16.00, 18.00, 20.00, 23.00) and was observed highest in the month of June i.e., (24.00).
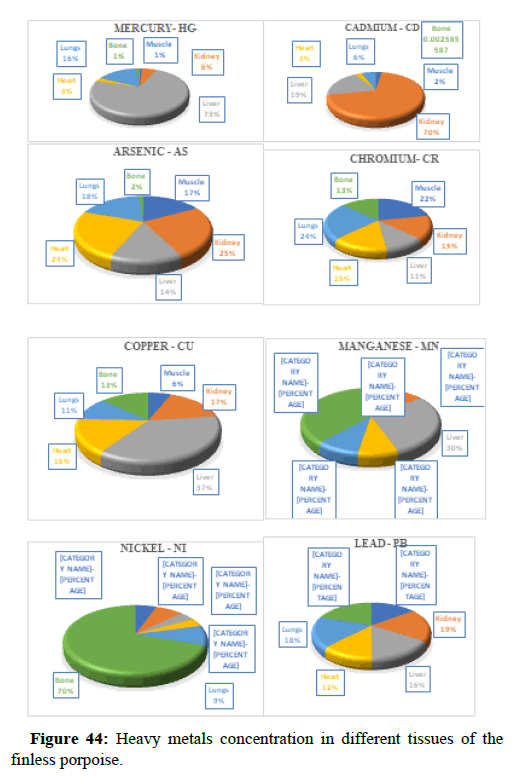
The tissue distribution of heavy metals accumulated in the finless porpoise an endangered species was taken from a secondary study. When comparing the proportion/percentage of Hg present in different tissues of FP, we found that Liver has the highest amount of mercury and copper as compared to other major tissues of the FP. 33.35 mg/kg of Hg of found in the major tissues of FP, out of which 73% was present in the liver, Zn was the dominant element in all tissues with a maximum mean concentration of 177.85 mg kg-1 dw in the liver. Overall, health risk assessment of trace elements in finless porpoises suggested that greater attention should be given to Hg, As, Cd and Se.
These metals are quint essential to maintain various biochemical and physiological functions in living organisms in very low concentrations only. These metals prove to be noxious when they exceed certain threshold concentrations. Heavy metals are significant environmental pollutants and their toxicity is a problem of increasing significance for ecological, evolutionary, nutritional and environmental reasons. The most commonly found heavy metals in waste water include copper, nickel, zinc, cobalt and cadmium all of which cause risks for human health and environment. Heavy metal toxicity can lower energy levels and damage the functioning of brain, lungs, kidney, liver and blood composition and other important organs. Long term exposure to high concentrations lead to gradual and progressive physical, muscular and neurological degenerative processes that initiate disease like multiple sclerosis, parkinson’s, alzheimer’s and muscular dystrophy. Repeated long term exposure of some metals and their compounds may cause cancer.
Keywords
Poyang lake; Water quality; Potential health risks; Finless porpoise; Trace elements accumulation; Heavy metals tissue dissemination
Introduction
In recent years, there has been an increasing ecological and global public health concern associated with heavy metal pollution of the environment; human exposure has risen dramatically as a result of an exponential increase in their use in a variety of industrial, agricultural, domestic and technological applications [1]. Geogenic, industrial, agricultural, pharmaceutical, home effluents and atmospheric sources have all been identified as sources of heavy metals in the environment [2]. Mining, foundries and smelters and other metal based industrial processes are notorious for polluting the environment [3].
Although heavy metals are naturally occurring elements found throughout the earth's crust, the majority of environmental contamination and human exposure are caused by anthropogenic activities such as mining and smelting, industrial production and use and domestic and agricultural use of metals and metal containing compounds [4-6]. Metal corrosion, air deposition, soil erosion of metal ions and heavy metal leaching, sediment resuspension and metal evaporation from water resources to soil and groundwater can all contribute to environmental pollution [7]. Weathering and volcanic eruptions have also been linked to heavy metal contamination.
Metal processing in refineries, coal combustion in power plants, petroleum combustion, nuclear power stations and high tension lines, plastics, textiles, microelectronics, wood preservation and paperprocessing factories are all industrial sources [8-10]. Metals such as Cobalt (Co), Copper (Cu), Chromium (Cr), Iron (Fe), Magnesium (Mg), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Selenium (Se) and Zinc (Zn) have been shown to be important nutrients needed for many biochemical and physiological processes [11]. A lack of essential micronutrients leads in a number of deficient illnesses or syndromes.
Heavy metals are also classified as trace elements due to their prevalence in trace amounts (ppb to less than 10 ppm) in a variety of environmental matrices [12]. Physical parameters such as temperature, phase association, adsorption and sequestration impact their bioavailability. It is also influenced by chemical parameters such as thermodynamic equilibrium speciation, complexation kinetics, lipid solubility and octanol/water partition coefficients [13]. Biological aspects including species traits, trophic relationships and biochemical/ physiological adaptability are also relevant [14]. In plants and animals, important heavy metals perform biochemical and physiological activities. They are essential components of numerous major enzymes and perform critical roles in a variety of oxidation reduction processes.
Metals in the environment
Metals are abundant in the environment and while they occur naturally, their release into the environment can be increased through human activity. High metal concentrations, of even metals that are essential for life, can adversely impact ecosystems. Metals can also interact with natural chemical constituents in ways that modify their toxicities to organisms. Because metals are distributed unevenly in the environment, their impact to ecosystem health varies from site to site. In fresh water bodies, such as rivers and lakes, differences in water chemistry on metal toxicity are profound metal toxicity to the same organism can vary by a factor of 50 depending on water chemistry in the area where the water has been collected. Identifying maximum metal concentrations that still allow for protection of ecosystems from metal toxicity is therefore challenging. Metals in water bodies are often managed by regulatory agencies through establishing numeric levels that the agency considers acceptable, which can be referred to generically as Protective Values for Aquatic Life (PVALs). PVALs that account for the influence of water chemistry on the toxicity of metals, broadly termed bioavailability-based approaches, are becoming more common [15].
Heavy metal pollution
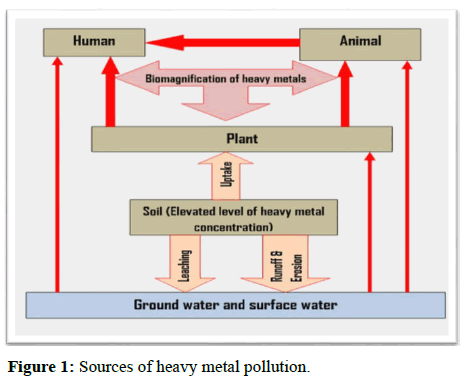
Heavy metals are characterized as metallic elements with a substantially high density in comparison to water [16]. With the notion that heaviness and toxicity are linked, heavy metals also include metalloids such as arsenic, which may cause toxicity at low levels of exposure [17]. Heavy metals occur naturally as lithosphere components and are expelled into the cosmos through volcanic activity and rock weathering. Heavy metals are by-products of numerous manufacturing processes that contribute varied amounts of various metals and as such, are expelled into the cosmos as waste. They enter the environment via air and land-based effluent. Heavy metal-induced toxicity and carcinogenicity has several molecular features, some of which are not well characterized or understood. Each metal, on the other hand, is recognized to have distinct characteristics and physicochemical qualities that bestow discrete toxicological modes of action. Heavy metals are dangerous when they are not absorbed by the body and instead accumulate in human organs. They can enter human organs through the intake of foodstuffs or inhalation of air when they come into contact in manufacturing processes in factories, urban and rural settings and so on. Mechanical vulnerability is a natural pathway for elderly people, whereas newborn vulnerability is caused through inhaling. Anthropogenic activities have damaged the environment and energy outputs have exceeded the ecosystem's capacity to govern [18]. Anthropogenic and natural processes can both produce heavy metals. Progress in industry, urbanization and commercial activity all contribute to heavy metal pollution. Natural heavy metal resources include industrial emissions, garbage dumps, dumping sites, metal cleaning, electrical appliances, cosmetics and various other sources [19]. These metals find their way into the water or through direct dumping in water, extraction, current flooding or through water flow processes [20]. In addition to heavy metals, large amounts of organisms are released into the water and may be reduced by bacterial activity leading to a decrease in oxygen levels or anaerobic conditions in a wide variety of water. All over the world, rivers and marine bodies serve as storage areas for the chemical, biological and pollutants of iron ore. This is because many industries and industries are located near the river bank (WHO/UNEP). This has resulted in a number of pollutants in the aquatic environment. Figure 1 shows some of the main origins of heavy metal systems in a water body.
Mining, domestic and industrial wastewater, irrigation, metal processing and flow also lead to the removal of heavy metals from lakes/rivers, ecosystems, etc. It has been observed that the anthropogenic processes of heavy metals exceed the natural occurrence of certain metals.
Man made habits, as well as cars/trucks/tractors/generators that emit toxic fumes into their ventilation, have a significant impact on the toxicity of these metals; a combination that releases Zn, As and Cu; Dichloro-Diphenyl-Trichloroethane (DDT) releasing as and the heating of natural gas emitting Ni, Ti, V, Hg and Se. Man-made practices have been found to contribute significantly to pollution as a result of the daily production of goods and services to meet the needs of growing citizens.
Compared to the rate of clean-up, the rate of global pollution has increased due to population growth, rapid economic growth; hazardous substances and chemicals used by farmers and waste disposal methods. The use of these toxic substances and hazardous metals in the area will lead to geo accumulation, biodiversity accumulation and bio enlargement (Table 1).
| Pollutants | Major sources | Effect on human health | Permissible levels (mg/L) |
|---|---|---|---|
| As | Pesticides, fungicides, metal smelters | Bronchitis, dermatitis, poisoning | 0.02 |
| Cd | Welding, electroplating, pesticides, fertilizers | Renal dysfunction, lung disease, lung cancer, bone defects, kidney damage | 0.06 |
| Pb | Paints, pesticides, automobile emissions, mining, burning of coal | Mental retardation in children, delay in development, fatal infant encephalopathy, chronic damage to nervous system, liver and kidney | 0.1 |
| Mn | Welding, fuel addition, ferromanganese production | Inhalation or contact damage to central nervous system | 0.26 |
| Hg | Pesticides, batteries, paper industry | Tremors, gingivitis, protoplasm poisoning, damage to nervous system, spontaneous abortion | 0.01 |
| Zn | Refineries, brass manufacture, metal plating | Damage to nervous system, dermatitis | 15 |
| Cr | Mine, mineral sources. | Damage to nervous system, irritability | 0.005 |
| Cu | Mining, pesticide production, chemical industry | Anemia, liver and kidney damage, stomach irritation | 0.1 |
Table 1: Source of heavy metals and their impacts on human health.
Environmental impacts of heavy metals
Heavy metals are classified as significant pollutants due to their destructive impact on living creatures and global ubiquity. The United States Environmental Protection Agency (US EPA) classifies Al, Fe, Cr, Sb, As, Be, Cd, Cu, Pb, Hg, Ni, Se, Ag and Zn as main contaminating metals. Furthermore, heavy metals have been included to the Agency for Toxic Substances and Disease Registry's (ATSDR) priority chemicals list due to their potential hazard to human health and toxicity. Unless the effects are mitigated, health and mortality issues, in addition to disrupting food networks, may occur. Several studies have shown that Reactive Oxygen Species (ROS) generation and oxidative stress are important factors in the toxicity and carcinogenicity of metals such as arsenic, cadmium, chromium, lead and mercury.
Because of their extreme toxicity, these five elements are considered priority metals with significant public health implications. They are all systemic toxins that have been shown to cause numerous organ harm at low levels of exposure. These metals are classified as known or probable human carcinogens by the US Environmental Protection Agency (US EPA) and the International Agency for Research on Cancer (IARC) based on epidemiological and experimental studies that show an association between exposure and cancer incidence in humans and animals.
Materials And Methods
Sample collection and determination
Five sites were arranged for field investigation to explore the fluctuation of water quality in Poyang Lake including Kangshan, Poyang, Benghu, Xingzi, Duchang and the monitoring at each section was conducted monthly during 2018-2020. Water samples were collected with 2 L Plexiglas water samplers at 0.5 m below the surface stored in 500 mL polyethylene bottles and acidulated using sulfuric acid. The acid washed polyethylene bottles were first disinfected by 70% alcohol and then washed sequentially with deionized water to eliminate the interference of any substances adhered to the bottle. Three indexes, COD (Chemical Oxygen Demand), NH3-N (Ammonia Nitrogen) and TP (Total Phosphorus), were determined within 72 hours by the typical dichromate method, Nessler's reagent calorimetry and ammonium molybdate spectrophotometric method, respectively. The routine measured data included concentration of heavy metals, which were obtained from the hydrological bureau of Jiangxi province and the commission of the ministry of water resources of China.
Sediments and erosion input
Residues enter the lake through natural drainage processes. The poyang basin, controlled by the S-N striking Ganjiang strike program and pre-Cretaceous counterparts, was the Cretaceous-Paleogene trans tensile fault depression that occurred in Indosinian intracontinental orogens in central Jiangxi. The pitcher had a tectonic structure for onestep increase between two depressions.
Poyang Lake is located on the south coast 2 km inland, reaching the Yangtze River, with a 162,000 catchment area accounting for 9% of the Yangtze river discharge area. Flowing rivers include the Xiu river, Gan river, Fu river, Xin river and Rao river. Enter the lake from Qiujin, Wanjiabu, Waizhou, Lijiadu, Meigang, Hushan and Dufengkeng stations and flow towards the mighty Yangtze river past Hukou station. The terrain of Poyang lake is complex and unchanging, consisting of numerous narrow canal rivers and lakes, indicating a perfect inclination toward the South and the North. The weather map uses the elevation of the Yellow Sea base level (the water level involved in the following adopts the same base level), which varies between 3.0 m and 14.0 m. The entire Poyang Lake can be divided into two standard sections, one of which is a large lake with shallow water and a shallow water south of Xingzi station and the other is a junction with a small river and a shallow water north of Xingzi. At the station. Influenced by the rain of hot weather, the flowing waters of Poyang lake are clearly marked by seasonal features. From June to August, the flow of rivers into the lake is relatively large, which is the rainy season of lake Poyang. From November to February, the river flow is very low, which is the dry season of Poyang lake. Many areas are exposed to water. Sometimes it is normal seasons. The largest difference in water level during wet and dry season in the lake area is about 8.8 meters. With the change in water flow, Poyang lake produces a shift of sections of lake and rivers and the water area also varies between 100 km2 and 3000 km2.
Study area
Poyang lake, with an area of 3583 km2 and an area of 27.6 km3 on average, is located on the southern bank of the Yangtze river in Jiangxi Province, China. A lake that holds millions of birds over 300 species plays a vital role in conserving and reducing flooding, regulating the climate and protecting the world's biodiversity. The lake receives water from five rivers (Raohe, Xinjiang, Fuhe, Ganjiang and Xiuhe) and flows into the Yangtze river a little way northward. Due to the interaction of the river, the area and volume of lake vary greatly throughout the year. It grows on high water during the wet season but shrinks above the river during the dry season. Poyang lake is characterized by significant annual and mid-year variations of the fixed level load. According to the estimated data from 1956 to 2015, the average concentration of the standard load at Poyang lake is approximately 0.120 kgm-3, high and valley values of 0.185 kgm-3 and 0.036 kgm-3 respectively. The sediment from the lake comes in a variety of forms including five rivers upstream, the return water from the Yangtze river, sand blown and washed off the shore. The rising rivers account primarily for 87.2% of the total. The amount of soil entering Poyang lake changed dramatically as human activities, especially resource changes such as intensive deforestation caused by anthropogenic activities. Deforestation in the lake waters has declined from 40.1% in 1950 to 31.5% in the early 1980s, leading to significant soil erosion. In recent years, the average volume of dry transport from the Ganjiang river, Fuhe river, Xinjiang river, Raohe river and Xiushui river to the lake annually can reach 9.16 × 106 t, 2.12 × 106 t, 1.43 × 106 t, 0.99 × 106 t and 0.80 × 106 t, respectively. The lake has a tropical climate, with an average annual temperature of 18°C, 1636 mm of rainfall and 1044 mm evaporation during 1953-2002. Due to the cold Siberian winds, the northern hemisphere is characterized by a high frequency of 56% in winter and spring. Due to controlling Pacific subtropical altitude, the south wind is the second most prevalent, especially in summer and autumn, when the probability of occurrence is almost 30%. According to data from 1964-2005, the average annual wind speed in Poyang Lake was approximately 3.8 ms-1 and times when the daily wind speed of ≥ 5 ms-1 could reach 99.4. A maximum of 31 ms-1 wind power was monitored at the Tangyin station on April 8, 1982. Investigation of the quality of water is critical to assessing water diversity, bio-geochemical cycling and environmental degradation.
Data acquisition
Five sites were arranged for field investigation to explore the fluctuation of water quality in Poyang Lake including Kangshan, Poyang, Benghu, Xingzi, Duchang and The monitoring at each section was conducted monthly during 2018-2020. Water samples were collected with 2 L Plexiglas water samplers at 0.5 m below the surface, stored in 500 mL polyethylene bottles and acidulated using sulfuric acid. The acid washed polyethylene bottles were first disinfected by 70% alcohol and then washed sequentially with deionized water to eliminate the interference of any substances adhered to the bottle. Three indexes, COD (Chemical Oxygen Demand), NH3-N (Ammonia Nitrogen) and TP (Total Phosphorus), were determined within 72 hours by the typical dichromate method, Nessler's reagent calorimetry and ammonium molybdate spectrophotometric method, respectively. The routine measured data included concentration of heavy metals, which were obtained from the hydrological bureau of Jiangxi province and the commission of the ministry of water resources of China. The location of the monitoring points is shown in Figure 2.
Water quality
Poyang lake water levels have been reported to be declining in recent years. This has led to many environmental problems. For example, rapid fish decline has been caused by declining water levels and environmental conditions. Poor water level also has a negative impact on the production of Vallisneria spiralis L. (a type of underwater plant), a food source for endangered porpoise. To date, however, there has been no systematic study of long-term changes in water quality in various parts of the lakes, despite the well-known effect of local sand spillage on water clearance. How and how water reacts to climate change and human impact on various parts of lakes is often unknown.
One key parameter of water quality is fixed turbidity (TSS) or turbidity, which controls the transmission of light to the water and thus the production of underwater vegetation. In addition, pollutants and heavy metals from the earth's surface are collected and transported by fossils in the aquatic environment, which affects the health of both marine wildlife and humans. Both climate change and human activities may influence short term and long term patterns of TSS distribution. For example, natural forces such as hurricanes or hurricanes may result in the formation of large sediment and mass evacuation. Human impacts such as blockage of dams and dams may affect water clearance in low-lying coastal waters and the environment. Even coastal feeding, which is used as an effective means of reducing coastal erosion problems, often leads to increased soil erosion in coastal areas and poses a threat to coastal areas. Therefore, it is highly desirable to document long-term changes in the distribution of TSS in order to detect possible interactions with climate variability and human activities.
Analysing water quality of Poyang lake
Water quality has been identified as an important factor in freshwater lakes, which play an important role in shaping the aquatic environment and governing its health and stability. However, due to population growth and industrial acceleration, many lakes around the world, e.g. Lake Burdur, lake Tai, lake Saimma and Poyang lake are considered to be declining water quality which led to a series of subsequent environmental problems including algal blooms, water inequality, deforestation and drinking water problem (Table 2).
| Monthly sampling (Year 2020) | Water transparency | Chlorophyll-a | Water temperature | Chemical oxygen demand |
|---|---|---|---|---|
| March | 0.55 | 0.00348 | 14.3 | 20.00 |
| April | 0.66 | 0.00276 | 16.0 | 13.00 |
| May | 0.42 | 0.00641 | 24.9 | 18.00 |
| June | 0.43 | 0.00301 | 27.9 | 24.00 |
| July | 0.43 | 0.00711 | 33.5 | 20.00 |
| August | 0.53 | 0.00688 | 29.7 | 21.00 |
| September | 0.65 | 0.00604 | 31.3 | 16.00 |
| October | 0.50 | 0.00617 | 23.3 | 13.00 |
| November | 0.50 | 0.00400 | 21.3 | 23.00 |
| December | 0.68 | 0.00578 | 13.1 | 23.00 |
Table 2: Water quality of Poyang lake-2020.
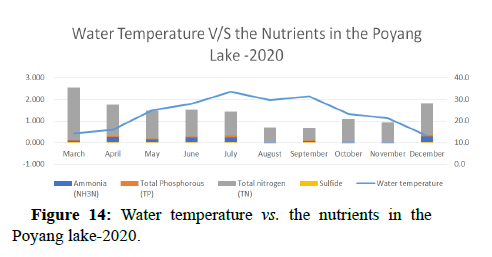
Water transparency decreased in the months December>April> September>March>August>October, November>June, July>May. The transparency of water was being affected by the presence of algal blooms which result due to the excessive amounts of nitrogen and phosphorous in the water (Figures 3-7). The presence of algal structures was identified by measuring chlorophyll-a, which was highest in July>August>May >October>September>December>November>March>June>April i.e., (0.00711>0.00688>0.00641>0.00617>0.00604>0.00578>0.00400>0.0034 8>0.0 0301>0.00276).
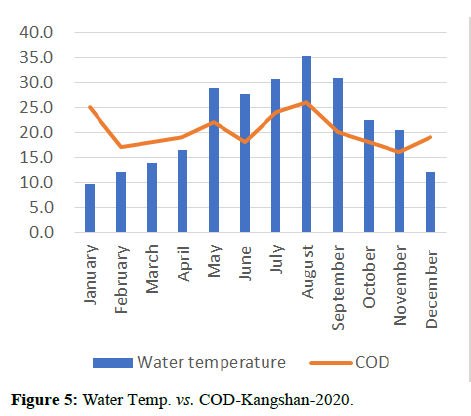
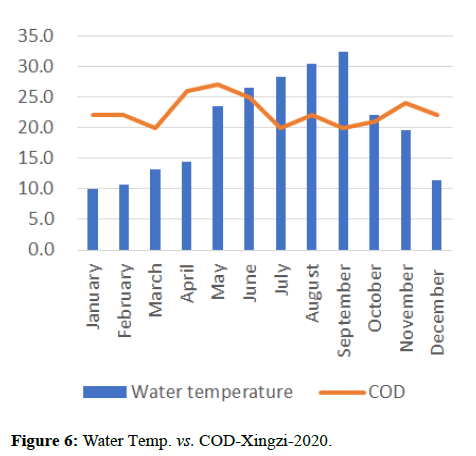
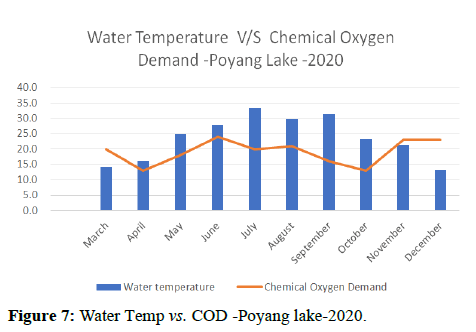
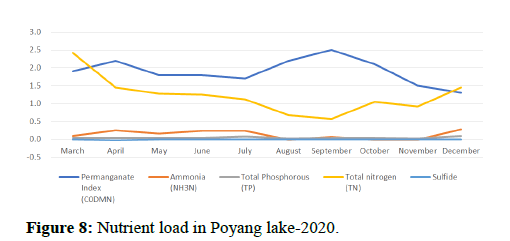
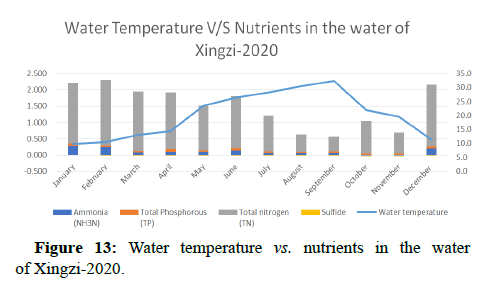
Temperature of a waterway is significant because it affects the amount of dissolved oxygen in the water. As the water temperature increases, the chemical oxygen demand increases COD (organics and inorganics) in the water, as shown in Figures 8 and 9. This means that the periods of extreme rainfalls i.e., April-June receive a large amount of chemicals through runoffs of agriculture and industrial sites thus leading to the accumulation of heavy metals and production of algal blooms. Chemical oxygen demand was observed the lowest in the months of April and October followed by September and May, March and July, November and December i.e., (13.00, 16.00, 18.00, 20.00, 23.00) and was observed highest in the month of June i.e., (24.00).
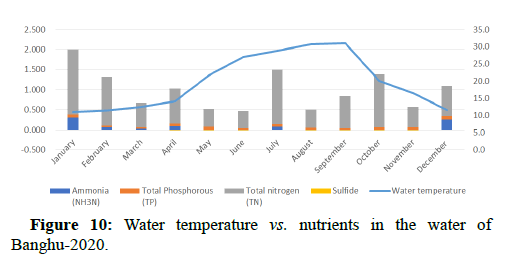
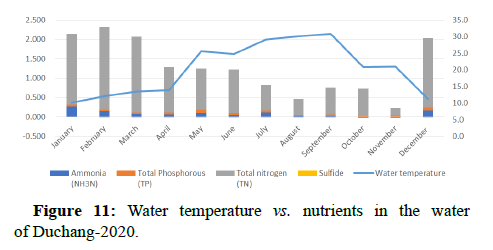
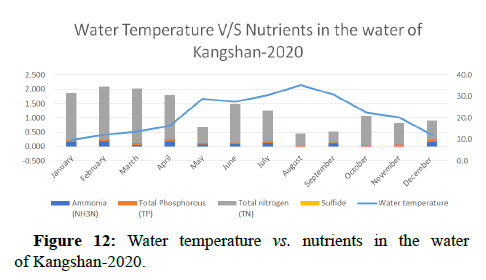
Water quality gets impacted by the presence of high nutrients of phosphorous, nitrogen and ammonia as it leads to the formation of algal blooms thus affecting the water temperature, transparency, increase in availability of chlorophyll-a and Chemical Oxygen demand as shown in Figures 10-14.
Other biological indicators: The species and abundance of freshwater algae are also good indicators of nutrient levels and general status of water bodies. Most freshwater algae are microscopic and only a few genera (e.g. Cladophora are large enough to be seen with the naked eye. There is usually a larger range of algal species in lakes than in moving water, particularly when the nitrogen and phosphorus levels in the water are low. When the nutrient levels are high eutrophication occurs, the range of species decreases, but certain species (e.g. Anabaena cicinalis and Microcystis spp. increase in abundance. This may create an algal bloom in the lake depending on water temperature and lake turbulence. According to the data, phosphorous levels are observed to be high during the time when the Poyang lake receives high amount of water from different tributaries i.e., in April-June, a change in trend is seen during the months of July and December which can be due to unexpected rainfalls experienced in the region, resulting in excessive amount of water coming to the Poyang lake and receiving nutrients thereby getting nutrient rich and leading to eutrophication. Total nitrogen levels are observed to be very less therefore it does not affect the species.
Analyzing the heavy metal load of Poyang lake
Heavy metals occur naturally and penetrate through geological processes such as rock climatic changes, gas extraction from the soil, forest fires and hot air extraction in tropical and volcanic areas. However, anthropogenic pollutants from industrial activities and the flow of agricultural water and storms also have a significant impact on metals in the environment. Major sources include mining, smelting and refining of metals; pollution from sewage treatment activities; vehicle extraction; the burning of fossil fuels in homes and industries and immersion of fertilizers and pesticides. Trace Elements (TEs exist in the focus of tracking on various natural matrices. Persistent exposure to non-essential TE such as As, Cd, Hg and Pb even at very low levels can have adverse effects on human health and aquatic mammals. Other important TE, such as Cu and Zn, at higher levels may be toxic.
Smaller cetaceans have been considered as indicators for monitoring coastal pollution due to their long-term accumulation of TE and predators. Collection of TEs was recorded in >>60 species of cetaceans over the past three decades; however, reports of toxic effects on marine mammals have not been found, because studies of toxicity in live cetaceans they are not morally acceptable. The toxicological risk posed by TEs is a problem that researchers are focusing on that (Table 3).
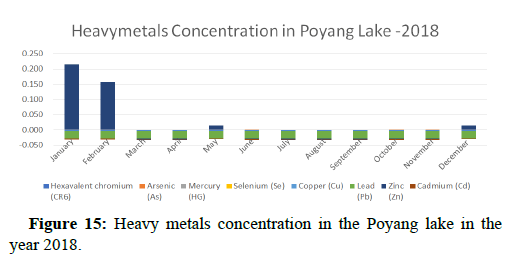
The numbers of heavy anthropogenic metals brought to ECS from the Yangtze River have increased from 5,000 tons in 2002 to 36,200 tons in 2012 (NBO. This increase highlights the urgent need for research to understand the potential health risks in the East China Sea (ECS. In addition to ECS, other studies have been conducted on TE levels in endangered porpoises from other Chinese waters, such as Bohai, Hong Kong, Beibu Gulf. Therefore, our research will close the information gap (Figure 15).
| Monthly sampling (Year 2018) | Hexavalent chromium (CR6) | Arsenic (As) | Mercury (HG) | Selenium (Se) | Copper (Cu) | Lead (Pb) | Zinc (Zn) | Cadmium (Cd) |
|---|---|---|---|---|---|---|---|---|
| January | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | 0.2161 | -0.0025 |
| February | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | 0.1562 | -0.0025 |
| March | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| April | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| May | -0.002 | 0.0004 | -5E-06 | -0.00015 | -0.0015 | -0.025 | 0.0135 | -0.0025 |
| June | -0.002 | 0.0005 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| July | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| August | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| September | -0.002 | -0.0001 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| October | -0.002 | 0.002694 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| November | -0.002 | 0.00104 | -5E-06 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
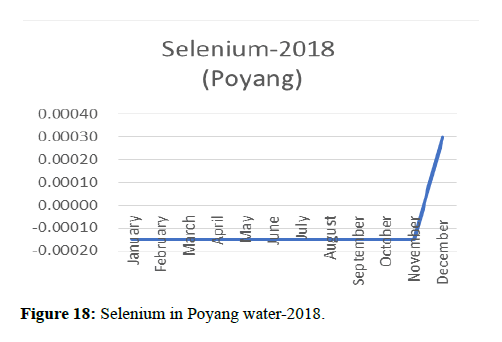
| December | -0.002 | 0.001 | -5E-06 | 0.0003 | -0.0015 | -0.025 | 0.0134 | -0.0025 |
Table 3: Monthly concentrations of heavy metals in Poyang lake-2018.
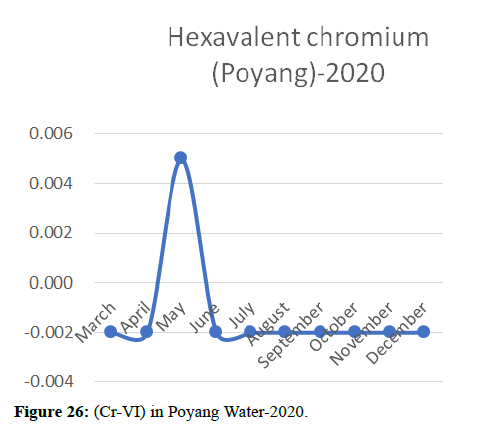
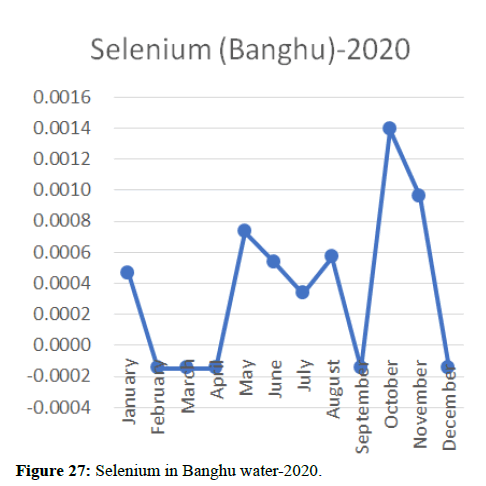
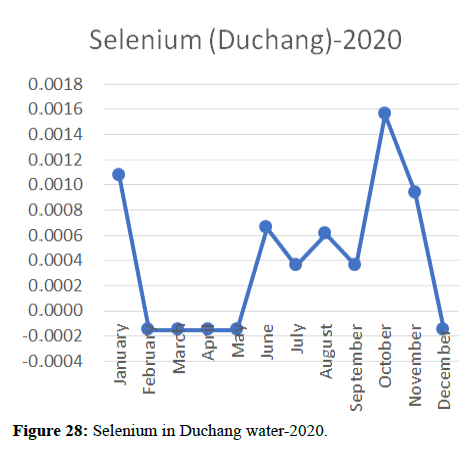
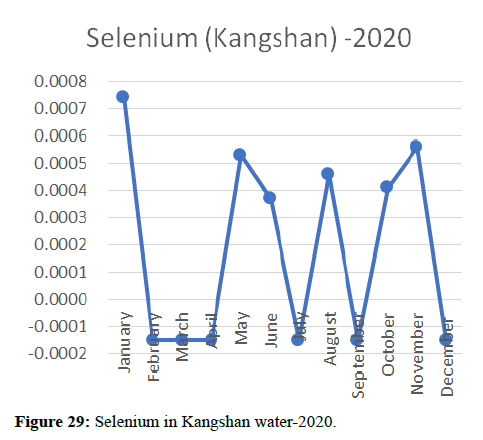
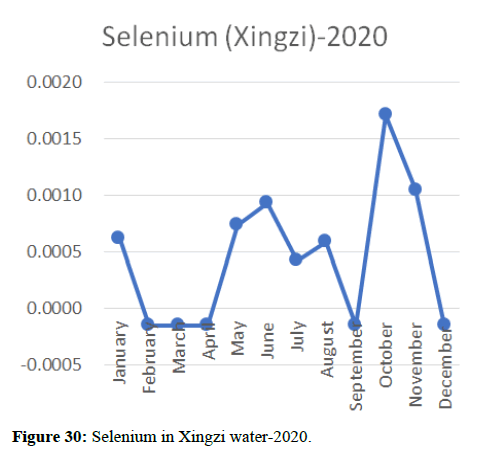
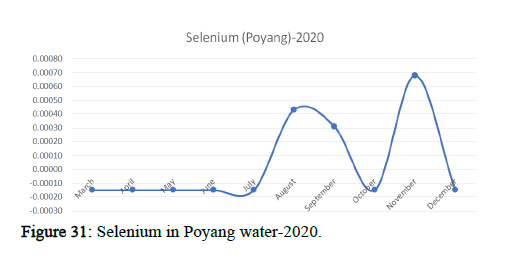
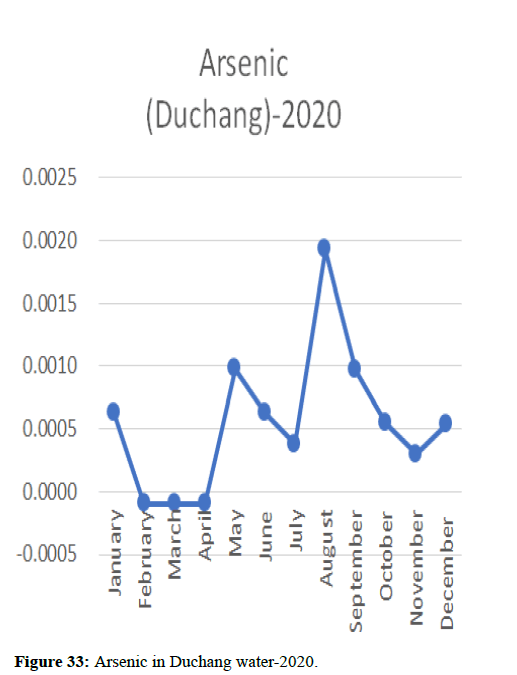
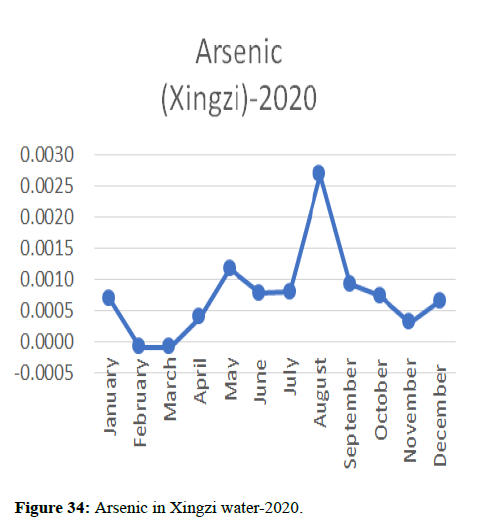
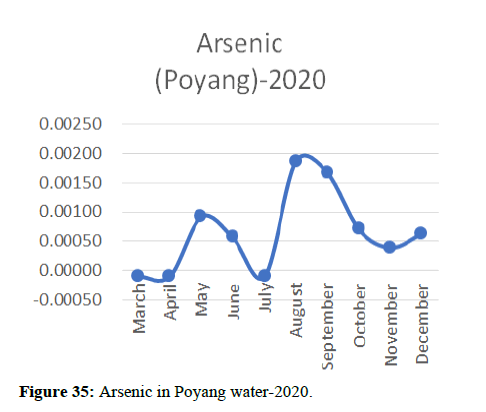
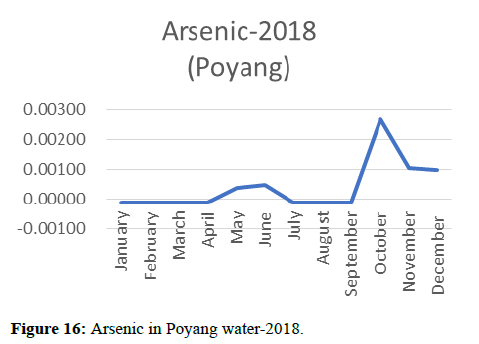
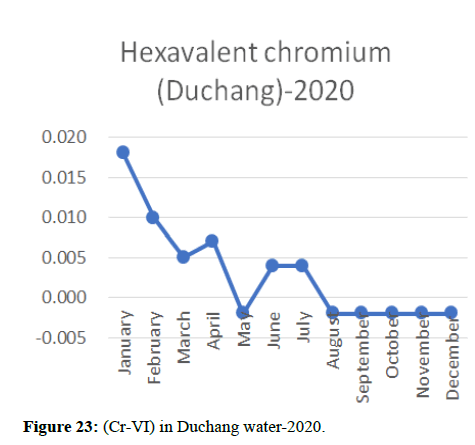
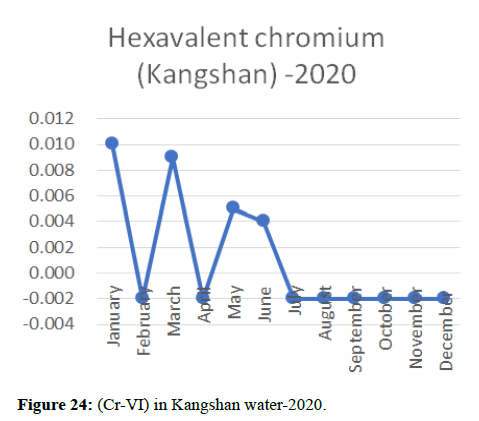
Heavy metal concentrations were experienced more in the water during the time periods that followed no rainfall and lead to a condition of draught. These time periods are considered to be more dangerous to the species living in the Poyang lake. Arsenic is observed to be high in the months of September-December due to lack of water in the lake, Zinc is experienced in high levels from January-March and Selenium from November-December (Figures 16-19 and Table 4).
| Monthly sampling (Year 2020) | Hexavalent chromium (CR6) | Arsenic As) | Mercury (HG) | Selenium (Se) | Copper (Cu) | Lead (Pb) | Zin (Zn) | Cadmium (Cd) |
|---|---|---|---|---|---|---|---|---|
| March | -0.002 | -0.0001 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| April | -0.002 | -0.0001 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| May | 0.005 | 0.00092 | 0.000080 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| June | -0.002 | 0.00056 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| July | -0.002 | -0.0001 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| August | -0.002 | 0.00187 | -0.000005 | 0.00043 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| September | -0.002 | 0.00167 | -0.000005 | 0.00031 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| October | -0.002 | 0.0007 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| November | -0.002 | 0.00039 | -0.000005 | 0.00068 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| December | -0.002 | 0.00063 | -0.000005 | -0.00015 | -0.0015 | -0.025 | -0.0025 | -0.0025 |
| Total | -0.013 | 0.00644 | 0.000 | 0.00037 | -0.015 | -0.25000 | -0.025 | -0.02500 |
Table 4: Monthly concentrations of heavy metals in Poyang lake-2020.
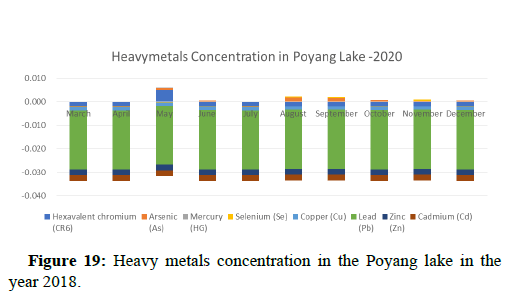
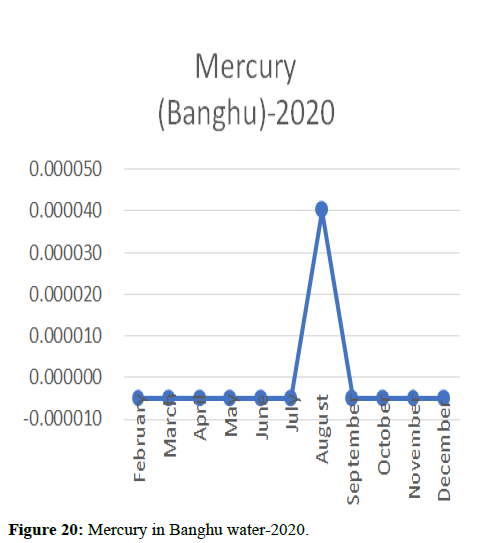
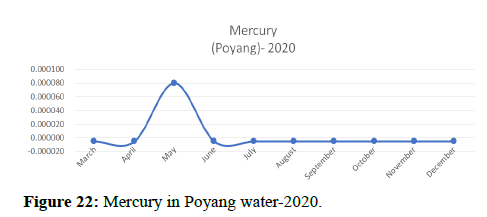
The 2018 annual data shows the concentration of trace heavy metals so we collected samples from sites that were considered to be contributing to the declining watershed of Poyang lake as the sites had more industrial and agricultural flows. Although comparing the data for the year 2018-2020, water levels have dropped significantly as heavy metallic levels exceeded in two years which could be due to rapid development and excessive water flow at the water level. High concentrations of mercury and chromium and arsenic were observed in the months of April-June, arsenic was also found at very high altitudes during the months of August-December which means that arsenic levels rose sharply in lake Poyang, Selenium strains. They were also acquired in very large quantities compared to the previous year where they were observed at innumerable prices (Figures 20-35).
Status and conservation of the Yangtze finless porpoise
The effects of climate change and population growth in freshwater reservoirs around the world are so great that in some places this vital resource is dwindling and in some cases, may be disappearing. Poyang Lake (PL), China's largest freshwater lake and extension of the Yangtze River, is an important habitat for Yangtze finless porpoise in addition to significant long-term water losses drought this important habitat is under increasing anthropogenic pressures in the form of sand mining, boat smuggling and deterioration of water quality due to water changes in the Yangtze yiver. In addition to the direct impacts on water quality, the fragmentation of settlements from this conversion may only increase with future hydropower projects. Such changes in the Yangtze river are believed to have contributed to the rapid decline in YFP population, which has been declining by an average of 13.73% per annum This decrease has led to the recent total number of YFP population being approximately 1000, approximately half (~450) of living population in PL. With the current human route, YFP may join the baiji (Lipotes vexillifer), a former resident of the Yangtze river to extinction.
Harmful mutations worldwide are believed to be responsible for nearly 40% of all species of mammals experiencing significant population decline and freshwater cetaceans, or river dolphins, which appear to be at high risk at these pressures. Degradation of habitats in an ecosystem can be catastrophic, especially in endangered species and habitats. Therefore, determining the current physical characteristics of individuals among the population at risk provides the basis for future research on the potential impact of anthropogenic activities on their health and well-being. It is widely accepted that the quality of habitat affects the physical fitness and life activities of animals living within these habitats. Therefore, monitoring physiologic factors can help us to understand and predict biological responses and demographics to environmental changes and pressures, causal relationship and the definition of management strategies (Figure 36).
Method for risk assessment
Sampling container pre-treatment: All glassware and sampling containers used were thoroughly washed with a warm detergent solution to removed dirt, followed by rinsing with tap water until free of every detergent. These were thoroughly rinsed again with ultrapure water and soaked in 10% (V/V nitric acid for 48 hours and finally rinsed with triply distilled water thrice and dried in the oven at 80°C to preclude any heavy metal contamination prior to sampling and analysis.
Finless Porpoise estimation in lake: Mercury is a heavy metal that belongs to the periodic table's transition element series. It is remarkable in that it may be found in three different forms in nature (elemental, inorganic and organic, each with its distinct toxicity profile. Elemental mercury exists as a liquid with a high vapor pressure at room temperature and is emitted into the atmosphere as mercury vapor. Mercury can also be found as a cation with the oxidation states +1 (mercurous or +2 (mercuric. Methylmercury is the most often encountered organic form of mercury in the environment and it is generated when inorganic (mercuric forms of mercury are methylated by microorganisms in soil and water.
Sample preparation and analysis: Sample adjustment, extraction and analysis of trace elements in marine living samples were based on secondary data from previous studies. In short, the tissues of the endangered water dolphin (i.e., Tissues, kidneys, liver, heart and lungs) are frozen to a constant weight and packaged homogenized using a meat grinder. Each minced tissue (approximately 0.3 g) was milled using 2 mL H2O2 and 5 mL HNO3 in a microwave digestive system (Yao, TOPEX 40, China. After grinding, the sample is reconstituted using ultrapure water. Prior to analysis, the samples were filtered using a 0.22 μm syringe filter. Trace elements were analyzed using ICP-MS and an atomic fluorescence spectrometer. Briefly, As, Cd, Co, Cr, Cu, Mn, Ni, Pb, Se, Sn, V and Zn were analyzed using ICP-MS (PerkinElmer, NEXION 300X, USA. Cd, Pb and Sn were analyzed in normal mode (STD), while As, Co, Cr, Cu, Mn, Ni, Se, V and Zn were analyzed in the Kinetic power Dissipation mode (KED). The ICP-MS settings were as follows: RF 1200 W power, plasma (argon) gas flow rate 15 L min-1, nebulizer gas flow rate 0.94 L min-1, analog stage voltage-1900 V, pulse stage voltage 950 V, scan mode, MCA 1 channels, 50 ms duration, 1000 ms integration time and readings taken as triple. However, Hg was analyzed using an atomic fluorescence spectrometer (JiTian, AFS-921, China). The main parameters of the fluorescence atom spectrometer were the current 30 mA lamp, photomultiplier tube voltage 270 V, reading time 7 s and the delay time was 0.5 s.
Data analysis-Tissue residue risk quotient: The health risk posed by trace elements in East Asian finless porpoise tissue was calculated using Risk Quotient (RQ) guidelines based on tissue residues, Reference volume (RfD) and Toxic Reference number (TRV). RQ is calculated from the rate of tissue concentration and Maximum Acceptable Concentration (MAC) for a particular follow-up factor. E.g. equation 1, equation 2:
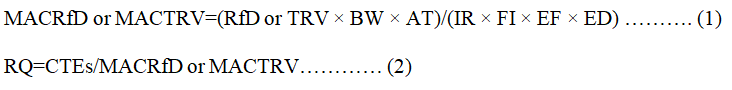
Where:
BW is the body weight of a chronic East Asian porpoise (estimated at about 60 kg in this study);
AT is an average life expectancy (10,220 days (365 days a year-1 × 28 years);
IR absorption rate, 3 kg per day-1 (5% body weight);
FI is the accepted fraction of a contaminant source (0.9);
EF frequency exposure (365 days year-1);
ED duration of exposure, 28 years.
The RQ variability was based on the physiological parameters of Neophocaena phocaenoides exposed to chemical contamination in Hong Kong coastal waters. RfD rates are obtained from the United States of America environmental protection agency's integrated hazardous information system. The TRV values used in this study were calculated based on the level of noninvasive adverse effect ((NOAEL) (Equation 3)) obtained from experimental mammals (mouse, mouse and mink) (Table 5).
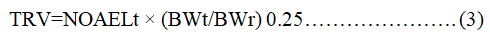
There,
NOAELt is a NOAEL test for mammals;
BWt is the body mass index of a mammal;
BWr body weight of infinite porpoise.
| Elements | Hg | As | Cd | Cr | Cu | Mn | Ni | Pb |
|---|---|---|---|---|---|---|---|---|
| Muscle | 0.3 | 1.38 | 1.39 | 0.78 | 5.38 | 1.49 | 0.31 | 0.40 |
| Kidney | 1.96 | 2.05 | 40.35 | 0.54 | 14.49 | 3.07 | 0.40 | 0.52 |
| Liver | 24.29 | 1.15 | 11.28 | 0.38 | 31.83 | 9.43 | 0.23 | 0.43 |
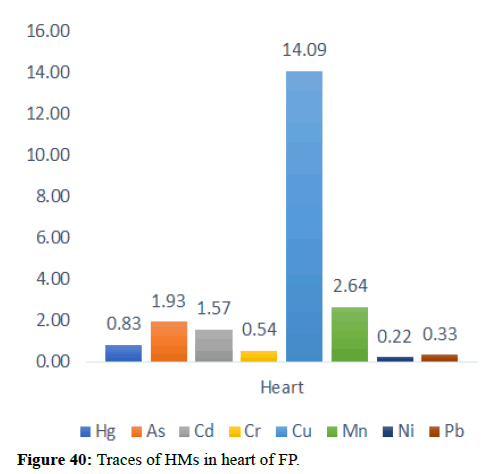
| Heart | 0.83 | 1.93 | 1.57 | 0.54 | 14.09 | 2.64 | 0.22 | 0.33 |
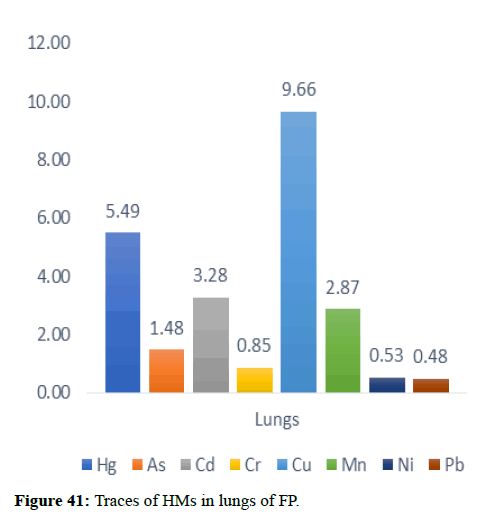
| Lungs | 5.49 | 1.48 | 3.28 | 0.85 | 9.66 | 2.87 | 0.53 | 0.48 |
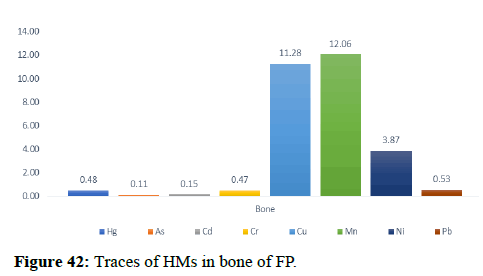
| Bone | 0.48 | 0.11 | 0.15 | 0.47 | 11.28 | 12.06 | 3.87 | 0.53 |
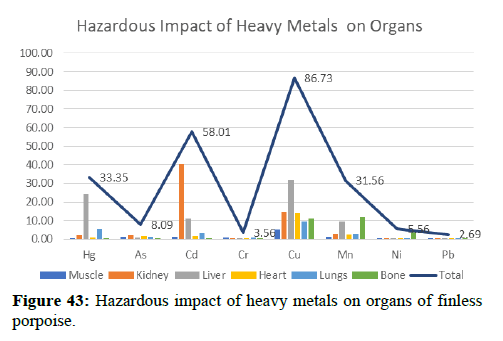
| Total | 33.35 | 8.09 | 58.01 | 3.56 | 86.73 | 31.56 | 5.56 | 2.69 |
Table 5: Trace element levels (mg/kg) in the tissues of the East Asian finless porpoises.
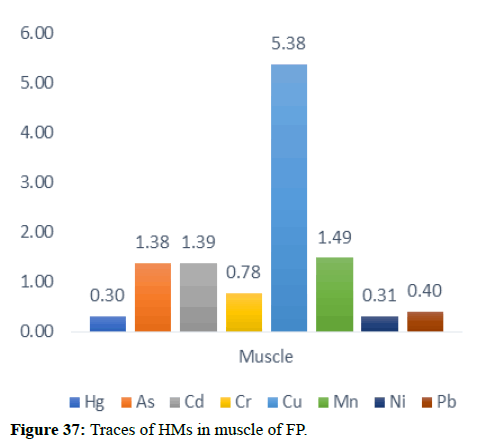
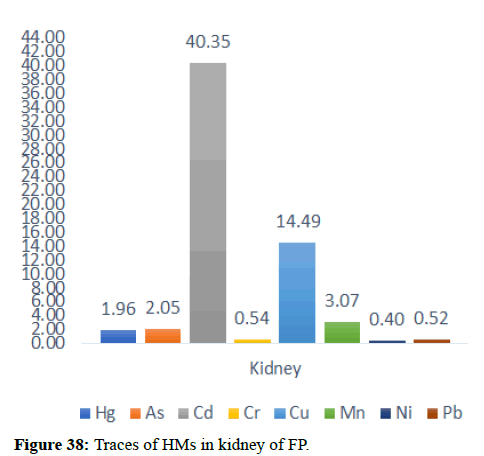
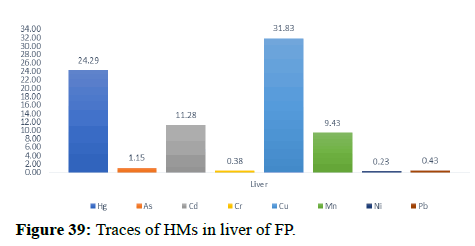
Table 5 illustrates the trace element concentrations in finless porpoise tissues (muscle, kidney, liver, heart and lung) obtained from strand lings in Liaodong Bay and the Yellow Sea's northern areas. The selected 8 trace elements were discovered at 100% frequency in all of the finless porpoise tissues studied. Cu was discovered in abundance throughout the FP body's tissues. This causes damage to practically all of the finless porpoise's tissues and organs. Cu, Hg, Cd and Mn are trace elements found in abundance in the liver (Table 6). The quantity of Hg in the lungs is likewise higher, resulting in neurological tissue damage. Because of the mining activities nearby massive levels of Fe is present in bones.
| Elements % | Hg | As | Cd | Cr | Cu | Mn | Ni | Pb |
|---|---|---|---|---|---|---|---|---|
| Muscle | 0.91% | 17.07% | 2.40% | 21.84% | 6.20% | 4.71% | 5.54% | 14.78% |
| Kidney | 5.87% | 25.30% | 69.55% | 15.19% | 16.71% | 9.73% | 7.22% | 19.20% |
| Liver | 72.83% | 14.15% | 19.44% | 10.71% | 36.70% | 29.89% | 4.17% | 15.93% |
| Heart | 2.50% | 23.89% | 2.71% | 15.25% | 16.24% | 8.36% | 3.93% | 12.43% |
| Lungs | 16.46% | 18.24% | 5.65% | 23.81% | 11.14% | 9.10% | 9.49% | 17.92% |
| Bone | 1.44% | 1.36% | 0.26% | 13.20% | 13.01% | 38.22% | 69.65% | 19.74% |
| Total | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
Table 6: Percentages of trace element levels (mg/kg) in the tissues of the East Asian finless porpoises.
When comparing the Hg dose/percentage present in different FP tissues, we found that the liver had significantly higher amounts of Mercury and Copper compared to other large FP tissues. 33.35 mg/kg of Hg found in the major FP tissues. FP, with 73% of its present in the liver, Zn was the dominant component of all tissues with a maximum dose of 177.85 mg kg-1 dw in the liver. A previous study in the northern yellow sea found that the concentration of Zn in seawater (3.81 μg L-1-21.2 μg L-1) was two orders higher in magnitude than the other, the higher concentrations of Zn were also found in the upper wall in Liaodong Bay 14.5 mg kg-1-145 mg kg-1. Recent research in Bohai and Yellow Seas has shown that apart from Ni and Cr, anthropogenic activities were a major source of trace elements in seawater (Zn, As, Cd, Cu, Hg and Pb) were mainly linked to anthropogenic river discharge is a major route. Therefore, the results suggest tracing the characteristics of anthropogenic origin that may be bio-accumulated in endogenous porpoises.
In general, specific tissue distribution of trace elements was observed in Table 6, as well as trace elements that often accumulate in internal organs. The total concentration of trace elements in the muscles was as follows: Liver>kidneys>heart>lung>muscles. Elements in cetaceans, high concentrations of other non-essential elements are expected in these organs. The concentration of hepatic Hg, Cd, Cu, Mn and Zn was significantly higher (p<0.05) than the concentration of other tissues, with the exception of renal Hg concentration, which was comparable to hepatic insufficiency. It was previously reported that the hepatic Zn and Cu concentrations of 70-350 and 10.5-105 mg kg-1 dw were concentrations suitable for small cetaceans that could not control themselves. In addition to inducing Zn in the liver at FP-6 (754 mg kg-1 dw) and FP-22 (424 mg kg-1 dw), Zn and Cu concentrations in all captive animals were within the self-regulatory range. Renal Co concentration was significantly higher (p<0.05) than other tissues, with concentrations up to 0.252 mg kg-1 dw in FP-14. The concentrations of Zn, Se, Cu, Mn, As, Ni and V between the liver and kidneys were compared with the levels found in the Indo-Pacific finless porpoises (Neophocaena phocaenoides) from the Pearl river Estuary. However, the concentration of hepatic and renal Hg and Cr in infinity East Asian porpoises was lower than N. phocaenoides, while the concentration of Pb and Cd was higher. As cephalopods, known to focus more on Cd than fish or crustaceans, are major N food components. Phocaenoides, Cd concentration in East Asian finless porpoise, which mainly eats Japanese sand shrimp (Crangon affinis) is expected to be low. Thus, high hepatic and renal cd in finless porpoises may be due to high Cd concentration in the Bohai and Yellow Seas or decreased metabolism and elimination levels in chronic porpoise. The concentrations of the hepatic Mn and Pb concentrations in the porpoises obtained in this study were compared with those found in other cetaceans such as S. chinensis west of the Pearl River Estuary collected between 2004 and 2015 and Hong Kong, 1993-1995, the striped dolphin (Stenella coeruleoalba) and the common dolphin (Tursiops truncates) from the Islands of the Pacific, 1997-2013, the dolphin with its short-beaked saddleback (Delphinus delphis) and the Indo-Pacific dolphin Tursi dolphindu South Australia (1988-2004). With the exception of Cd, liver concentrations among the remaining trace elements (Hg, Cr, Ni and Se) were lower than those reported in S. chinensis, S. coeruleoalba, D. delphis and T. truncatus (Figures 37-42).
The difference observed in the hepatic focus of trace elements in cetaceans was probably due to differences in diet, location and digestive capacity. Previous research found that fish came from the Pearl river Estuary, a S-residence. Chinensis, with significantly higher levels of Cr, Ni and Pb compared to Bohai and Yellow seas results suggested that S. chinensis had a high risk of exposure to these trace elements, hence the high concentration of hepatic Cr and Ni tissues. In contrast, the total Hg concentration in seawater from the Pearl river Estuary and Bohai and the Yellow Seas has been compared, suggesting that habitat did not significantly affect the difference between hepatic Hg concentrations in S. chinensis and Asian finless porpoises. Similarly, the lower concentration of Cr and Ni in seawater off the South Australian coast compared with those from Bohai and the Yellow Seas suggests that habitat did not play a significant role in liver disparity between the Eastern predators. Asia and D. delphis and T. truncatus. East Asian food finless porpoises are mainly pelagic and similar to D. delphis. However, T. truncatus contains benthic foods that include fish and cephalopods, while S. chinensis has a piscivorous diet. The concentration of trace element in seawater columns often varies in depth due to changes in water chemistry and physicochemical properties of the elements. For example, the concentration of Ni has been shown to increase slightly with the depth of the seawater column. The previous studies found that the concentration of trace elements varied between benthic, demersal and pelagic fish, with the concentration of Cr high in demersal. These results suggest that food may have contributed to differences between species in the focus of the trace element. There has been a significant positive correlation in the concentration of Hg, As, Cd and Zn in paired samples of kidneys and kidneys. This is probably because blood circulation facilitates the distribution of trace elements in muscle tissue.
Results and Discussion
Heavy metal load in Poyang lake
Heavy metal concentrations were experienced more in the water during the time periods that followed no rainfall and lead to a condition of draught. These time periods are considered to be more dangerous to the species living in the Poyang lake. Arsenic is observed to be high in the months of September-December due to lack of water in the lake, zinc is experienced in high levels from January-March and selenium from November-December. 2018-2020, water levels have dropped significantly as heavy metallic levels exceeded in two years which could be due to rapid development and excessive water flow at the water level. High concentrations of mercury and chromium and arsenic were observed in the months of April-June, arsenic was also found at very high altitudes during the months of August-December which means that arsenic levels rose sharply in lake Poyang, selenium strains. They were also acquired in very large quantities compared to the previous year where they were observed at innumerable values.
Behavioral characteristics of finless porpoise
Their behavior, like that of other porpoises, is less active and flashy than that of dolphins. They don't ride bow waves and appear to be afraid of boats in some regions. On the denticulate part of their backs, mothers have been spotted holding calves. YFPs have been observed leaping from the Yangtze river and performing tail stands. Swimming is the most crucial characteristic of aquatic creatures like finless porpoises. Aquatic creatures can use swimming to get food, seek advantages and avoid damage and actively pick suitable habitat regions. To scan the beam axis, freshwater finless porpoises have been observed rolling their bodies and shaking their heads. Finless porpoises are usually seen alone, in pairs, or in groups of up to 12, while aggregations of up to 50 have been documented. According to recent research, a mother/calf pair or two adults form the fundamental unit of a finless porpoise school and schools of three or more individuals are aggregations of these units or solitary individuals. The species social system appears to be immature, with the mother/calf pair likely being the sole permanent social unit. In most cases, reproduction has not been thoroughly investigated. Calving occurs on the Yangtze river between April and May, on the Pacific coast of Japan between May and June and in western Kyushu between November and December, according to reports. Kyushu animals live for about 25 years and reach sexual maturity between the ages of 4-9 Gestation lasts 11 months. Due to habitat fragmentation, genetics may also be used to show where and how populations are divided into different units that can no longer interbreed. In the Yangtze river, genetic research revealed that obstacles to gene flow exist between the three remaining fragmented Yangtze finless porpoise populations.
Fish and shrimp are eaten by finless porpoises in the Yangtze river, as well as fish, shrimp and squid in the Yellow Sea/Bohai region and off the coast of Pakistan. They are known to consume fish, shrimp, squid, cuttlefish and octopus in Japanese waters. Finless porpoises are opportunistic feeders that eat whatever is available in their environment. Seasonal diet variations have not been investigated, however they do appear to consume some plant material, such as leaves and grains populations. Poyang lake, with its high biodiversity and ecological appropriateness, is an important home for a variety of aquatic animals and plants. The material delivered from the top reaches of the Yangtze river has been intercepted by the three Gorges dam in recent years, resulting in aggravation of riverbed erosion in the middle and lower sections of the riverbed. The water level at Hukou station has been steadily dropping, causing an increase in discharge from Poyang Lake into the Yangtze river.
Poyang Lake's water surface area continues to decrease. The occurrence of low flow happens earlier than previously and the length of the low flow in Poyang lake increases. This tendency is just getting worse. However, as the water environment deteriorates, Poyang lake's ecological habitat deteriorates and biological variety and quantity of benthic animals and fish decline. The Yangtze finless porpoise is the world's only freshwater dolphin. It's a migratory aquatic creature found in rivers and lakes and it's found exclusively in the Yangtze river's middle and lower sections, as well as two huge connecting lakes. Through the Hukou station, the Yangtze finless porpoise enters Poyang lake from the Balijiang river portion. The population of Yangtze finless porpoises in the Yangtze river's main channel has declined from over 2500 in 1990 to around 500 in due to loss of water biological habitat function. According to prior survey data, the number of space scales increased in November of each year. For prediction and conservation, habitat models for evaluating the abundance and geographical distribution of aquatic creatures are required. The Yangtze finless porpoise's most significant feeding and activity environment is Poyang lake. However, no study on a habitat model for Yangtze finless porpoises has been done in the Poyang lake area. This study will also analyze the quantitative effects of water environment on biological health risks of finless porpoise.
Porpoises are reportedly eating fish and shrimps in the Yangtze river and fish, shrimp and squid in the Yellow Sea/Bohai area and the coast of Pakistan. In Japanese waters, they are known to eat fish, shrimp, squid, cuttle fish and squid. They are opportunistic feeders who use a variety of locally available foods especially for cephalopods, crustaceans and small fish. Seasonal changes in diet have not been studied however; they also consume other plant substances, including leaves and rice. Porpoises are opportunistic feeders that mainly feed on cephalopods, crustaceans and small fish. They live in shallow coastal waters severely affected by human activity. Therefore, the IUCN red list of threatened species classified East Asian finless porpoise as endangered species (IUCN). In general, crustaceans have a higher trace element higher than fish and cephalopods, probably because crustaceans come in direct contact with sediments and primarily feed on benthic organisms.
Health risk determination
The levels of Cr, Ni and Pb in all five organs were almost identical, especially those between the muscle and internal organs samples (p>0.05). As Cr is important, cetaceans such as East Asian finless porpoise have developed bio-regulate mechanisms to regulate the form of Cr (i.e., Cr (III) or Cr (VI)), distribution, accumulation and termination. The form of Cr (III) is more lyophilic and tends to accumulate tissue, although less toxic, than Cr (VI). Like Cr, Ni is an important bio-regulatory factor; therefore, small tissue specific variations in the expected concentration. Pb and As exist in marine environments in the form of organic and inorganic, as well as inorganic forms that dominate seawater and soil. Inorganic As is sometimes converted by aquatic organisms to form more bioaccumulative organo arsenic species. Finless porpoise tissues probably performed the excretion function of As and Pb as tissue-specific variants were not present. However, since the present study measured the complete concentration of As, Cr, Ni and Pb without distinguishing biological and inanimate species, the exact role of genetic factors such as physicochemical properties in tissue distribution could not be fully established (Figures 43 and 44).
In many chemicals, there is a lack of relevant information on the effects on cetaceans due to the difficulty of conducting research on these species. In this study, reference to trace element level thresholds on a human rib bone was included in the infinitive porpoise health examination. In addition, the Lowest Adverse Effect Level (LOAEL) and No Adverse Effect level (NOAEL) was observed and the Tolerable Daily Intake (TDI) was also found in the risk profile of follow-up exposure to chronic porpoise. The value 10 is commonly used in the transition between NOAEL and LOAEL.
Conclusion
Heavy metals, which may have accumulated through the food chain, are a particular concern because dolphins are long lived and at the top of the food chain and are therefore susceptible to such pollutants. The effects of heavy metals on marine life may be acute or long-term and may have a significant impact on the health and productivity of the entire ecosystem.
The amounts of arsenic, selenium and zinc were present at higher levels. The Poyang lake water was then tested in 2020 which showed high level of heavy metals added up in the coming years which were mercury, chromium, selenium and arsenic this was a matter of concern as heavy metals.
Protection, counter measures and suggestions
• As mentioned earlier Yangtze's endangered species are classified as endangered on the IUCN red list. These animals face a number of threats, including illegal fishing, poaching directly using dolphin meat as a practicing fishery, dam construction, deforestation and contamination by pollutants. Examining the saturation of lead in dolphin bones over a period of five years may indicate whether cessation of lead use has resulted in a reduction in bioavailable lead in the marine environment. It was found that this high concentration of heavy metals was caused by natural events, accelerated industrial growth, anthropogenic processes, contributions from tributaries and the Yangtze River and the operation of the Dexing and Yongping copper mines near Poyang lake, industries and urban waste, etc. When combined with food, water and mud, the hazard quotient of Hg, Pb, Cu, Cr and Zn was found to be at high risk exposure spectrum (3.2-6.5) and a harmful impact on finless porpoise, while Cd and the As hazard quotient is considered to be the safe spectrum of finless porpoise as its values (1.4-1.8) were within the safe range.
• Rapid urban development and coastal industries have contributed to increased emissions (e.g. As, Cu, Cd, Hg, Ni and Pb) nearby Poyang Lake threatens the health and survival of endangered Poyang. Zn bioaccumulation is high in chronic porpoise and their predisposition is consistent with previous studies that have found high Zn concentration in the region. Other important trace elements are collected in cetaceans in a tissue-specific manner and based on age, kidney and liver and older cetaceans accumulate high concentration. High renal load and hepatic trace element may lead to age-related kidney and liver damage as well as potential immunotoxic, genotoxic and neurotoxic effects to chronic porpoise. However, further research is needed on the long-term monitoring of non-trace elements in order to better understand the threats that may be posing to endangered mammals. Further research is also needed to assess age-related differences in food behavior and habitats for endangered porpoise and other cetaceans in general.
• Overall, there is an urgent need to enforce strict environmental laws against the use of non-essential items, especially in coastal cities, to protect endangered species.
References
- Chaney RL (1991) The heavy elements: Chemistry, environmental impact and health effects. J Environ Qual 20: 876-876. [Crossref]
- He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19: 125-140. [Crossref][Googlescholar][Indexed]
- Herawati N, Suzuki S, Hayashi K, Rivai IF, Koyoma H, et al. (2000) Cadmium, copper and zinc levels in rice and soil of Japan, Indonesia and China by soil type. Bull Environ Contam Toxicol 64: 33-39. [Crossref][Googlescholar][Indexed]
- Shallari S, Schwartz C, Hasko A, Morel JL (1998) Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci Total Environ 19209: 133-142. [Crossref][Googlescholar][Indexed]
- Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338: 47-49. [Crossref][Googlescholar]
- Arruti A, Fernández-Olmo I, Irabien A (2010) Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain). J Environ Monit 12: 1451-1458. [Crossref][Googlescholar][Indexed]
- Sträter E, Westbeld A, Klemm O (2010) Pollution in coastal fog at Alto Patache, Northern Chile. Environ Sci Pollut Res Int 17: 1563-1573. [Crossref][Googlescholar][Indexed]
- Diagomanolin V, Farhang M, Jafarzadeh N (2004) Heavy Metals in the Karoon waterway river, Iran. Toxicol Lett 15: 63-68. [Crossref][Googlescholar][Indexed]
- Duffus JH (2002) Heavy metals-a meaningless term? Pure Appl Chem 74: 793-807. [Crossref][Googlescholar]
- Verster C, Bouwman H (2020) Land-based sources and pathways of marine plastics in a South African context. S Afr J Sci 116: 25-33. [Crossref][Googlescholar]
- Hutton M, Simon C (1986) The quantities of cadmium, lead, mercury and arsenic entering the UK environment from human activities. Sci Total Environ 57: 129-150. [Crossref][Googlescholar][Indexed]
- Bradley SB (1984) Flood effects on the transport of heavy metals. Int J Environ Studies 22: 225-230. [Crossref][Googlescholar]
- Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: Impacts of excess nutrient inputs on freshwater, marine and terrestrial ecosystems. Environ Pollut 100: 179-196. [Crossref][Googlescholar][Indexed]
- Velez D, Montoro R (1998) Arsenic speciation in manufactured seafood products. J Food Prot 61: 1240-1245. [Crossref][Googlescholar][Indexed]
- Conacher HB, Page BD, Ryan JJ (1993) Industrial chemical contamination of foods. Food Addit Contam 10: 129-143. [Crossref][Googlescholar][Indexed]
- He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19: 125-140. [Crossref][Googlescholar][Indexed]
- Lokeshwari H, Chandrappa GT (2006) Impact of heavy metal contamination of Bellandur lake on soil and cultivated vegetation. Curr Sci Assoc 91: 622-627. [Googlescholar]
- Dökmeci AH (2017) An assessment of the heavy metals in wild Mussels Mytilus galloprovincialis from the Marmara sea coast of Tekirdag, Turkey. Fresenius Environ Bull 26: 7608-7612. [Googlescholar]
- Türkdogan MK, Kilicel F, Kara K, Tuncer I, Uygan I, et al. (2003) Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ Toxicol Pharmacol 13: 175-179. [Crossref][Googlescholar][Indexed]
- Kueh CS, Lam JY (2008) Monitoring of toxic substances in the Hong Kong marine environment. Mar Pollut Bull 57: 744-757. [Crossref][Googlescholar][Indexed]
Citation: Sabir A, Hua W (2023) Determining the Health Risks of Heavy Metals on Freshwater Dolphin (Finless Porpoise) in Poyang Lake. J Marine Sci Res Dev 13:380
Copyright: © 2023 Sabir A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2784
- [From(publication date): 0-2023 - Oct 18, 2025]
- Breakdown by view type
- HTML page views: 2336
- PDF downloads: 448






-69408-g025.png)