Research Article Open Access
Determining and Estimation of Antibody Production in the Bubble Eye, Goldfish
Natsuki Nukada1, Eriko Avsar-Ban1, and Yutaka Tamaru1,2,3*1Department of Life Sciences, Graduate School of Bioresources, Mie University, 1577 Kurimamachiya, Tsu, Mie 514-8507, Japan
2Department of Bioinfomatics, Mie University Advanced Science Research Centre, Mie University, 1577 Kurimamachiya, Tsu, Mie 514-8507, Japan
3Laboratory of Applied Biotechnology, Mie University Industrial Technology Innovation Institute, Mie University, 1577 Kurimamachiya, Tsu, Mie 514-8507, Japan
- *Corresponding Author:
- Yutaka Tamaru
Graduate School of Bioresources
Mie University
1577 Kurimamachiya, Tsu
Mie 514-8507, Japan
Tel: +81 59 231 9560
Email: ytamaru@bio.mie-u.ac.jp
Received date: December 05, 2016; Accepted date: December 22, 2016; Published date: December 28, 2016
Citation: Nukada N, Avsar-Ban E, Tamaru Y (2016) Determining and Estimation of Antibody Production in the Bubble Eye, Goldfish. J Marine Sci Res Dev 6:219. doi:10.4172/2155-9910.1000219
Copyright: © 2016 Nukada N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Development of antibody production technologies is necessary for diagnostic treatments and drug discovery. In general, mammals are used as host animals to produce antigen-specific antibody. However, such host animals have never produced several specific antibodies because host animals may not recognize foreign proteins. To overcome this problem, we used teleost to produce antibodies because teleost are evolutionally localized in the origin of vertebrates and have an acquired immune system in addition to the innate immune system. In particular, we attempt to produce antibody using “Bubble Eye” as a kind of goldfish (Carassius auratus), which has sacs filled with lymph liquid, as an immune animal. In this study, a recombinant EGFP-His was expressed in E. coli and then injected into Bubble Eye’s sac in every two weeks. The antibodies were collected from sac instead of blood. Furthermore, a sandwich dot blotting was developed for detection of antibodies against EGFP-His. The antigen-specific antibodies were detected after 42 days from first immunization.
Keywords
Antibody; Immunization; Fish; Goldfish; Vaccination
Introduction
Development of antigen-specific antibody production is necessary for research tools, diagnostic treatments, drug discovery, and so on. However, host animals have never produced specific antibodies against well-conserved proteins between human and other mammals, because host animals do not often recognize foreign proteins, so-called immune tolerance. To overcome this problem, the procedures on antibody production have recently been researched using various immune animals such as chicken, ostrich, shark, camel, and so on. In general, mammals are used as host animals to produce polyclonal antibodies. These host animals have some advantages to produce the antibodies. For example, camelids and sharks are known to possess functional homodimeric antibodies composed of only heavy chains in addition to classical heterodimeric immunoglobulin (Ig) antibodies [1,2]. Such as small molecular antibodies make it possible to easily produce the recombinant antibody in the heterologous expression system by Escherichia coli [3]. On the other hand, chicken and ostrich antibodies can be harvested from their egg yolks instead of blood, and the productivity of antibody in their eggs is much higher than that in a similar sized mammal [4,5].
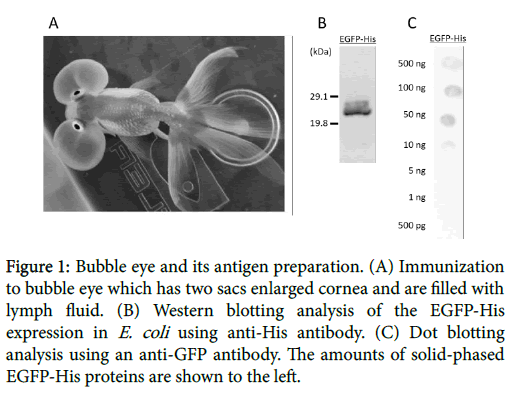
In this study, we attempted to produce the antigen-specific antibodies using fish. Teleost are evolutionally localized in the primitive and diverse groups of vertebrates, and have an acquired immune system in addition to the innate immune system. Mammals possess five functionally distinct immunoglobulin (Ig) isotypes (IgM, IgD, IgG, IgA and IgE), while fish Ig is identified as IgM in cartilaginous and teleosts [6,7]. In a recent study, several Ig isotypes have been identified in different species of teleosts, such as IgZ in zebrafish (Danio rerio ) [8], IgT in rainbow trout (Oncorhynchus mykiss) [9] and the novel IgH in fugu (Fugu rubripes) [10] and chimeric IgM-IgZ in common carp (Cyprinus carpio) [11]. Therefore, teleosts have a potentially of unique immune responses. Previously, we reported to produce specific antibody using zebrafish against human leucine-rich repeat-containing G-protein-coupled receptor 3 (LGR3) [12]. However, zebrafish is too small to take blood including antibody. So we focused on goldfish belonging to Cyprinidae, the same species as carp and zebrafish. Goldfish was first domesticated in China more than a thousand years ago, and several distinct breeds have so far been developed. 23 types of goldfish has been produced by selective breeding and certified by Japan Ornamental Fish Association, while more than 100 types were not certified. In particular, we focused on ‘’Bubble Eye’’ as a new antibody producer which has two large fluidfilled sacs containing lymph under each eye (Figure 1).
Figure 1: Bubble eye and its antigen preparation. (A) Immunization to bubble eye which has two sacs enlarged cornea and are filled with lymph fluid. (B) Western blotting analysis of the EGFP-His expression in E. coli using anti-His antibody. (C) Dot blotting analysis using an anti-GFP antibody. The amounts of solid-phased EGFP-His proteins are shown to the left.
Eye has several advantages as an immune host, the antigen injection and the antibody collection can be performed through the sacs including lymph, antibody can be continuously collected because the sacs after collecting lymph would recover again within about a week or two, and sampling is easier and less damage to the fish than through blood. Here, we report that Bubble Eyes immunized with EGFP-His as antigen generated antigen-specific antibody.
Material and Methods
Recombinant EGFP-His was used as the antigen in this study. The EGFP-His gene was amplified from pXI-EGFP plasmid [14] with primer set as follows: EGFP_rest9U, 5′- aaaaaacccgggGTGAGCAAGGGCGAGGAG-3′ and EGFP_His6, 5′- ctagtggtggtgatgatgatgCTTGTACAGCTCGTCCATGC-3′ and subsequently amplified with an SfiI restriction site at the downstream of His-tag using these primers; EGFP_rest9U and His6_rest1D, 5′- ttttggccgaggcggcCTAGTGGTGGTGATGATGATG-3′. For expression in Escherichia coli (E. coli ), the EGFP-His gene was cloned into the modified pCold TF DNA (TaKaRa Bio Inc.) vector that the SmaI site was bound to the translation enhancing element (TEE) and the SfiI site was bound to upstream of the transcription terminator using these primers; pCold TF_rest1U, 5′- aaaaaaggccgcctcggccGTAATCTCTGCTTAAAAGCACAGAATC-3′ and 5′-ttttttcccgggCACTTTGTGATTCATGGTGTATTACC-3′. E. coli BL21 strain was transformed with pCold TEE EGFP-His plasmid and was cultured at 37°C in 2 × YT medium (1.6% Bacto Trypton, 1% dry yeast extract (Nacalai Tesque, Inc.), and 0.5% NaCl, pH 7.0) containing 50 μg/ml ampicillin. When the OD600 of the culture was reached from 0.4 to 0.5, isopropyl β-D-1-thiogalactopyranoside (IPTG) was added at the final concentration of 0.1 μM and was incubated at 15°C for 24 h.
The cells were collected by centrifugation at 3,000 × g for 15 min at 4ºC and were resuspended in a Ni+-chelating column binding buffer (20 mM sodium phosphate, 0.5 M NaCl, and 20 mM imidazole, pH 7.4). After sonication, the crude extracts were collected by centrifugation at 12,000 × g for 30 min at 4ºC. The supernatant was loaded onto the Ni+-column charged with Ni+-Sepharose 6 Fast Flow (GE healthcare) and was eluted by the Ni+-column elution buffer (20 mM sodium phosphate, 0.5 M NaCl, and 500 mM imidazole, pH 7.4). Furthermore, the target fraction was replaced with the DEAE-column buffer (20 mM 2-amino-2-(hydroxymethyl) propane-1,3-diol (Tris)- HCl, pH 8.0) using a ultrafiltration membrane (Millipore). The EGFPHis protein was purified using DEAE-Sepharose Fast Flow (GE healthcare) with the DEAE-column buffer and a linear elution by the buffer (20 mM Tris-HCl, 1 M NaCl, pH 8.0). The eluted fraction was replaced with a divalent ion-free goldfish Ringer’s solution (125 mM NaCl, 2.6 mM KCl, 10 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES), pH 7.4).
Purified fractions were separated by an SDS-PAGE (10% gel) and were transferred onto a PVDF membrane. The membrane was blocked in PBS containing 5% skim milk and 0.5% Tween 20, incubated with an anti-His antibody (Amersham Biosciences, 1:3,000) at room temperature for 1 h, and subsequently incubated with an anti-mouse IgG HRP-conjugated antibody (Cell Signaling Technology, 1:25,000) at room temperature for 1 h. The dot blots were developed using an Amersham ECL plus Western Blotting Detection Reagent (GE healthcare) and the chemiluminescent was captured by Light Capture II Cooled CCD Camera System (ATTO).
Adult bubble eyes (Figure 1A) were purchased from local breeders at the Maruteru fish farm (Aichi, Japan). Bubble eyes were maintained in a freshwater at 25ºC under light condition of 14-h light period and at 10-h dark period. Bubble eyes were injected at the 100-μg purified recombinant EGFP-His with an adjuvant directly into bubble eye’s sacs. Six groups were prepared, i.e., only EGFP-His antigens, antigens with oil-based adjuvant (egg-yolk lecithin, peanut oil, and glycerol) [15], antigens with oil base adjuvant including inactivated E. coli , Aspergillus oryzae , or Mycobacterium tuberculosis, or goldfish Ringer’s solution instead of the antigens with oil-based adjuvant as a control. The E. coli DH5α strain was cultured in LB-medium at 37ºC for 16 h and the A. oryzae OSI-1013 strain was cultured in DPYmedium at 28ºC for 18 to 20 h. These bacteria were sonicated for inactivating. M. tuberculosis H37 Ra (dry, Difco Laboratories) was used as inactivated cells. Each bacterial cells were added to the oil base in an amount of 0.5 mg/ml and mixed with antigen in a volume ratio of 1:1 using 1-ml syringe (Termofisher Scientific) and an 18 G-reagent mixing needle to prepare with water-in-oil emulsions. The bacterial cells were used as only for an initial immunization, and only the oilbased adjuvant was subsequently used. Booster injections were then given for every 14 days. The bubble eye’s lymph was collected from their sacs before immunization and 14 days after each injection up to 70 days. The collected lymph was centrifuged at 1,500 × g for 10 min at 4ºC to remove precipitates and stored at -30ºC.
For inferring detection limit of the dot-blotting using an anti-GFP antibody, dot blotting solid-phased EGFP-His recombinant protein was performed. The PVDF membrane was soaked in methanol and was transferred to distilled water and soaked in PBS with shaking. A total of 2-μl EGFP-His solutions diluted with PBS were spotted onto the membrane and dry-up overnight. The membrane was blocked with TBST (0.05% [v/v] Tween 20, 20 mM Tris-HCl, 150 mM NaCl, pH 7.5) containing 5% skim milk at room temperature for 1 h. After washing with TBST, the membrane was incubated with anti-GFP antibody (MBL) diluted 1:3,000 with Can Get Signal Solution 1 (TOYOBO) at room temperature for 1 h. Next, the membrane was washed and incubated with an anti-rabbit IgG HRP-linked antibody diluted (1:100,000) with Can Get Signal Solution 2 (TOYOBO) at room temperature for 1 h. After through washing, the dot blots were developed using an Amersham ECL plus Western Blotting Detection Reagent and detected by Light Capture II Cooled CCD Camera System.
For detecting specific antibodies against EGFP-His of immunized bubble eye’s lymph, sandwich dot blotting was performed. 2-μl aliquots of bubble eye’s lymph diluted to 1:100 with PBS were spotted onto the membrane prepared as same, as the above method and dry-up overnight. The membrane was blocked in TBST including 5% skim milk at room temperature for 1 h. After washing, the membrane was incubated with the EGFP-His protein solution diluted with Can Get Signal Solution 1 at room temperature for 1 h, incubated with an anti- GFP antibody (MBL) diluted 1:3,000 with Can Get Signal Solution 1 at room temperature for 1 h, and subsequently incubated with an antirabbit IgG HRP-linked antibody with the dilution (1:100,000) in Can Get Signal Solution 2 at room temperature for 1 h. The dot blots were developed by using an Amersham ECL plus Western Blotting Detection Reagent and detected by Light Capture II Cooled CCD Camera System.
Results
The recombinant EGFP-His protein was successfully expressed in E. coli transformed by the pCold TEE-EGFP-His plasmid, with molecular mass of around 28 kDa. The identity of expressed proteins was further confirmed by Western blotting using the anti-His antibody. This result suggested that the purified proteins were detected by the His-tagged EGFP protein (Figure 1B).
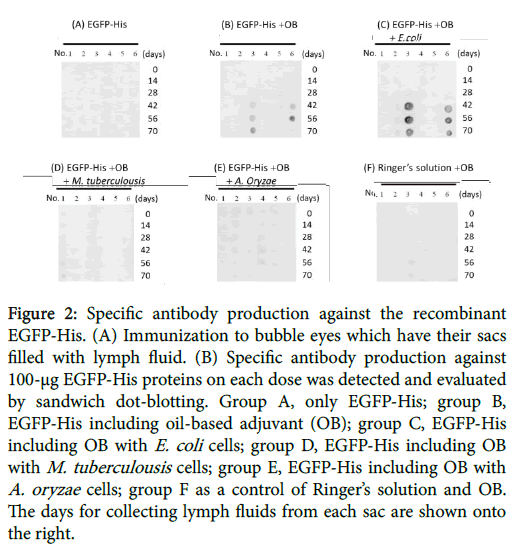
For calculating detection limit of the dot blots using the anti-GFP antibody, dot blotting solid-phased by the EGFP-His recombinant protein was performed. The results showed that anti-GFP antibody detected over 5-ng EGFP-His proteins under the condition (Figure 1C). For detecting antigen as specific antibodies, the dot blotting of solid-phased bubble eye’s lymph fluid was performed. Dot blotting showed a significant response to the EGFP-His protein in experimental group B, while the antigen with oil base adjuvant, and group C, and the antigen with oil-based adjuvant including E. coli . Both groups indicated that the titer of antigen specific antibodies were clearly higher after 42 days from first immunization (Figure 2).
Figure 2: Specific antibody production against the recombinant EGFP-His. (A) Immunization to bubble eyes which have their sacs filled with lymph fluid. (B) Specific antibody production against 100-μg EGFP-His proteins on each dose was detected and evaluated by sandwich dot-blotting. Group A, only EGFP-His; group B, EGFP-His including oil-based adjuvant (OB); group C, EGFP-His including OB with E. coli cells; group D, EGFP-His including OB with M. tuberculousis cells; group E, EGFP-His including OB with A. oryzae cells; group F as a control of Ringer’s solution and OB. The days for collecting lymph fluids from each sac are shown onto the right.
However, experimental groups which were injected only antigen, the antigens with the adjuvant including A. oryzae or M. tuberculosis , and Ringer’s solution with those adjuvant was detected with no signals.
Discussion
To our knowledge, this is the first description of a successful method to generate the antigen-specific antibody by employing bubble eye’s sacs. Therefore, the recombinant EGFP-His proteins were directly injected into their sacs. The results on dot blotting suggested that the adjuvants at a first antigen injection were needed to produce some specific-antibody in bubble eyes. In addition, the antigen-specific antibodies were raised after third antigen injection in the experimental group B, the EGFP-His proteins with an oil-based adjuvant, and group C as EGFP-His with an oil-base adjuvant including E. coli . Gramnegative bacterium in outer membrane of cell walls consists of lipopolysaccharides which are involved in some immunostimulation. Mycobacterium is also known to have the adjuvanticity, although the present study suggested that the antibody’s titers were higher when the adjuvant including E. coli was used of injection into their sacs. Some of high antigenicity could be blamed on the existent of other gramnegative bacteria, through the freshwater such as a Gram-negative bacterium such as Aeromonas spp. which was caused on the opportunistic infection. Bubble eyes have a potential of immune animals as alternative to mammals, because the antibodies of immunoglobulin M (IgM) could be easily collected through their sacs without any killing. Therefore, these fish would be no more out of animal welfares. Further work is needed to generate other antigens such as GPCRs and developing more highly sensitive assay methods. Cyprinidae contributes over 20 million metric tons to fish production in the worldwide and accounts for approximately 40% of total global aquaculture production and 70% of total freshwater aquaculture production [16]. However, goldfish farming have declined in recent years, so it is hoped that the researching of goldfish utilization as an immune hosts contributes to the revitalization of goldfish farming.
Acknowledgement
This research was supported by Ministry of Agriculture, Forestry and Fisheries in Japan and Japan Science and Technology Agency.
References
- De Genst E, Saerens D, Muyldermans S, Conrath K (2006) Antibody repertoiredevelopment in camelids. Dev Comp Immunol 30: 187-198.
- Dooley H, Flajnik M (2006) Antibody repertoire development in cartilaginous fish.Dev Comp Immunol. 30:43-56.
- Hoogenboom HR (2005) Selecting and screening recombinantantibody libraries.Nat Biotechnol. 23: 1105-1116.
- Gassmann M, Thömmes P, Weiser T, Hübscher U (1990) Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J 4:2528-2532.
- Adachi K, Handharyani E, Sari DK, Takama K, Fukuda K, et al. (2008) Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthiocamelus) yolk. Mol Med Rep 1:203-209.
- Kokubu F, Hinds K, Litman R, Shamblott MJ, Litman GW (1988) Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. EMBO J 7:1979-1988.
- Wilson MR, Marcuz A, van Ginkel F, Miller NW, Clem LW, et al. (1990) The immunoglobulin M heavy chain constant region gene of the channel catfish, Ictaluruspunctatus: an unusual mRNA splice pattern produces the membrane form of the molecule. Nucleic Acids Res 18:5227-5233.
- Danilova N, Bussmann J, Jekosch K, Steiner LA (2005) The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol 6:295-302.
- Hansen JD, Landis ED, Phillips RB (2005) Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. ProcNatlAcadSci U S A 102:6919-6924.
- Savan R, Aman A, Sato K, Yamaguchi R, Sakai M (2005) Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol35:3320-3331.
- Savan R, Aman A, Nakao M, Watanuki H, Sakai M (2005) Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinuscarpio L.). Immunoqenetics57:458-463.
- Nukada N, Avsar-Ban E, Ishikawa H, Tamaru Y (2014) Research and Development of Antibody Production Using Zebrafish against Human hLGR3. Ann Vaccines Immunization 1:1003.
- Sawatari E, Hashimoto H, Matsumura T, Iwata Y, Yamamoto N, et al. (2009) Cell growth-promoting activity of fluid from eye sacs of the bubble-eye goldfish (Carassiusauratus). ZoologSci 26:254-258.
- Avsar-Ban E, Ishikawa H, Akiyama S, Manya H, Endo T, et al. (2012) Functional and heterologous expression of human protein O-linked mannose β-1,2-N-acetylglucosaminyltransferase 1 in zebrafish. J BiosciBioeng114:237-239.
- Reynolds JA, Harrington DG, Crabbs CL, Peters CJ, Di Luzio NR (1980) Adjuvant activity of a novel metabolizable lipid emulsion with inactivated viral vaccines. Infect Immun28:937-943.
- Xu P, Zhang X, Wang X, Li J, Liu G, et al. (2014) Genome sequence and genetic diversity of the common carp, Cyprinuscarpio. Nat Genet 46:1212-1219.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 4579
- [From(publication date):
December-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 3650
- PDF downloads : 929