Research Article Open Access
Determination of Total and Free Concentrations of Flucloxacillin and Cefazolin in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry
Mei Zhang1,2*, Grant A Moore2, Richard Everts3 and Evan J Begg11Department of Medicine, University of Otago−Christchurch, Christchurch, New Zealand
2Toxicology, Canterbury Health Laboratories, Christchurch, New Zealand
3Infectious Diseases Specialist and Medical Microbiologist, Nelson Bays Primary Health, Nelson, New Zealand
- *Corresponding Author:
- Dr. Mei Zhang
Clinical Pharmacology, Department of Medicine
University of Otago - Christchurch/Toxicology
Canterbury Health Laboratories, Christchurch, New Zealand
Tel: 0064 3 364 0640 ext 89746
Fax: 0064 3 364 1003
E-mail: mei.zhang@cdhb.health.nz
Received date: January 23, 2014; Accepted date: February 15, 2014; Published date: February 18, 2014
Citation: Zhang M, Moore GA, Everts R, Begg EJ (2014) Determination of Total and Free Concentrations of Flucloxacillin and Cefazolin in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry. J Anal Bioanal Tech 5:182. doi: 10.4172/2155-9872.1000182
Copyright: © 2014 Zhang M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid, simple and sensitive liquid chromatography/tandem mass spectrometry (LC-MS/MS) assay was developed for the determination of total and free concentrations of flucloxacillin and cefazolin in human plasma. Free flucloxacillin and cefazolin were separated from bound drug by ultra filtration. Samples of plasma and plasma ultra filtrate were pre-treated with acetonitrile (sample: acetonitrile = 1:4). Flucloxacillin, cefazolin and butobarbitone, the internal standard, were then resolved on a C18 (2)column using gradient elution of 0.05% formic acid and methanol. The three compounds were detected using electrospray ionisation in the positive mode. Standard curves were linear over the concentration range 0.2 to 100 mg/L (r>0.99) in plasma and 0.005 to 10 mg/L (r>0.99) in plasma ultra filtrate for both flucloxacillin and cefazolin. For both total and free flucloxacillin and cefazolin, bias was <±10%, and intra- and inter-day coefficients of variation (imprecision) were <10%. The limit of quantification was 0.2 mg/L for both flucloxacillin and cefazolin in plasma. The limit of quantification was 0.005 mg/L for both flucloxacillin and cefazolin in plasma ultra filtrate. The assay has been used successfully in a clinical pharmacokinetic study of flucloxacillin, and in clinical practice to enhance the effective use of flucloxacillin and cefazolin.
Keywords
Flucloxacillin; Cefazolin; Plasma; LC-MS/MS
Introduction
Flucloxacillin and cefazolin are widely used β-lactam antibiotics for the treatment of bacterial infections. Maximizing the effectiveness while minimizing the toxicity of antimicrobial agents is important in the treatment of infections, and requires an understanding of the pharmacokinetics and pharmacodynamics of the prescribed antimicrobial [1]. It is particularly important for the free concentration of β-lactam antibiotics to be above the minimum inhibitory concentration (MIC) of the bacteria at the site of the infection for a sufficient period time over the dosing interval. For moderately severe infection the period of time should be at least 50% [2,3]. Although not commonly utilised for β-lactam antibiotics, therapeutic drug monitoring (TDM) may enhance the likelihood of efficacy and minimize the toxicity of antimicrobial therapy in individual patients.
Pharmacokinetic studies and TDM of antibiotics require a simple and rapid analytical method to analyse the concentrations in plasma that is sensitive enough to measure accurately the free concentrations which are 4-5% of total concentrations for flucloxacillin [4,5] and ~20% for cefazolin [6]. Various analytical methods have been developed for measuring flucloxacillin or cefazolin alone [7-9] and in combination with other drugs [10-12] in human plasma, including HPLC [7,9] and LC-MS/MS [8,10-12]. It appears that no method has been reported for the simultaneous determination of flucloxacillin and cefazolin for total- and free-concentrations in plasma. In our clinical practice, requests for flucloxacillin or cefazolin monitoring are received almost daily.It is time consuming and costly to analyse the samples required for flucloxacillin test and the samples required for cefazolin test by two separate methods. Therefore, it was considered worthwhile to develop a simple, rapid, robust and reliable LC-MS/MS method for simultaneous determination of flucloxacillin and cefazolin in plasma, suitable for routine use in busy hospital laboratories, as well as for clinical pharmacokinetic studies.
Materials and Methods
Materials
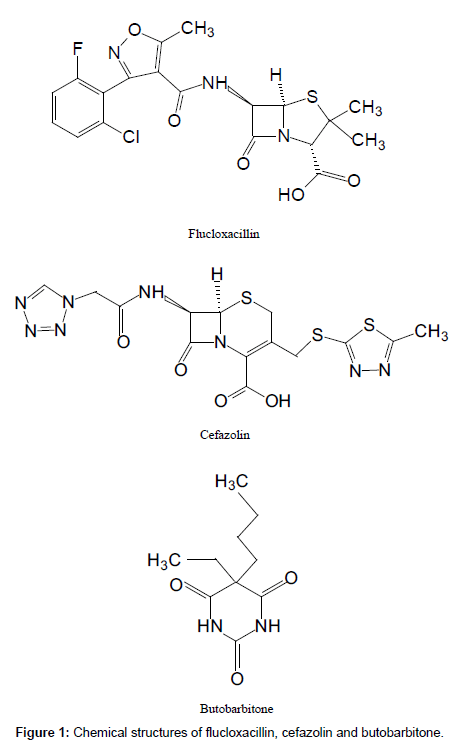
Flucloxacillin and cefazolin (Figure 1) were purchased from Toronto Research Chemicals Inc. (Toronto, Canada) and Sigma Co. (Australia) respectively. Both are commercially available as the sodium salts. Butobarbitone (Figure 1) was kindly donated by the Institute of Environmental Science and Research, New Zealand (Wellington, New Zealand). HPLC-grade acetonitrile, methanol and formic acid were purchased from BDH (Poole, UK). Distilled, deionised water was produced by a Milli-Q Reagent Water System (Millipore, MA, USA). The human plasma used as the assay blank and for the preparation of standards was obtained from New Zealand Blood Services (Christchurch, New Zealand).
Instrumentation and analytical conditions
The LC-MS/MS system consisted of a Shimadzu LC-20AD HPLC system (Shimadzu Corporation, Kyoto, Japan) interfaced with an API 4000™ triple quadrupole mass spectrometer (Applied Biosystems, Foster City, Canada) equipped with a Turbo Ion Spray® source. Analyst software (Applied Biosystems, Foster City, Canada) was used to control equipment, to coordinate data acquisition, and to analyse data. Flucloxacillin, cefazolin and the internal standard butobarbitone were separated under gradient elution using a Luna C18(2)5 μm, 50 × 2.0 mm internal diameter analytical column equipped with a C18 4.0 × 2.0 mm internal diameter guard column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of solvent A (0.05% formic acid) and solvent B (methanol). The flow rate was set at 0.3 mL/min. The initial condition was 70% solvent A and 30% solvent B. A linear gradient was performed with mobile phase B increasing from 30% to 90% within 1 min. After 4 min, the mobile phase was returned to the initial condition and re-equilibrated for 2 min. The total analysis time was 6 min.
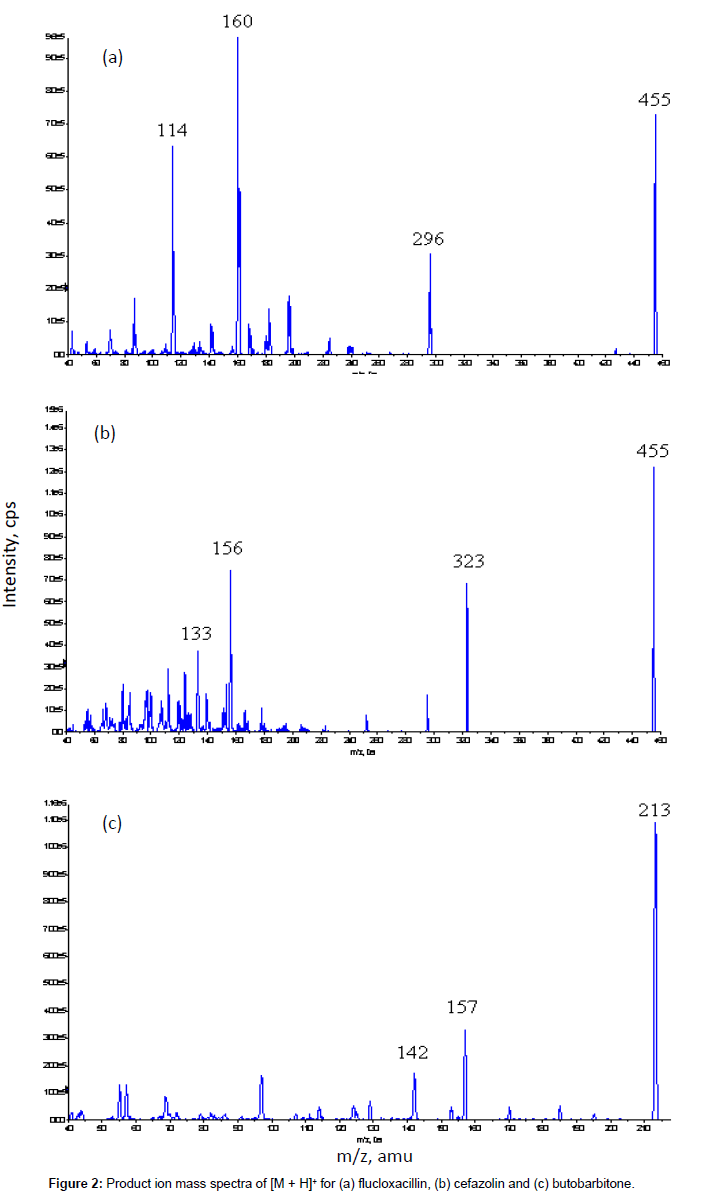
The mass spectrometer was operated in the positive ion mode with curtain gas, Gas 1 and Gas 2 flow rates of 20, 45 and 60 psi, respectively. The ion spray voltage was 5000 V and the source temperature was 500°C. Data acquisition was performed via multiple reactions monitoring (MRM). The optimized precursor-to-product ion transitions monitored for flucloxacillin [M + H]+ , cefazolin [M + H]+ and butobarbitone [M + H]+ were m/z 455 → 160 with declustering potential (DP) 51 V and collision energy (CE) 19 V, m/z 455 →323 with DP 46 V and CE 17 V, and m/z 213 →157 with DP 71 V and CE 21 V, respectively.
Standards
The stock solutions of flucloxacillin and cefazolin (both 5.0 mg/ mL as free base) were prepared by dissolving 26.2 mg of the respective sodium salt standards in 5.0 mL of water. Two sets of the same standard stock solutions were prepared for standard curves and for quality control (QC) samples respectively. The intermediate standard solution of flucloxacillin and cefazolin (0.5 mg/mL of flucloxacillin and cefazolin) was prepared by diluting 100 μL of flucloxacillin stock solution and 100 μL of cefazolin stock solution to 1.0 mL of water. The stock internal standard butobarbitone solution (2.0 mg/mL) was prepared by dissolving 20 mg of butobarbitone in 10.0 mL of methanol. A working solution of the internal standard (0.2 mg/mL) was prepared by diluting the stock solution with water.
For the analysis of total-concentration in plasma, the standard curves of flucloxacillin and cefazolin were prepared to cover a calibration range of 0.2 to 100 mg/L by spiking appropriate aliquots of flucloxacillin and cefazolin stock solutions to drug-free plasma. Flucloxacillin and cefazolin plasma quality control (QC) standards were prepared in single 5 mL aliquots in concentrations of 0.2, 2.0, 20.0 and 100 mg/L and stored at -80°C until analysed.
For the analysis of free-concentration in plasma, the ultra filtrate of drug-free human plasma for the preparation of standards was prepared by ultra filtration (2600 g for 30 min at 37°C) using Centrifree device, 30KDa (Millipore, MA, USA). The standard curves of flucloxacillin and cefazolin were prepared to cover a calibration range of 0.05 to 10 mg/L by spiking appropriate aliquots of the intermediate standard solution of flucloxacillin and cefazolin to drug-free plasma ultra filtrate. The quality control (QC) standards of flucloxacillin and cefazolin in plasma ultra filtrate were prepared in single 2 mL aliquots in concentrations of 0.005, 0.01, 0.1, 1.0 and 10.0 mg/L and stored at -80°C until analysed.
Sample preparation
For the analysis of total-concentration in plasma: The internal standard butobarbitone, 50 μL of 0.2 mg/mL, was added to 50 μL of each of the blank, standard, QC and patient samples. The mixtures were vortexed briefly. Two hundred microliters of acetonitrilewas added to precipitate the proteins. After centrifugation at 15,000 g for 5 min, a 50 μL aliquot of clear supernatant was mixed with 500 μL of water and transferred to the 96 well microtiter autosampler plate. A volume of 10 μL was injected into the LC-MS/MS system.
For the analysis of free concentration in plasma: Free drug was separated from total drug by ultra filtration (2600 g for 30 min at 37°C) using Centrifree device, 30KDa (Millipore, MA, USA). Before the Centrifree devices were chosen for the preparation of plasma ultra filtrate of patient samples, the adsorption of flucloxacillin and cefazolin to the ultra filtration membrane during the filtration process (non-specific binding) was checked using aqueous standards. The non-specific binding was measured by comparing the peak areas of flucloxacillin and cefazolin in the samples with ultra filtration to those in the samples without ultra filtration. No non-specific binding was observed.
The internal standard butobarbitone, 50 μL of 0.1 mg/mL, was added to 50 μL of each plasma ultra filtrate of blank, standard, QC or patient samples. After the mixture was vortexed briefly, 200 μL of acetonitrilewas added. The plasma ultra filtrate was comparatively clear and no protein precipitation was observed after adding extraction solution (200 μl of acetonitrile). Therefore, it was not necessary to centrifuge the samples. After vortexing for 30 seconds, a 50 μL aliquot was mixed with 200 μL of water and transferred to the 96 well microtiter autos ampler plate. A volume of 10 μL was injected into the LC-MS/MS system.
Validation
The standard curves were the plot of the peak area ratios (analyte/internal standard) of flucloxacillin and cefazolin versus the corresponding concentrations of flucloxacillin and cefazolin. To evaluate the assay recoveries and matrix effects, three sets of standards were prepared using a modification of the method of Matuszewski et al. [13] for flucloxacillin, cefazolin and the internal standard butobarbitone. For the plasma assay (for total concentration in plasma), the standards of flucloxacillin and cefazolin were prepared in concentrations of 0.2, 2.0, 20 and 100 mg/L, and butobarbitone at 0.2 mg/mL, the concentration used in the assay. The first set was prepared in plasma from six different sources, the second set in blank extracted plasma (the extracts of blank plasma after-protein precipitation) from the same six different sources as in first set, and the third set in water. Every set included six samples at each concentration. Absolute recoveries at each concentration were measured by comparing the peak areas of flucloxacillin, cefazolin and butobarbitone in plasma to those in the spiked plasma extracts at the corresponding concentrations (n = 6) [Absolute recovery = (peak area of analyte in spiked plasma sample)/(peak area of analyte in spiked plasma extract sample) × 100%]. The matrix effects were assessed by comparing the peak areas of flucloxacillin, cefazolin and butobarbitone from the spiked plasma extracts with the response of standard solution at the same concentration in water (n = 6) [Matrix effect = (peak area of analyte in spiked plasma extract sample)/(peak area of analyte in spiked water sample) × 100%]. A value of 100% indicates that the responses in water and in spiked plasma extract were the same and no absolute matrix effect is observed. A value of >100% indicates ionization enhancement and a value of <100% indicates ionization suppression. Currently, no guidelines exist for the assessment of matrix effect. In our work, matrix effect value 100 ± 15% are acceptable. For the plasma ultra filtrate assay (for free concentration in plasma), only the second and third sets of standards were prepared for the matrix effect evaluation and the standards of flucloxacillin and cefazolin were prepared in concentrations of 0.005, 0.01, 0.1, 1.0 and 10.0 mg/L, and butobarbitone at 0.2 μg/mL, the concentration used in the assay. The procedure was the same as for plasma assay but using plasma ultra filtrate instead of plasma. The plasma ultra filtrate was comparatively clear and no protein precipitation was observed after adding extraction solution (200 μL of acetonitrile). Therefore, there was no difference between the prepared plasma ultra filtrate standard samples and the spiked plasma ultra filtrate extract samples and it was not necessary to evaluate the recoveries of flucloxacillin, cefazolin and butobarbitone from plasma ultra filtrate. Quality control was assessed by analysis of six samples at each concentration on the same day (intra-day) and of one sample at each concentration on six different days (inter-day). Bias was determined as the measured minus the actual concentration, expressed as a percentage of the actual concentration. Imprecision was measured as intra- and inter-day coefficients of variation. The lowest concentration for both flucloxacillin and cefazolin standard curves was considered to be the lower limit of quantitation (LLOQ) at which the concentration of flucloxacillin, and cefazolin could be determined with acceptable accuracy and precision. According to the US Food and Drug Administration guidance for bioanalytical method validation [14], the mean value determined at LLOQ should not deviate by more than 20% of the actual value, and the precision determined at LLOQ should not exceed 20% of the coefficients of variation (CV).
The effects of freezing and thawing on the concentrations of flucloxacillin and cefazolin were studied using plasma QC samples at 0.2, 2.0, 20 and 100 mg/L and plasma ultra filtrate QC samples at 0.005, 0.01, 0.1, 1.0 and 10.0 mg/L, which were subjected to four freeze-thaw cycles before analysis. The stability of QC samples at -80°C was evaluated by concentration analysis at weekly intervals for four months. The stability of the stock standard solutions of flucloxacillin and cefazolin at -80°C for six months was evaluated by comparing the response with that of the freshly prepared standard solutions. The stability of the processed samples at 4°C (the temperature of the auto sampler) for 24 hours was evaluated by comparing the results with the original results. In all cases, flucloxacillin and cefazolin were considered to be stable as long as degradation was <10% of the concentration at day 0. Probenecid was analysed to investigate possible interference because it is the most likely co-administered drug.
Results and Discussion
Mass spectrometry and chromatography
The MS/MS parameters were optimised to produce maximum responses for flucloxacillin, cefazolin and the internal standard butobarbitone using electrospray ionisation in the positive ion mode. The protonated molecular ions [M + H]+ were m/z 455 for both flucloxacillin and cefazolin, and m/z 502 for butobarbitone. The transitions yielding the most abundant product ions were 455 → 160 for flucloxacillin, 455 → 323 for cefazolin and 213 → 157 for butobarbitone. The product ion spectra of [M + H]+ for three compounds are shown in Figure 2.
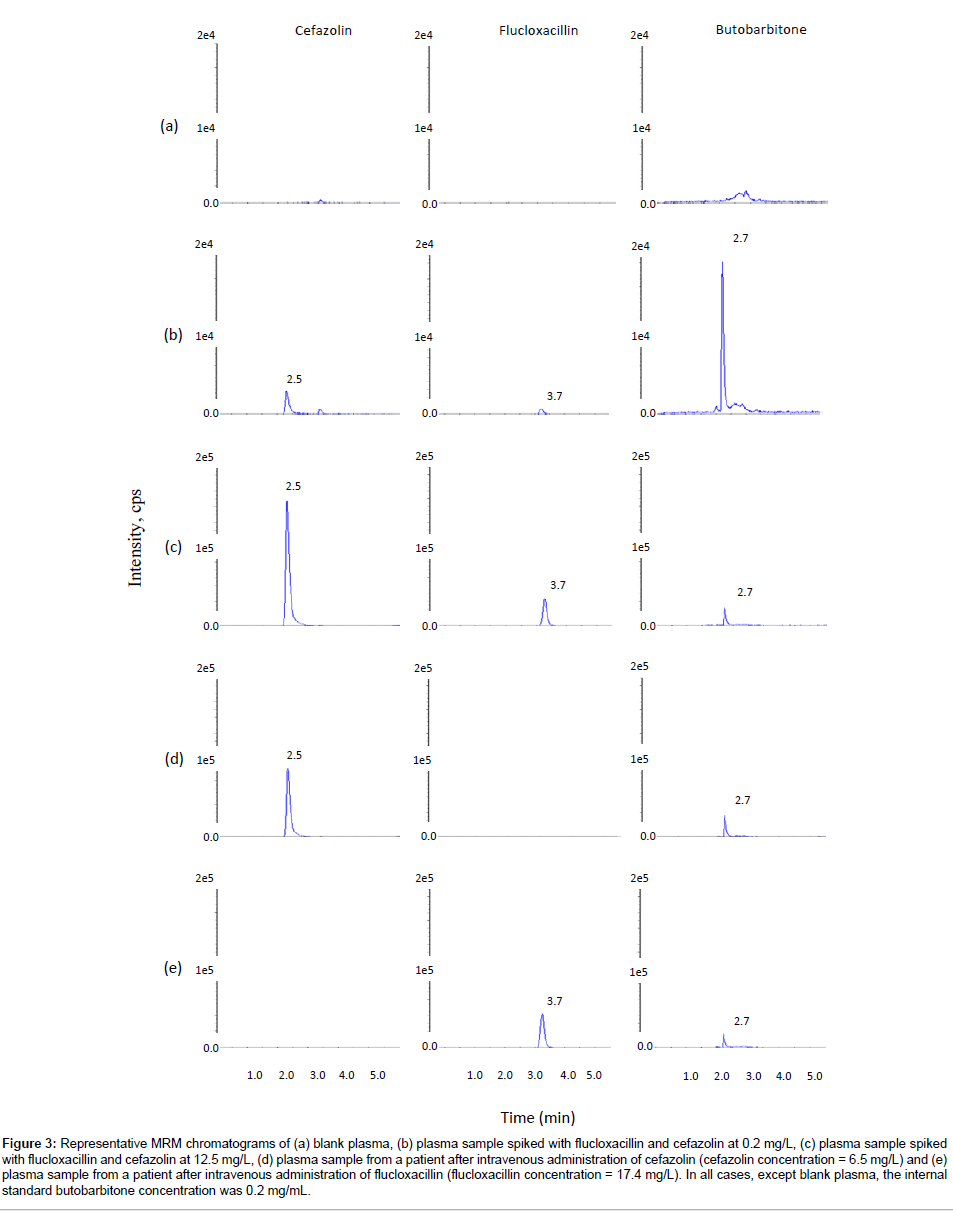
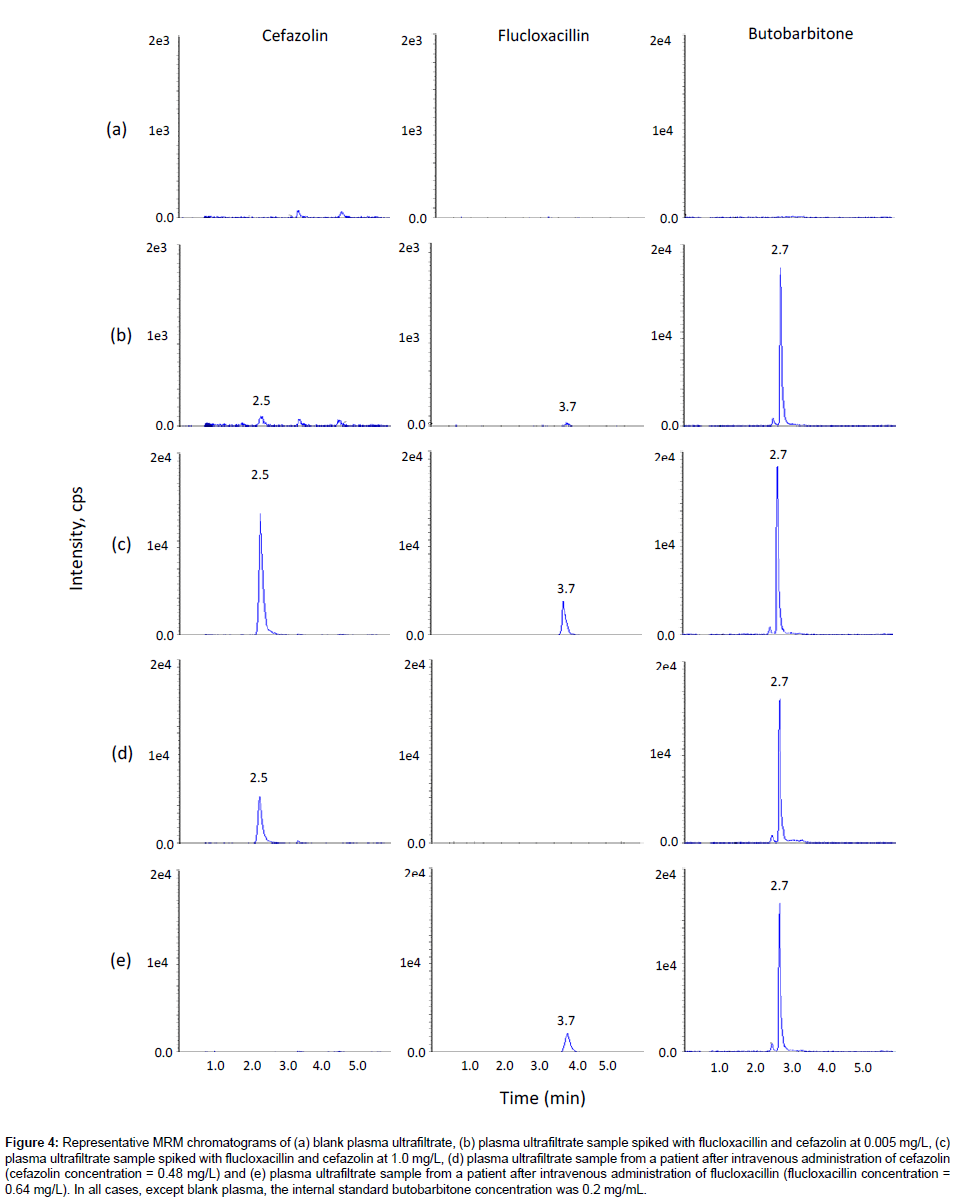
To achieve optimized chromatographic resolution, peak sharpness and signal intensity, a variety of LC analytical columns, including Phenomenex Gemini C6-Phenyl and Luna C18(2) and C8 columns, and mobile phase compositions, including 5 mM ammonium acetate, 0.05% formic acid and methanol, were evaluated. The Phenomenex Luna C18 (2) and C8 columns gave the best chromatographic resolution and sharp peaks. Choosing the Phenomenex Luna C18 (2) column over the Phenomenex Luna C8 column enabled this method to share the same column with another routine method in our laboratory, so that we could run both methods overnight without the need of changing columns. The mobile phase consisting of 0.05% formic acid and methanol gave higher signal intensity. Gradient elution with increasing methanol decreased the retention of the later-eluting components, flucloxacillin and the internal standard butobarbitone, so that they eluted faster with sharper peaks, speeding up the analysis time for each sample. The optimized LC condition chosen was therefore a mobile phase consisting of 0.05% formic acid and methanol with gradient elution on a Phenomenex Luna C18 (2) column. Under these conditions, the retention times were approximately 2.5, 2.7, and 3.7 min for cefazolin, the internal standard butobarbitone and flucloxacillin, respectively (Figure 3 and 4). Blank plasma samples and plasma ultra filtrate samples from more than six different sources of the same matrix were tested for interference, and flucloxacillin, cefazolin and the internal standard peaks were free of interference from any other peaks present in the blanks (Figures 3 and 4). There was no interference of probenecid.
Figure 3: Representative MRM chromatograms of (a) blank plasma, (b) plasma sample spiked with flucloxacillin and cefazolin at 0.2 mg/L, (c) plasma sample spiked with flucloxacillin and cefazolin at 12.5 mg/L, (d) plasma sample from a patient after intravenous administration of cefazolin (cefazolin concentration = 6.5 mg/L) and (e) plasma sample from a patient after intravenous administration of flucloxacillin (flucloxacillin concentration = 17.4 mg/L). In all cases, except blank plasma, the internal standard butobarbitone concentration was 0.2 mg/mL.
Figure 4: Representative MRM chromatograms of (a) blank plasma ultrafiltrate, (b) plasma ultrafiltrate sample spiked with flucloxacillin and cefazolin at 0.005 mg/L, (c) plasma ultrafiltrate sample spiked with flucloxacillin and cefazolin at 1.0 mg/L, (d) plasma ultrafiltrate sample from a patient after intravenous administration of cefazolin (cefazolin concentration = 0.48 mg/L) and (e) plasma ultrafiltrate sample from a patient after intravenous administration of flucloxacillin (flucloxacillin concentration = 0.64 mg/L). In all cases, except blank plasma, the internal standard butobarbitone concentration was 0.2 mg/mL.
Sample preparation
Protein precipitation is the simplest and most rapid method of plasma sample preparation for the measurement of drug concentrations. Acetonitrile is one of the most widely used precipitating agents. Precipitation with acetonitrile (4:1 ratio of acetonitrile to plasma) gave the best sample clean-up and highest recoveries for flucloxacillin, cefazolin and the internal standard butobarbitone.
Method validation
Standard curves of flucloxacillin and cefazolin were adequately fitted by 1/x2 weighted quadratic regressions (r>0.999) for plasma over the concentration range of 0.2 to 100 mg/L, and for plasma ultra filtrate over the concentration range of 0.005 to 10.0 mg/L. The LLOQ was around 0.2 mg/L for both flucloxacillin and cefazolin in plasma, and 0.005 mg/L for both flucloxacillin and cefazolin in plasma ultra filtrate. The accuracy and precision were assessed at LLOQ and the low, medium and high level QCs. For both flucloxacillin and cefazolin in both plasma and plasma ultra filtrate, there was no constant direction to the bias (i.e. + or -) for LLOQ and QCs and the mean values were within ± 10% of the spiked values (Tables 1-4). Imprecision was small, as indicated by both intra- and inter-day coefficients of variation of <10% at concentrations of LLOQ and QCs (Tables 1-4). The absolute recoveries of flucloxacillin and cefazolin from plasma at concentrations of 0.2, 2.0, 20 and 100 mg/L were similar and consistent, with mean values of around 100%. The absolute recovery of the internal standard butobarbitone at the concentration employed was 102 ± 5.9% (n = 6).
| Sample | Concentration spiked (mg/L) | Concentration found (mg/L) (Mean ± SD) | Bias (%) | Imprecision CV (%) | |
|---|---|---|---|---|---|
| Flucloxacillin | |||||
| LLOQ | 0.200 | 0.190 ± 0.0136 | -4.9 | 7.2 | |
| QC1 | 2.00 | 1.86 ± 0.090 | -6.8 | 4.8 | |
| QC2 | 20.0 | 18.7 ± 0.81 | -6.6 | 4.3 | |
| QC3 | 100 | 101 ± 5.53 | 1.3 | 5.5 | |
| Cefazolin | |||||
| LLOQ | 0.200 | 0.212 ± 0.0142 | -5.8 | 6.7 | |
| QC1 | 2.00 | 2.00 ± 0.135 | 0.0 | 6.7 | |
| QC2 | 20.0 | 19.9 ± 0.61 | -0.3 | 3.0 | |
| QC3 | 100 | 101 ± 4.61 | 1.5 | 4.5 | |
Table 1: Intra-day assay variance of the determination of flucloxacillin and cefazolin in plasma (n = 6).
| Sample | Concentration spiked (mg/L) | Concentration found (mg/L) (Mean ± SD) | Bias (%) | Imprecision CV (%) |
|---|---|---|---|---|
| Flucloxacillin | ||||
| LLOQ | 0.200 | 0.208 ± 0.0092 | 3.92 | 4.4 |
| QC1 | 2.00 | 2.14 ± 0.083 | 6.83 | 3.9 |
| QC2 | 20.0 | 20.8 ± 1.48 | 4.00 | 7.1 |
| QC3 | 100 | 102 ± 7.95 | 2.43 | 7.8 |
| Cefazolin | ||||
| LLOQ | 0.200 | 0.201 ± 0.0065 | 0.33 | 3.3 |
| QC1 | 2.00 | 1.99 ± 0.112 | -0.33 | 5.6 |
| QC2 | 20.0 | 18.7 ± 0.34 | -6.42 | 1.8 |
| QC3 | 100 | 93.9 ± 3.49 | -6.15 | 3.7 |
Table 2: Inter-day assay variance of the determination of flucloxacillin and cefazolin in plasma (n = 6).
| Sample | Concentration spiked (mg/L) | Concentration found (mg/L) (Mean ± SD) | Bias (%) | Imprecision CV (%) |
|---|---|---|---|---|
| Flucloxacillin | ||||
| LLOQ | 0.0050 | 0.0051 ± 0.00032 | 2.0 | 6.3 |
| QC1 | 0.0100 | 0.0095 ± 0.00062 | -4.7 | 6.5 |
| QC2 | 0.100 | 0.102 ± 0.0045 | 1.5 | 4.5 |
| QC3 | 1.00 | 1.05 ± 0.054 | 1.5 | 5.1 |
| QC4 | 10.0 | 10.1 ± 0.57 | 1.4 | 5.7 |
| Cefazolin | ||||
| LLOQ | 0.0050 | 0.0052 ± 0.00029 | 4.0 | 5.6 |
| QC1 | 0.0100 | 0.0102 ± 0.00063 | 2.0 | 6.2 |
| QC2 | 0.100 | 0.102 ± 0.0055 | 2.1 | 5.4 |
| QC3 | 1.00 | 1.05 ± 0.048 | 5.3 | 4.5 |
| QC4 | 10.0 | 10.1 ± 0.57 | 1.4 | 5.7 |
Table 3: Intra-day assay variance of the determination of flucloxacillin and cefazolin in plasma ultrafiltrate (n = 6).
| Sample | Concentration spiked (mg/L) | Concentration found (mg/L) (Mean ± SD) | Bias (%) | Imprecision CV (%) |
|---|---|---|---|---|
| Flucloxacillin | ||||
| LLOQ | 0.0050 | 0.0052 ± 0.00017 | 4.5 | 3.2 |
| QC1 | 0.01 | 0.0105 ± 0.00048 | 4.7 | 4.6 |
| QC2 | 0.100 | 0.108 ± 0.0071 | 7.6 | 6.6 |
| QC3 | 1.00 | 1.02 ± 0.075 | 2.0 | 7.3 |
| QC4 | 10.0 | 10.2 ± 0.67 | 2.2 | 6.6 |
| Cefazolin | ||||
| LLOQ | 0.0050 | 0.0051 ± 0.00011 | 2.0 | 2.1 |
| QC1 | 0.0100 | 0.0102 ± 0.00037 | 1.9 | 3.6 |
| QC2 | 0.100 | 0.099 ± 0.0059 | -1.3 | 6.0 |
| QC3 | 1.00 | 0.94 ± 0.025 | -5.7 | 2.6 |
| QC4 | 10.0 | 9.3 ± 0.23 | -6.7 | 2.4 |
Table 4: Inter-day assay variance of the determination of flucloxacillin and cefazolin in plasma ultrafiltrate (n = 6).
The matrix effects (mean ± SD%) for plasma at concentrations of 0.2, 2.0, 20 and 100 mg/L and plasma ultra filtrate at concentrations of 0.005, 0.01, 0.1, 1.0 and 10 mg/L for flucloxacillin and cefazolin were similar and consistent, with mean values of around 100% (Table 5). The matrix effect for the internal standard butobarbitone was 96.1 ± 5.8% (n = 6). No significant matrix effects were evident.
| Concentration spiked (µg/L) | Flucloxacillin (Mean ± SD%) | Cefazolin (Mean ± SD%) |
|---|---|---|
| Plasma | ||
| 0.20 | 96.4 ± 9.7 | 97.2 ± 4.5 |
| 2.00 | 97.9 ± 8.7 | 96.5 ± 5.4 |
| 20.0 | 111 ± 6.8 | 101.5 ± 1.9 |
| 100 | 104 ± 8.6 | 99.1 ± 2.9 |
| Plasma ultrafiltrate | ||
| 0.005 | 99.6 ± 4.8 | 96.6 ± 2.7 |
| 0.01 | 95.4 ± 7.1 | 97.2 ± 8.8 |
| 0.10 | 102 ± 7.1 | 93.7 ± 6.5 |
| 1.00 | 95.9 ± 6.9 | 101 ± 10.1 |
| 10.0 | 98.0 ± 5.1 | 101 ± 4.6 |
Table 5: Matrix effect data for flucloxacillin and cefazolin (n = 6, from six different sources).
Flucloxacillin and cefazolin were found to be stable in plasma and plasma ultra filtrate for at least four freeze-thaw cycles when stored at -80°C. The QC samples for both plasma and plasma ultra filtrate were stable for at least four months at -80°C. Stock standard solutions of flucloxacillin and cefazolin remained stable for at least six months at -80°C. The processed samples were stable for at least 24 hours at 4°C. In all cases, the concentrations of flucloxacillin and cefazolin of stored samples deviated <10% from the freshly prepared samples.
Application of the assays
The method has been used in a clinical study to examine the pharmacokinetics over 12 h of a single 1000 mg dose of oral flucloxacillin given alone on empty stomach, with probenecid 500 mg orally on empty stomach, and with probenecid 500 mg orally and a standard breakfast. The plasma and plasma ultra filtrate standard curves of flucloxacillin covered the entire range of concentrations in patient samples. All patient samples were analysed in duplicate and the variations between duplicate analysis results were <10%. A paper arising from this clinical study is in preparation. Also, this method is currently being used in our laboratory therapeutic drug monitoring (TDM) service to monitor the free and total concentrations of flucloxacillin or cefazolin in plasma in patients on flucloxacillin or cefazolin therapy. Requests for flucloxacillin or cefazolin assays are received almost daily in our local clinical practice. The concentration ranges of patient samples were well covered by the concentration ranges of the standard curves and there were no samples with concentrations above the upper limit of quantitation. To ensure the accuracy and reproducibility of the method for patient samples, some patient samples were reanalysed. All the repeat values were ≤ 15% difference from the initial value.
Conclusions
A validated LC-MS/MS method for the determination of flucloxacillin or cefazolin in plasma and plasma ultra filtrate has been described. The method has been used in a clinical study and the TDM service, and proven to be rapid, sensitive, specific and accurate.
References
- Roberts JA, Norris R, Paterson DL, Martin JH (2012) Therapeutic drug monitoring of antimicrobials. Br J Clin Pharmacol 73: 27-36.
- Craig WA (1995) Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22: 89-96.
- Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1-10.
- Barza M, Weinstein L (1976) Pharmacokinetics of the penicillins in man. Clin Pharmacokinet 1: 297-308.
- Røder BL, Frimodt-Møller N, Espersen E, Rasmussen SN (1995) Dicloxacillin and flucloxacillin: pharmacokinetics, protein binding and serum bactericidal titers in healthy subjects after oral administration. Infection 23:107-112.
- Vella-Brincat JW, Begg EJ, Kirkpatrick CM, Zhang M, Chambers ST, et al. (2007) Protein binding of cefazolin is saturable in vivo both between and within patients. Br J Clin Pharmacol 63:753-757.
- Kunicki PK, WaÅ› J (2012) Simple HPLC method for cefazolin determination in human serum - validation and stability testing. J Chromatogr B Analyt Technol Biomed Life Sci 911: 133-139.
- Douglas A, Udy AA, Wallis SC, Jarrett P, Stuart J, et al. (2011) Plasma and tissue pharmacokinetics of cefazolin in patients undergoing elective and semielective abdominal aortic aneurysm open repair surgery. Antimicrob Agents Chemother 55: 5238-5242.
- Landersdorfer CB, Kirkpatrick CM, Kinzig-Schippers M, Bulitta JB, Holzgrabe U, et al. (2007) Population pharmacokinetics at two dose levels and pharmacodynamic profiling of flucloxacillin. Antimicrob Agents Chemother 51: 3290-3297.
- Mastovska K, Lightfield AR (2008) Streamlining methodology for the multiresidue analysis of beta-lactam antibiotics in bovine kidney using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1202: 118-123.
- Fagerquist CK, Lightfield AR, Lehotay SJ (2005) Confirmatory and quantitative analysis of beta-lactam antibiotics in bovine kidney tissue by dispersive solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Chem 77: 1473-1482.
- Huang C, Gao J, Miao L (2012) Simultaneous determination of flucloxacillin and ampicillin in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and subsequent application to a clinical study in healthy Chinese volunteers. J Pharm Biomed Anal 59: 157-161.
- Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75: 3019-3030.
- US Department of Health and Human Services Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Method Validation. Center for Drug Evaluation and Research (CDER).
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17513
- [From(publication date):
February-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12803
- PDF downloads : 4710