Research Article Open Access
Determination of Sulfur in Bio-Samples by ICP-QMS/QMS with an ORC
Yanbei Zhu*, Yuko Kitamaki, Megumi Kato, Tomoya Kinumi, Akiharu Hioki and Koichi Chiba
National Metrology Institute of Japan, National Institute of Advanced Industrial Science and Technology, Umezono, Tsukuba, Ibaraki, Japan
- *Corresponding Author:
- Yanbei Zhu
National Metrology Institute of Japan
National Institute of Advanced Industrial Science and Technology
1-1-1 Umezono, Tsukuba, Ibaraki 305-8563, Japan
Tel: 81-29-861-4130
E-mail: b-zhu@aist.go.jp
Received date: July 25, 2015; Accepted date: September 29, 2015; Published date: October 05, 2015
Citation: Zhu Y, Kitamaki Y, Kato M, Kinumi T, Hioki A, et al. (2015) Determination of Sulfur in Bio-Samples by ICP-QMS/QMS with an ORC. J Anal Bioanal Tech 6:282. doi:10.4172/2155-9872.1000282
Copyright: © 2015 Zhu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A method for determination of sulfur was investigated using an inductively coupled plasma tandem quadrupole mass spectrometer (ICP-QMS/QMS) with an octopole reaction/collision cell (ORC). It helped to achieve a lower background equivalent concentration (BEC) and a lower detection limit (DL) when 10% HNO3 with 10% ethanol was applied as the rinse solution. Sulfur measurement was carried out by shifting the measured mass from 32S+ to 32S16O+ by reaction with O2 gas. The introduction of H2 together with O2 as reaction gases provided a lower DL. The BEC and DL of 32S were 0.83 ng g-1 and 0.03 ng g-1, respectively. The validation of the present method was confirmed by determining sulfur in certified reference materials, NIST SRMs 2773, 2298, and 2299. The results of sulfur in amino-acids and protein indicate that determination of sulfur could be an effective technique for quantification of sulfur-containing amino acids and proteins.
Keywords
ICP-QMS/QMS; Sulphur; ORC; Detection limit; Certified reference material
Introduction
Sulfur is a major target element in metallomics research [1-3]. One of the reasons might be that the sulfur content in human-body is approximately 2%, while two sulfur-containing amino acids cysteine and methionine contribute to the contents of sulfur. Sulfur has four stable isotopes, i.e., 32S, 33S, 34S, 36S [4]. Due to the high abundances of 32S and 34S, respectively ca. 95% and 4%, they are usually analysed for metallomics purpose.
Inductively coupled plasma mass spectrometry (ICP-MS) is one of the most sensitive techniques for elemental measurement due to high temperature of the ICP for ionisation, relatively simple spectra than optical analysis, and wide linearity of the detector. However, the measurement of sulfur isotopes is often challenging for ICPMS with a single quadrupole mass spectrometer (i.e., ICP-QMS) because of the polyatomic spectral interferences, especially 16O2+ on 32S+.
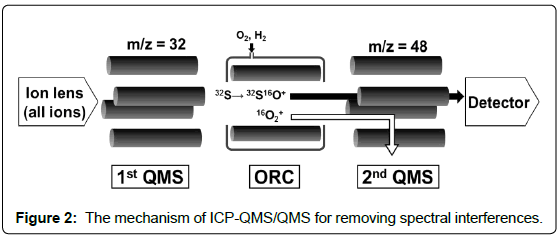
Many researchers had tried to improve the detection limits of 32S+ by ICP-QMS using O2 as the reaction cell gas [5-15] to shift 32S+ to 32S16O+ or Xe gas as the collision gas [15-18] to remove the interferences of 16O2+. High resolution (HR-) ICPMS [6,19-25] providing a resolution over 1800 could separate the spectrum of 16O2+ from that of 32S+, which can be calculated from the relative atomic masses of 16O and 32S, ca. 15.9949 and 31.9721, respectively [26]. In recent years, an alternative choice for sulfur measurement is an ICP-MS equipped with tandem quadrupole mass spectrometers (ICP-QMS/QMS) along with an octopole reaction/collision cell (ORC) [27-29]. In the measurement by ICP-QMS/QMS, the m/z of 32 was permitted to pass the first QMS and transferred to the ORC for reaction with oxygen gas, after which 32S+ was shift to 32S16O+ and measured by the second QMS at the m/z of 48.
To the best of the present authors’ knowledge, the best detection limits of 32S obtained by ICP-QMS with O2 as reaction cell gas [5] and with Xe as the collision gas [16] were 0.2 ng g-1 and 3.2 ng g-1, respectively. The best detection limits obtained by HR-ICP-MS without [19] and with [20] a desolvation unit were 0.6 ng g-1 and 0.01 ng g-1, respectively. The improvement of detection limit by using the desolvation unit could be attributed to the removal of oxygen introduction to the HR-ICP-MS, removing the tailing of 16O2 + which resulted in a relatively high background signal of 32S+ [20]. The reported detection limit [27-29] obtained by ICPQMS/ QMS was in the range of 0.33 ng g-1 to 4.3 ng g-1, which is comparable to the best performance obtained using other approaches but still higher than that obtained by HR-ICP-MS with using a desolvation unit. These facts might indicate that the background signals were not controlled sufficiently in the reports by ICP-QMS/QMS up to date. From the mechanism of spectral interference separation in the ICP-QMS/QMS with and ORC, the present authors speculated that the background signal of sulfur might be achieved at the same level as that obtained by HR-ICP-20MS with using a desolvation unit
In the present work, the authors tried to establish a measurement method for sulfur using ICP-QMS/QMS, where optimisation of the operation conditions was carried out to get a better detection limit (DL). For such purpose, the m/z of the first QMS was set to 32 permitting the pass of 32S+ and 16O2+ as the majority of the ions; H2 was additionally used as the reaction gas along with 16O2 to prevent the transfer of 16O2+ to 16O3 + in the ORC; the m/z of the second QMS was set to 48 for measuring the signal of sulfur as 32S16O+. In the present work, the precursor ions generating spectral interferences were also investigated, which may provide valuable information for ICP-QMS measurement.
The validity of the present method was confirmed by analysing three certified reference materials, NIST SRMs 2773, 2298, and 2299. The measurement of sulfur content was applied to the quantitation of two sulfur-containing amino acids, cystine and methionine, and a sulfur-containing protein.
Experimental
Instrumentation
An ICP-QMS/QMS with an ORC (Agilent 8800s, Agilent Technologies, Japan) was applied to the measurement of sulfur isotopes and the spectral interferences. The typical operating conditions are summarised in Table 1. The operating condition for each parameter was daily optimised. A microwave digestion instrument (ETHOS 1, Milestone General K.K., Kawasaki, Japan) and TFM® digestion vessels were utilised for the digestion of the certified reference material (CRM) samples. The cleaning of digestion vessels was carried out using an automatic cleaning system (TraceClean system, Milestone General K.K.) and then cleaned with the microwave irradiation whose program is identical to that used for the digestion of the samples.
| Instrument parameter | Operating condition |
|---|---|
| RF power (W) | 1600 |
| Plasma gas flow rate (L min-1) | 18 |
| Auxiliary gas flow rate (L min-1) | 1.8 |
| Carrier gas flow rate (L min-1) | 0.9 |
| Sampling depth (mm) | 8.0 |
| Cell gas (mL min-1) | O2 0.3, H2 1.0 |
| Nebulizer | Micro flow 200 |
| Selected mass at 1st QMS | 32,34 |
| Selected mass at 2nd QMS | 48,50 |
| Accumulation-time/mass (s) | 3 |
| Number of replicates | 10 |
Table 1: Typical operating conditions of ICP-QMS/QMS.
Chemicals and materials
An elemental standard solution of sulfur (as SO42-, guaranteed by the Japan Calibration Service System, JCSS) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Ultrapur® grade of HNO3 (13 mol L-1) for digestion of the samples and making sample solutions was also purchased from Kanto Chemical Co., Inc. Pure water used throughout the present study was prepared using a Millipore purification system (Element, Nihon Millipore Kogyo, Tokyo, Japan). The validity of the present method was confirmed by analysing three CRMs, i.e., NIST SRMs 2773 (bio-diesel), 2298 (high octane gasoline), and 2299 (reformulated gasoline), issued by the National Institute of Standards and Technology (Gaithersburg, USA). The present method was applied to the analysis of three CRMs for sulfur-containing compounds, i.e., two amino acids (NMIJ CRM 6025-a, L-Cystine and NMIJ CRM 6023-a, L-Methionine) and a protein (NMIJ CRM 6202-a, human serum albumin), issued by the National Metrology Institute of Japan (Tsukuba, Japan).
Microwave acid digestion
In microwave acid digestion, 0.5 mL of the sample was taken for each sub-sample and the mass was precisely measured using an electronic balance. After that, 5 mL of Ultrapur® HNO3 was added and subjected to the microwave irradiation process (ramp: 180°C for 30 min, hold: for 15 min; cool down: for 50 min; 200°C for 30 min, hold: for 15 min; cool down: for 50 min). After the microwave irradiation, pure water was added to each sample to achieve a 50 mL digested solution. At least two blank tests were carried out in each batch of acid digestion, where quantities of HNO3 equivalent to the samples were added into empty digestion vessels and those were subjected to all of the operations in the acid digestion.
Results and Discussion
Cleaning of the instrument for sulfur measurement
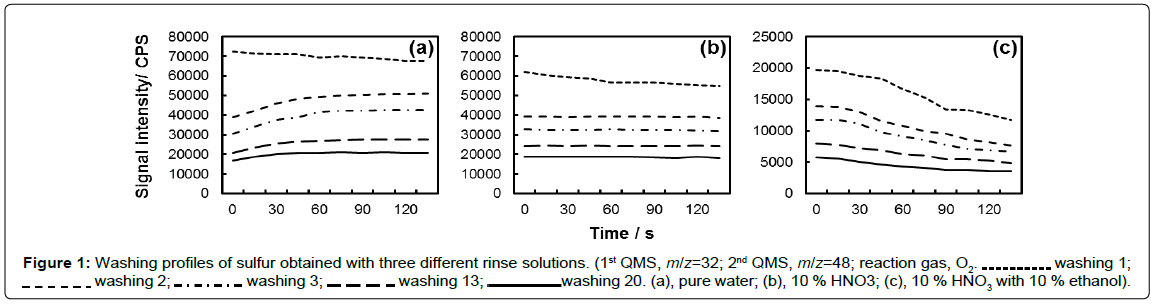
In order to achieve a lower detection limit (DL) for sulfur measurement, the instrument background should be well-controlled. In the present experiment, the instrument was repeatedly washed by introduction of three rinse solutions in turn, i.e., pure water, 10% HNO3, and 10% HNO3 with 10% ethanol. The washing was repeated until the background equivalent concentration (BEC) was lower than 2 ng g-1.
The signal intensities of sulfur obtained with different rinse solution are plotted in Figure 1, where the washing procedure was carried out in the order of “pure water washing 1→10% HNO3 washing 1→10% HNO3 with 10% ethanol washing 1→pure water washing 2→”.
For each set of washing procedure, it is noted that the signal intensity of sulfur obtained by 10% HNO3 with 10% ethanol was much lower than that by 10% HNO3, while the later one was slightly lower than that by pure water. These facts could be attributed to the matrix effect caused by ethanol and HNO3, respectively. It can be seen that sulfur signal intensity increased in each washing with pure water (except for washing 1) from 0s to 60s, which could be attributed to the matrix transfer from 10% HNO3 with 10% ethanol to pure water.
In Figure 1, it is notable that the signal intensity of sulfur obtained for “washing 1” of each rinse solution decreased to some extent with the increase of washing time. However, the signal intensity of sulfur after “washing 1” was respectively stable for pure water and 10% HNO3 in each washing, which indicate that the residual sulfur was difficult to remove by washing with pure water or 10% HNO3. By contrast, the signal intensity of sulfur apparently decreased in each washing of 10% HNO3 with 10% ethanol, indicating the effective removal of residual sulfur in the instrument.
Major precursor ions for the spectral interferences of sulfur isotopes
Figure 2 shows the mechanism of the ICP-QMS/QMS for removing the spectral interferences with 32S+ measurement. Oxygen was solely used as the reaction gas in the researches using ICP-QMS/ QMS published up to date [26-28]. In the present research, H2 was used together with O2 as the reaction gases.
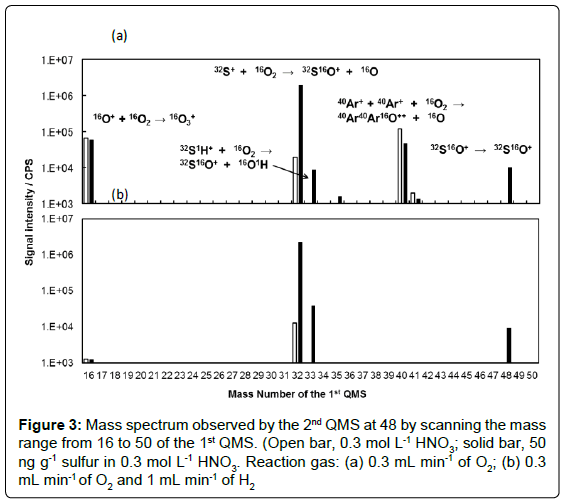
The 1st QMS and the 2nd QMS could be individually controlled: the instrument permits the mass scan by both QMS’s. In the present work, mass scan was carried out for the 1st QMS followed by the 2nd QMS filtering at the m/z 48 for the measurement of 32S16O+. The flow rate of O2 as reaction gas was optimised in advance. As the result, 0.3 mL min-1 of O2 was found to be the optimum and applied in the following experiments. Mass scan of the 1st QMS was carried out for the mass range from 2 to 260, using a pair of blank (0.3 mol L-1 HNO3) and standard (50 ng g-1 sulfur in 0.3 mol L-1 HNO3) solutions. Since the signal intensities for the mass range lower than 15 and those for the mass range higher than 51 were very low and negligible, the results for the mass range from 16 to 50 are shown in Figure 3 for a clearer comparison.
The results in Figure 3(a) were obtained using O2 as the reaction gas. Very high signals for m/z of 48 at the 2nd QMS were obtained when the m/z of the 1st QMS were 16, 32, 33, 40, and 48. The precursor ions at m/z of 16, 32, 33, and 48 could be attributed to the ions of 16O+, 32S+ (with 16O2+), 32S1H+, and 32S16O+, respectively. The precursor ion at the m/z of 40 has not been reported up to date. One of the possibilities of this precursor ion might be 80Kr++, which could be the impurities in Ar gas used in ICP-QMS/QMS. A further experiment was carried out by setting the m/z of the 1st QMS and the 2nd QMS as 42 and 50, respectively. Signal of 84Kr16O++ was not observed. This result might indicate that either 84Kr++ was not present in the ions passing through the 1st QMS or 84Kr++ was not react with 16O to form 84Kr16O++ ion. Therefore, it could be concluded that the precursor ion at the m/z of 40 in Figure 3(a) was not 80Kr++. The present authors speculate that the precursor ion at the m/z of 40 was 40Ar+ (maybe with minute 40Ar2++), which might transfer to 40Ar2 16O++ and be observed at the m/z of 48 at the 2nd QMS. The authors also carried out a measurement by an HR-ICP-MS to check whether the 40Ar2 16O++ ion was generated in the plasma. The 40Ar2 16O++ ion was not found in the HR-ICP-MS measurement. This fact indicated that the 40Ar216O++ ion was not generated in the plasma but generated in the ORC by the reaction with 16O2. Another possibility of 40Ar+ related interferences might be 16O3+ produced by asymmetric charge transfer. It should be noted that the ionization energies of Ar and O are 15.759 eV and 13.61806 eV, respectively [30]. Therefore, charge transfer from Ar+ to O+ is an endothermic reaction. Such kind of reactions is possible to happen in the ORC with enough collision energy [31].
Furthermore, it can be seen from Figure 3(b) that the precursor ions at the m/z of 16 and 40 were removed by using H2 as the reaction gas together with O2. The signal intensity obtained for the m/z of 32 slightly decreased when H2 was additionally applied as the reaction gas.
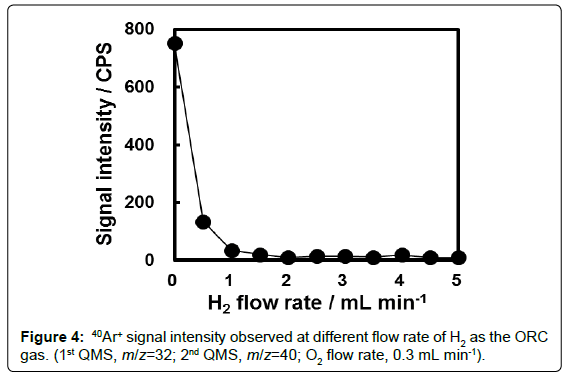
In the measurement of ICP-QMS/QMS with an ORC, the 1st QMS could be set to permit the pass of ions with m/z of 32 and contributes to the removal of 16O+ and 40Ar+ generated in the plasma. However, 40Ar+ signal could still be observed at the 2nd QMS, the reason for which is not clear up to now. The effect of H2 on removal of 40Ar+ was investigated and the results are plotted in Figure 4. As can be seen, apparent 40Ar+ signal was observed when H2 was not introduced as reaction gas.Introduction of 1 mL min-1 H2 as the reaction gas was enough to remove the 40Ar+ signal. Therefore, H2 was used as the reaction gas along with O2 in the present experiment to ensure the removal of 40Ar+ related ions.
Optimisation of H2 flow rate for the measurement of sulfur
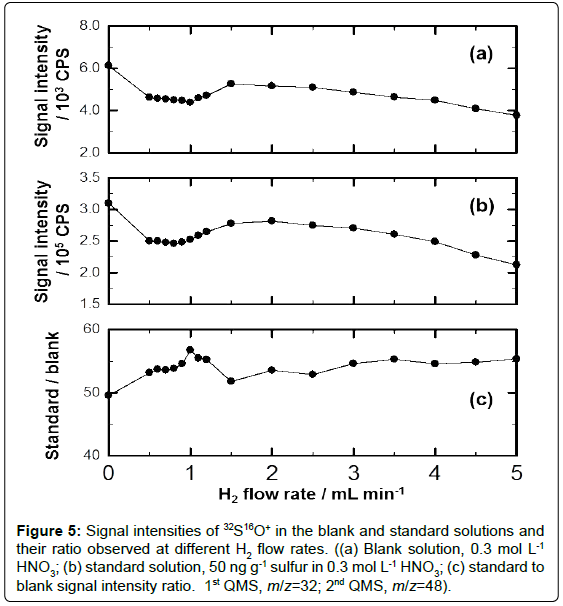
The signal intensities of sulfur in a pair of blank and standard solutions were investigated at different H2 flow rates from 0 to 5 mL min-1. The results of 32S in the blank and standard solutions are plotted in Figure 5(a) and 5(b), while the (standard/blank) signal intensity ratio (S/B) is plotted in Figure 5(c).
Figure 5: Signal intensities of 32S16O+ in the blank and standard solutions and their ratio observed at different H2 flow rates. ((a) Blank solution, 0.3 mol L-1 HNO3; (b) standard solution, 50 ng g-1 sulfur in 0.3 mol L-1 HNO3; (c) standard to blank signal intensity ratio. 1st QMS, m/z=32; 2nd QMS, m/z=48).
It can be seen from Figure 5(a) to 5(c) that the signal intensities of 32S16O+ in both the blank solution and the standard one decreased gradually when the H2 flow rate increased from 0 to 1.0 mL min-1. By contrast, the S/B increased gradually and the maximum ratio was obtained at H2 flow rate of 1.0 mL min-1. These results might indicate that the application of H2 as the reaction gas helped to supress the mass shift from 16O2+ to 16O3+ or help to suppress the formation of other spectral interferences induced by 16O2+. The signal intensities of 32S16O+ in the blank solution and the standard solution increased to some extent when the H2 flow rate increased from 1.0 mL min-1 to 2.0 mL min-1, while the S/B decreased. When the H2 flow rate exceeded 2.0 mL min-1, the signal intensities of 32S16O+ in the blank solution and the standard solution decreased gradually. At the same time, the S/B increased slightly when the H2 flow rate increased from 2.0 mL min-1 to 3.0 mL min-1, while the S/B was almost stable and independent of the H2 flow rate when it was over 3.0 mL min-1.
As it can be seen in Figure 5(c), the maximum S/B was achieved at the H2 flow rate of 1.0 mL min-1. This condition provided the best detection limit for sulfur measurement and was applied to the following experiments. The DL(3σ) and BEC of 32S were 0.03 ng g-1 and 0.83 ng g-1, respectively. The calibration curve obtained with 0 ng g-1, 50 ng g-1, 100 ng g-1, and 150 ng g-1 sulfur in 0.3 mol L-1 HNO3 gave a correlation factor with R2=0.9999.
A comparison of the present DL and BEC with those reported with various techniques is summarised in Table 2. As can be seen in Table 2, the present DL is comparable with the best performance reported in Reference [20] obtained by HR-ICP-MS with the assist of a desolvation unit to remove the 16O2+ interference. The present method provides better DL and BEC than other techniques without using a desolvation unit. The reason could be attributed to the effective removal of spectral interferences by the QMS/QMS system with the ORC using H2 and O2 as the reaction gases. The 1st QMS selecting m/z of 32 permitted the pass of 16O2+ and 32S+ while blocked 16O+ and reduced the possibility of transference from 16O+ to 16O3+ in the ORC. The introduction of H2 as the reaction gas might further reduced the possibility of transference from 16O2+ to 16O3+ in the ORC and/or the formation of other spectral interferences induced by 16O2+. It is noted that the DL in the present work was much lower than those reported in References [27-29] based on the ICP-QMS/QMS technique. These results might indicate that the measurements in References [27-29] were carried out with a relatively higher blank signal, i.e., a higher BEC, as mentioned in Reference [27].
| Reference Number | Instrument | Technique | Analyte | DL/ngg-1 | BEC/ngg-1 |
|---|---|---|---|---|---|
| [5] | Elan DRC | O2 reaction | 32S16O+ | 0.2 | 4.8 |
| [6] | Elan DRC-II | O2 reaction | 32S16O+ | 4.3 | NAa |
| [7] | HP 4500 | O2 reaction | 32S16O+ | 35 | NAa |
| [9] | Thermo X7 | O2 reaction | 32S16O+ | 11 | NAa |
| [10] | Elan DRC-2 | O2 reaction | 32S16O+ | 30 | NAa |
| [12] | Agilent 7500ce | O2 reaction | 32S16O+ | 3.4 | NAa |
| [14] | Agilent 7500a | O2 reaction | 32S16O+ | 13 | 84 |
| [15] | Agilent 7500ce | O2 reaction | 32S16O+ | 0.7 | NAa |
| [24] | Elan DRC-plus | O2 reaction | 32S16O+ | 10 | NAa |
| [15] | Agilent 7500ce | Xe collision | 32S+ | 10.9 | NAa |
| [16] | Agilent 7500c | Xe collision | 32S+ | 3.2 | NAa |
| [17] | Agilent 7500ce | Xe collision | 32S+ | 45 | NAa |
| [18] | Micromass | He, H2, Xe reaction/collision | 32S+ | 48 | NAa |
| [6] | Element | HR (4500) | 32S+ | 14 | NAa |
| [19] | Elemental Axiom | HR (6000) | 32S+ | 0.6 | NAa |
| [20] | Element | HR (4000), desolvation | 32S+ | 0.01 | NAa |
| [24] | Element XR | HR (4000) | 32S+ | 1 | NAa |
| [25] | Element XR | HR (4500) | 32S+ | 0.7 | NAa |
| [27] | Agilent 8800 | QMS/QMS, O2 reaction | 32S16O+ | 1.2 | NAa |
| [28] | Agilent 8800 | QMS/QMS, O2 reaction | 32S16O+ | 0.33 | NAa |
| [29] | Agilent 8800 | QMS/QMS, O2 reaction | 32S16O+ | 4 | NAa |
| Present work | Agilent 8800 | QMS/QMS, H2, O2 reaction | 32S16O+ | 0.03 | 0.83 |
Table 2: The DLs and BECs for measuring 32S by various techniques. a: Not available.
Validity of the method for measurement of sulfur
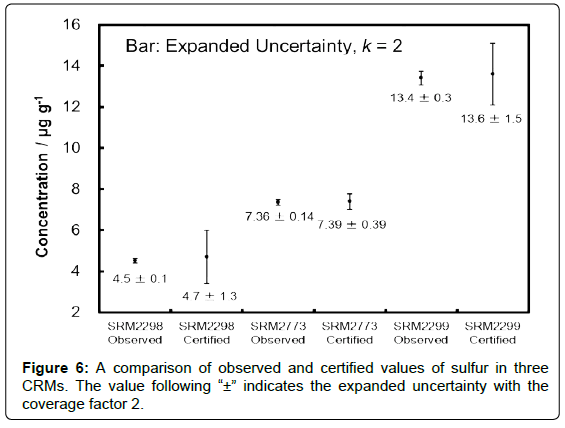
In order to confirm the validity of the present method, each content of sulfur in three CRMs, NIST SRMs 2773 (bio-diesel), 2298 (high octane gasoline), and 2299 (reformulated gasoline), was measured after acid digestion.
The observed values of sulfur contents in these CRMs are plotted in Figure 6 in comparison to the certified values, along with the expanded uncertainty of each value. It can be seen that the observed value for each CRM agreed with its certified one considering the uncertainty. Because the CRMs were analysed after microwave acid digestion (sample 0.5 g, solution 50 g), the concentration of sulfur in the sample solution of NIST SRM 2298 was approximately 45 ng g-1. These results showed that the present method is valid for measuring sulfur at 50 ng g-1 with the expanded uncertainty of approximately 2%.
Quantitation of sulphur-containing amino acids and sulfur containing proteins by measuring sulfur contents
The present method was applied to the quantitation of sulfurcontaining amino acids (NMIJ CRM 6025-a, L-Cystine and NMIJ CRM 6023-a, L-Methionine) and a protein (NMIJ CRM 6202- a, human serum albumin). The amino acid samples were dissolved in 0.1 mol L-1 HCl. The protein sample was digested with Ultrapur® HNO3 in the same way as that used for the NIST SRMs. The samples were further diluted to obtain the analysis solutions containing approximately 50 ng g-1 of S.
The results are summarized in Table 3 along with the certified values. It can be seen that the observed values were in agreement with the certified values considering the measurement uncertainty. This fact indicates that the measurement of S content could be applied to the quantitation of sulfur-containing compounds.
| Sample code | Observed valuea | Certified valuea | Unit |
|---|---|---|---|
| NMIJ CRM 6025-a | 0.982±0.021 | 0.998±0.003 | kg kg-1 |
| NMIJ CRM 6023-a | 0.991±0.022 | 0.999±0.002 | kg kg-1 |
| NMIJ CRM 6202-a | 1130±23 | 1098±31 | μmol kg-1 |
Table 3: Observed and certified values of sulfur-containing amino acids and protein. a: The value following “±” indicates the expanded uncertainty with the coverage factor 2.
Conclusion
ICP-QMS/QMS with an ORC system was investigated to establish a method for the measurement of sulfur in bio-samples. Washing procedure using 10% HNO3 with 10% ethanol helped to obtain a lower BEC and a lower DL for sulfur measurement. The interference of 16O2+ with the measurement of 32S+ could be effectively removed by setting the m/z of the 1st QMS as 32 to permit the pass of 16O2+ and 32S+, while the 32S+ reacted with O2 gas and transferred to 32S16O+ in the ORC and then measured at the m/z of 48 in the 2nd QMS. The introduction of H2 gas in the ORC helped to remove 40Ar+ related interferences with the measurement of 32S+. The DL and BEC of 32S in the present work were 0.03 ng g-1 and 0.83 ng g-1, respectively. The DL was comparable with that obtained by an HR-ICP-MS with the assist of a desolvation unit and better than those obtained with other techniques. This result could be attributed to the fact that the measurement of 32S+ in the present work was shifted to 32S16O+ and the transfer from 16O2+ to 16O3 + was effectively suppressed by the introduction of H2; i.e., the measurement by ICP-QMS/QMS with an ORC did not suffer from 16O2+ signal tailing as that observed in HR-ICP-MS. The validity of the present method was confirmed by analysing sulfur in three NIST SRMs, for which the observed values agreed with their certified values. The results of analysis of sulfur-containing amino acids and protein showed that the measurement of sulfur content could be applied to the quantitation of these compounds.
The analysis in the present work was designed for the quantitation of total sulfur in the sample, which is effective for the quantitation of sulfur-containing compounds of high purity for metrological purpose. Hyphenation of ICP-QMS/QMS with separation techniques such as high pressure liquid chromatography could provide further information for the species of sulfur in the sample.
Acknowledgements
The present research was partly supported by the International Cooperation Project for Research and Standardisation of Clean Energy Technologies (FY2013- 2014). The authors express their sincere gratitude to Drs. Stephen E. Long and John L. Molloy of NIST for their cooperation in the project. The authors also appreciate the technical support for using the ICP-QMS/QMS by Mr. Yasuyuki Shikamori at Agilent Technologies Japan.
References
- Rappel C, Schaumlöffel D (2008) The role of sulfur and sulfur isotope dilution analysis in quantitative protein analysis. Anal Bioanal Chem 390: 605-615.
- Szpunar J (2005) Advances in analytical methodology for bioinorganic speciation analysis: metallomics, metalloproteomics and heteroatom-tagged proteomics and metabolomics. Analyst 130: 442-465.
- Prange A, Profrock D (2008) Chemical labels and natural element tags for the quantitative analysis of bio-molecules. J Anal At Spectrom 23: 432-459.
- Berglund M, Wieser ME (2011) Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure Appl Chem 83: 397-410.
- Bandura DR, Baranov VI, Tanner SD (2002) Detection of ultratrace phosphorus and sulfur by quadrupole ICPMS with dynamic reaction cell. Anal Chem 74: 1497-1502.
- Hann S, Koellensperger G, Obinger C, Furtmuller PG, Stingeder G (2004) SEC-ICP-DRCMS and SEC-ICP-SFMS for determination of metal-sulfur ratios in metalloproteins. J Anal At Spectrom 19: 74-79.
- Divjak B, Goessler W (1999) Ion chromatographic separation of sulfur-containing inorganic anions with an ICP-MS as element-specific detector. J Chromatogr A 844: 161-169.
- El Balkhi S, Poupon J, Trocello JM, Massicot F, Woimant F, et al. (2010) Human plasma copper proteins speciation by size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. Solutions for columns calibration by sulfur detection. Anal Chem 82: 6904-6910.
- Wang M, Feng W-Y, Wang HJ, Zhang Y, Li J, et al. (2008) Analysis of mercury-containing protein fractions in brain cytosol of the maternal and infant rats after exposure to a low-dose of methylmercury by SEC coupled to isotope dilution ICP-MS. J Anal At Spectrom 23: 1112-1116.
- Hann S, Falta T, Boeck K, Sulyok M, Koellensperger G (2010) On-line fast column switching SEC × IC separation combined with ICP-MS detection for mapping metallodrug–biomolecule interaction. J Anal At Spectrom 25: 861-866.
- Rampler E, Dalik T, Stingeder G, Hann S, Doellensperger G (2012) Sulfur containing amino acids – challenge of accurate quantification. J Anal At Spectrom 27: 1018-1023.
- Persson DP, Hansen TH, Laursen KH, Schjoerring JK, Husted S (2009) Simultaneous iron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics 1: 418-426.
- da Silva MA, Arruda MA (2013) Laser ablation (imaging) for mapping and determining Se and S in sunflower leaves. Metallomics 5: 62-67.
- Ciavardelli D, Sacchetta P, Federici G, Di Ilio C, Urbani A (2010) Protein phosphorylation stoichiometry by simultaneous ICP-QMS determination of phosphorus and sulfur oxide ions: a multivariate optimization of plasma operating conditions. Talanta 80: 1513-1525.
- Suzuki Y, Nobusawa A, Furuta N (2014) Quantification of proteins by measuring the sulfur content of their constituent peptides by means of nano HPLC-ICPMS. Anal Sci 30: 551-559.
- Pröfrock D, Leonhard P, Prange A (2003) Determination of sulfur and selected trace elements in metallothionein-like proteins using capillary electrophoresis hyphenated to inductively coupled plasma mass spectrometry with an octopole reaction cell. Anal Bioanal Chem 377: 132-139.
- Schaumloffel D, Giusti P, Preud'Homme H, Szpunar J, Lobinski R (2007) Precolumn isotope dilution analysis in nanoHPLC-ICPMS for absolute quantification of sulfur-containing peptides. Anal Chem 79: 2859-2868.
- Mason PRD, Kaspers K, van Bergen MJ (1999) Determination of sulfur isotope ratios and concentrations in water samples using ICP-MS incorporating hexapole ion optics. J Anal At Spectrom 14: 1067-1074.
- Evans EH, Wolff JC, Eckers C (2001) Sulfur-specific detection of impurities in cimetidine drug substance using liquid chromatography coupled to high resolution inductively coupled plasma mass spectrometry and electrospray mass spectrometry. Anal Chem 73: 4722-4728.
- Prohaska T, Latkoczy C, Stingeder G (1999) Precise sulfur isotope ratio measurements in trace concentration of sulfur by inductively coupled plasma double focusing sector field mass spectrometry. J Anal At Spectrom 14: 1501-1504.
- Riondato J, Vanhaecke F, Moens L, Dams R (1997) Determination of Trace and Ultra trace Elements in Human Serum With a Double Focusing Magnetic Sector Inductively Coupled Plasma Mass Spectrometer. J Anal At Spectrom 12: 933-937.
- Wildner H (1998) Application of inductively coupled plasma sector field mass spectrometry for the fast and sensitive determination and isotope ratio measurement of non-metals in high-purity process chemicals. J Anal At Spectrom 13: 573-578.
- Becker JS, Boulyga SF, Becker JS, Pickhardt C, Damoc E, et al.(2003) Int J Mass Spectrom 228: 985-997.
- Wolf KD, Balcaen L, Walle EVD, Cuyckens F, Vanhaecke F (2010) A comparison between HPLC-dynamic reaction cell-ICP-MS and HPLC-sector field-ICP-MS for the detection of glutathione-trapped reactive drug metabolites using clozapine as a model compound. J Anal At Spectrom 25: 419-425.
- Amais RS, Long SE, Nóbrega JA, Christopher SJ (2014) Determination of trace sulfur in biodiesel and diesel standard reference materials by isotope dilution sector field inductively coupled plasma mass spectrometry. Anal Chim Acta 806: 91-96.
- Coplen TB, Bohlke JK, De Bievre P, Ding T, Holden NE, et al. (2002) Isotope-abundance variations of selected elements (IUPAC Technical Report). Pure Appl Chem 74: 1987-2017.
- Diez Fernández S, Sugishama N, Ruiz Encinar J, Sanz-Medel A (2012) Triple quad ICPMS (ICPQQQ) as a new tool for absolute quantitative proteomics and phosphoproteomics. Anal Chem 84: 5851-5857.
- Amais RS, Amaral CDB, Fialho LL, Schiavo D, Nobrega JA (2014) Determination of P, S and Si in biodiesel, diesel and lubricating oil using ICP-MS/MS. Anal Methods 6: 4516-4520.
- Balcaen L, Woods G, Resano M, Vanhaecke F (2013) Accurate determination of S in organic matrices using isotope dilution ICP-MS/MS. J Anal At Spectrom 28: 33-39.
- Agilent Technologies, Technical Overview, Understanding oxygen reaction mode in ICP-MS/MS.
- Boting K, Treu S, Leonhard P, Hei C, Bings NH (2014) First experimental proof of asymmetric charge transfer in ICP-MS/MS (ICP-QQQ-MS) through isotopically enriched oxygen as cell gas. J Anal At Spectrom 29: 578-582.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15493
- [From(publication date):
December-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10790
- PDF downloads : 4703

 washing 1;
washing 1; washing 2;
washing 2; washing 3;
washing 3; washing 13;
washing 13; washing 20. (a), pure water; (b), 10 % HNO3; (c), 10 % HNO3 with 10 % ethanol).
washing 20. (a), pure water; (b), 10 % HNO3; (c), 10 % HNO3 with 10 % ethanol).