Research Article Open Access
Determination of Specific Activity and Tellurium (IV) Concentration in Non-Carrier Added Iodine-124 Solutions, Via UV-Vis Spectroscopy
Atilio I Anzellotti*, Robert Ylimaki and Alexander Yordanov
IBA Molecular, Virginia Commonwealth University, Richmond, USA
- *Corresponding Author:
- Atilio I. Anzellotti
IBA Molecular, Virginia Commonwealth
University, Richmond, VA 23298, USA
Tel: 8652061839
E-mail: aanzellotti@abt-mi.com
Received Date: December 23, 2015; Accepted Date: January 19, 2016; Published Date: January 26, 2016
Citation: Anzellotti AI, Ylimaki R, Yordanov A (2016) Determination of Specific Activity and Tellurium (IV) Concentration in Non-Carrier Added Iodine-124 Solutions, Via UV-Vis Spectroscopy. J Anal Bioanal Tech 7:298. doi:10.4172/2155-9872.1000298
Copyright: © 2016 Anzellotti AI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In this work, two fast and highly sensitive analytical methods, based on UV-VIS spectroscopy, were adapted for its use on the determination of specific activity (SA) and Tellurium (IV) concentration, in 124I- solutions produced by the 124Te(p,n)124I reaction. The solutions analyzed showed high specific activity (209 - 216 Ci/mg) and Te(IV) contents below the regulatory acceptable limit (1 ppm).
Keywords
Iodine-124; Tellurium (IV); HPLC; UV-Vis spectroscopy; Specific activity
Introduction
Positron Emission Tomography (PET) is a highly sensitive imaging modality that provides functional information about pathologically relevant processes. The procedure generally involves the injection of a positron-emitting radiotracer and further detection/image reconstruction of the gamma rays produced upon electron-positron annihilation [1,2]. Currently the most used radionuclides for PET applications have relative short half-lives, for instance 11C (20.4 min half-life), 13N (9.97 min) and 18F (110 min). The use of radiotracers with longer half-lives involves a range of potential benefits, such as an increased time available for synthesis and quality control, imaging of long metabolic/physiologic processes, etc. [3,4].
Iodine-124 (124I-) is an attractive isotope of iodine due to its complex radioactive decay scheme and convenient half-life (4.18 d). There are several modes for the production of Iodine-124, but the nuclear reaction 124Te(p,n)124I offers the highest purity (with only few other radionuclidic impurities, <0.1%) and also the most convenient one, since the maximum cross-section for the reaction is below 14 MeV placing it within the capacity of most available cyclotrons [5,6].
With the increasing use of positron emission tomography (PET) in nuclear medicine, medical oncology, pharmacokinetics and drug metabolism, 124I-labeled radiopharmaceuticals could be most useful for PET imaging [7-9]. Furthermore, the 4.18 d half-life would permit their use in PET facilities far away from the radionuclide production centers (satellite concept). Sodium [124I] iodide is already reportedly used for diagnosis and treatment planning in thyroid disease [10,11]. In addition to the growing number of [124I]-labeled small molecules studied in recent years, notably [124I]m-iodobenzylguanidine ([124I]MIBG) [12] and [124I]2’-fluoro-2’-deoxy-5’-iodo-1β-D-arabinofuranosyl-uracil ([124I]FIAU) [13]. There is also a strong interest in the high selectivity/ sensitivity features from [124I]-labeled antibodies [14,15]. Limited availability of this radionuclide so far has been a hindrance to its wider development and clinical use, for example its commercially available from just a couple of producers worldwide (IBA Molecular, Perkin Elmer, etc.).
There are two key quality attributes that need to be determined in 124I- solutions to be used in radiotracer synthesis:
1) Specific Activity (SA), it is defined as the radioactivity per unit mass of a radionuclide or a radiotracer. In practice, the radioactivity and mass of a sample are determined by different methods in order to get the final SA expressed as a ratio, where the radioactivity is expressed in Curies (Ci) or Becquerels (Bq), and the mass can be expressed in grams or moles.
2) Amount of tellurium present in the form Te(IV), potentially coming from the staring material (enriched Tellurium oxide), it can potentially interfere with labelling reactions and also represents a chemical impurity (heavy metal), so it needs to be taken into account in an eventual clinical application.
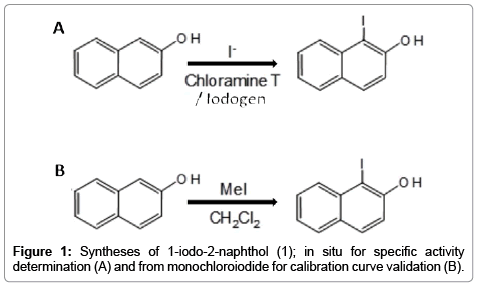
In our case, the SA is obtained from the radioactive measurement of 124I- using a dose calibrator or another appropriate instrument; however the measurement of the often small amount of matter (nonradioactive iodine, ng to fg range) requires very sensitive methods of detection. For this purpose spectrophotometric methods are preferred due to its simplicity and low-cost, but the extinction coefficient for the analyte of interest (I-) is not appropriate for the working range intended and therefore the sensitivity needs to be increased. To this end we use a derivatization process in which the iodine is reacted in-situ with 2-naphthol or β-naphthol using either Chloramine T (C-T) or Iodogen as oxidation reagents. The resulting product 1-iodo-2-naphthol (Figure 1A) has a very large extinction coefficient, thanks to the 2-naphthol moiety and since the reaction is quantitative, it can be used to measure the concentration of iodine in an indirect mode.
Another important characteristic of 124I- solutions to be used as raw material in radiotracer development would be a minimal Te(IV) content, due to chemical and biological reasons (vide supra). We adapted a highly sensitive UV-VIS method to evaluate the Te(IV) levels in 124I- solutions by the formation of an association complex with sodium tungstate in the presence of poly(vinyl alcohol) [16].
Experimental
Instruments
A Waters 515 HPLC system equipped with an Alltech 460 UV-vis detector and radioactivity detector (Bioscan, model FC-3300), was used for determination of specific activity in 124I- batches. The column used was an Alltech lichrosorb RP-18 5 μ (length of 250 mm, ID 4.6 mm). For Te(IV) determination was used a Genesys 10 UV-VIS spectrometer (Thermo Spectronic) with multi-wavelength capability, the wavelength of interest was set to 580 nm.
Materials and reagents
All solvents and reagents (highest purity grade) were purchased from Sigma-Aldrich and used without further purification. 1-iodo- 2-naphthol (used for the calibration curve) was synthesized from 2-naphthol and iodine monochloride in dichlorometane according to Figure 1B. The solvent was evaporated and the product purified by gradient elution flash chromatography (hexane/acetone). The final characterization was done by Elemental analysis (C,H,N) and 1H-NMR spectroscopy, for the latter observing shifts between 0.10-0.14 ppm compared to free 2-naphthol; solvent residual peak D2O at 4.69 ppm was taken as reference: 7.92 (1H, d), 7.74 (1H, d), 7.73 (1H, d), 7.54 (1H, d), 7.37 (1H, d) and 7.25 (1H, d). HPLC analysis revealed that the product was 98% pure and eluted in a different retention time compared to the starting material. Tellurium dioxide 99.9995% (Sigma Aldrich, part # 435902) was used in the calibration curve for Te(IV) determination.
124I- solution batches were prepared by the 124Te(p,n)124I nuclear reaction using the COSTIS Compact Solid Target Irradiation System (IBA S.A., Louvain-La-Neuve, Belgium). 124I- was separated from the target using the Reetz GmBH (Berlin, Germany) quartz thermochromatographic furnace for radio-iodine recovery from solid targets [16]. Typically 5 μL of the concentrated solution are diluted to 50 μL with sodium hydroxide 0.02M, this volume is enough to perform SA and Te(IV) determination in duplicate (if needed), since only 20 μL are needed for SA determination and 5 μL for Te(IV) determination.
SA and Te(IV) determination were evaluated under the international guidelines for method validation [17,18]. Data analysis suite from Microsoft Excel (Microsoft office professional plus 2010, ver. 14.0.71635000 32 bit) was used to obtain calibration data including LOQ and LOD values. Resolution (Rs) between peaks was calculated according to the following formula:
Rs=1.18[(tRA-tRB)/(WA1/2h+WB1/2h)],
Where, Rs=resolution
t=elution time for species A or B
W=width of the peak at half height for A or B
Specific activity determination
Solutions for calibration curve using the standard 1-iodo- 2-naphthol were prepared by dilution of a 1 mg/mL solution in methanol/water mixture 1:1. Blank injections were performed between data points to account for residual 1-iodo-2-naphthol in the column. Injection volume was 20 μL, typical volume and radioactivity in a sample was 50 μL and 5.0-1.0 mCi, respectively.
Linearity was evaluated based on the linear regression coefficient value (R2 ≥ 0.99) obtained for average data points performed along seven different 124I- values (i.e., 500, 250, 150, 100, 50, 20 and 3 ng, for a 500 to 3 ng working range). Every data point was performed in triplicate. Repeatability was evaluated through relative standard deviation (RSD) calculations at three different 124I- values (high, mid and low section of the working range, i.e., 500, 150 and 3 ng). Every data point was performed six times LOQ and LOD were calculated based on the residual standard deviation (σ) and slope (S) of the calibration curve obtained for the linearity section.
Mobile phase used was methanol 70% in water and the flow was adjusted to 1 ml/min. UV-detector was set at 254 nm. System suitability test for the HPLC system in reverse-phase conditions was performed at the beginning of each day using a mixture of different ketones (Waters Co; part # WAT042887). A criteria of resolution (Rs=4.0) was established for the propiophenone and butyrophenone peaks using the same method for SA. 1 was produced in situ using 10 μL of a chloramine-T solution (1 mg/mL) and 10 μL of concentrated trifluoroacetic acid, or alternatively by addition of 25 μL of Iodogen solution (0.5 mg/mL) in ethanol, both in the presence of excess 2-naphthol. Dilution was accounted for in the iodine concentration calculation.
Determination of Te(IV) content
This method was scale-down from the literature in order to work with 4-5 μL aliquots with an activity <0.5 mCi, the samples were diluted up to 1.5 mL prior to measurement giving concentrations in the range 0.5-4.0 ng/mL. Briefly to 500 μL of the diluted sample in water it was added 120 μL of Na2WO4 solution (1 μg/mL in sulfuric acid 2M); in addition to 60 μL of poly(vynil alcohol) solution (1 mg/mL). 120 μL of nile blue solution (0.5 mg/mL) were added within 5 minutes of measurement. Disposable cuvettes with 100 L capacity on the path length were used for UV-VIS measurement, and fresh solutions for the calibration curve were prepared before measuring each new batch of 124I-. Absorption was measured at 580 nm.
Results and Discussion
Specific activity determination
124I- solutions prepared by the 124Te(p,n)124I nuclear reaction are considered non-carrier added (nca), in principle this means that no iodine was added to the matrix and we could achieve the theoretical value for maximum specific activity in iodine, which is 251.6 Ci/mg or 31,200 Ci/mmol [19]. In practice this value is never achieved but it is useful to establish a limit for the detection of the method. Given the conditions of the method and the amount of sample tested the minimum amount of iodine that can be found is in the range of 116 to 23 ng of 124I-.
We adapted an analytical approach that has been used for SA determination in solutions of 123I- and 131I-, and modified the procedure to customize the quality control in non-carrier added 124I- [20]. In order to assess the potential of this derivatization method for 124I- solutions, the reference compound 1-iodo-2-naphthol (1) was first prepared and characterized by 1H-NMR spectroscopy and HPLC. The linearity, accuracy and repeatability of the method was tested using solutions in the concentration range of 22.5-0.15 μg/mL or actual 450 to 3.0 ng analyzed. From the results obtained in Table 1 it can be seen that the method was linear in the tested range, with a limit of quantification (LOQ) of 9.3 ng and limit of detection (LOD) of 2.6 ng. The method was linear, accurate (recovery % 90-110, n=9) and repeatable (Relative Standard Deviation <5%, n=18) in the working range, which was appropriate for the iodine concentrations expected.
| Method | Slope | Intercept | Correlation coefficient | Working range |
|---|---|---|---|---|
| 1 | 13,154 | 12,205 | 0.9997 | 3-450 ng |
| C-T | 14,797 | 2,295 | 0.999 | 3-500 ng |
| IG | 13,572 | 16,862 | 0.993 | 3-500 ng |
Table 1: Comparison between the linearity parameters found in the different SA determination methods. 1-iodo-2-naphthol (1), Chloramine-T (C-T) and Iodogen® (IG).
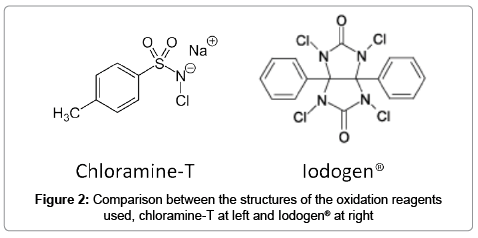
After the method was validated with the compound 1, additional calibration curves were obtained in which 1 was produced in situ via the derivatization reaction of 2-naphthol, from known amounts of potassium iodine and either oxidation agent chloramine-T (C-T) or Iodogen (IG) (Figure 2). Usually the latter is considered milder since it does not need the presence of strong acids such as trifluoroacetic acid. A comparison between the linearity values and working ranges for all the calibration curves found is presented in Table 1. It can be observed that all three methods are equivalent, in terms of response obtained vs. concentration of iodine, independently of compound 1 being prepared in situ.
Optimization for the derivatization reaction in the SA method was made for the following variables: time of injection after mixing (2 to 15 min), amount of oxidation agent used (5 to 25 μg, total mass) and temperature (25°C to 45°C). Iodine was introduced as potassium iodine. The derivatization reaction to produce 1 in situ was fast, the time was set to 5 minutes after mixing, for both oxidation agents, by comparing the peak areas obtained to the reference compound 1. In the case of chloramine-T the time for injection mixing was important since it was observed that the peak for 1 was not stable with time, eventually decreasing in favor of the specie 1-chloro-2-naphthol (2) which is also formed in excess given the concentration of C-T and presence of trifluoroacetic acid (see structure of both oxidation agents used in Figure 2). No significant change in the peak for 1 was observed up to 15 minutes after mixing when IG was used as an oxidant, and negligible amounts of 2 were also noted, possibly due to the milder nature of this reagent and the absence of trifluoroacetic acid in the matrix mixture. Finally temperature was found to not modify results obtained in the range tested, 25°C to 45°C, as a default 25°C was chosen due to convenience. Blanks for both methods without potassium iodide as a source of iodide did not produce any peak in the window for compound 1.
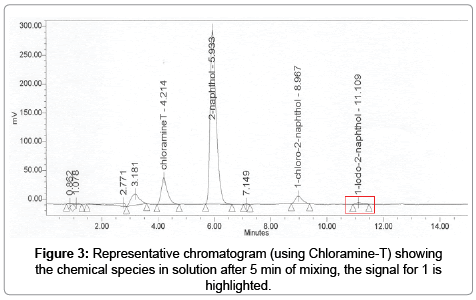
In a typical chromatogram the peak for the specie of interest 1 was in a very consistent window, determined from specificity experiments (n=6), eluting between 11.0 to 11.3 min. The elution of this peak using the reference compound was generally seen later compared to the production of 1 in-situ for both C-T and IG methods, possibly because of the absence of organic solvents in the matrix, although for all cases the peak for 1 remained inside the aforementioned window. For chromatograms obtained using dilutions of the reference compound there was only one peak present for 1, for the other methods peaks identified with unreacted 2-naphthol and other negligible and unidentified peak was detected.
As seen in Figure 3, the chromatogram for the chloramine-T method exhibited the greater amount of peaks, although given the resolution with the peak of interest (Resolution of 1 and 2 was Rs = 3) this did not represented an issue.
In Table 2 the main differences in quality attributes validated for the chloramine-T and Iodogen® methods are summarized, in general both methods comply with general requirements for analytical methods in terms of specificity, linearity and range. The Iodogen® method exhibits lower limits of quantification and detection and more importantly it showed to be more repeatable and accurate than the chloramine-T method. These differences could be due to the instability of 1 after mixing. Specifically Robustness was evaluated fin both methods by changes in flow rate (10%) and mobile phase composition (10%), these changes caused the peak for 1 to go out of the specified window obtained from specificity experiments. It is therefore demonstrated that such variations in either mobile phase composition or flow would impact the result of the method. An appropriate generic System Suitability Test was used at the beginning and end of day of experiments to assure consistency of results.
| Quality Attribute | Chloramine-T | Iodogen® |
|---|---|---|
| Specificity | Specific | Specific |
| Linearity | R2=0.999 | R2=0.993 |
| Range | 3-500 ng | 3-500 ng |
| LOQ | 9 ng | 6 ng |
| LOD | 4 ng | 3 ng |
| Accuracy | 90-110% | 95-105% |
| Precision (Repeatability) | RSD<20% | RSD<10% |
| Intermediate Precision | RSD<25% | RSD<10% |
RSD: relative standard deviation
Table 2: Comparison between the qualities attributes found in the validation of SA determination using chloramine-T and Iodogen®.
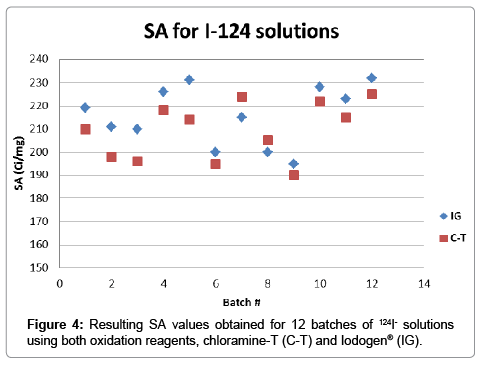
After validation of both methods for SA determination, twelve different batches of 124I- solutions prepared by the 124Te(p,n)124I nuclear reaction, were analyzed in parallel. In Figure 4 it can be observed a plot for the SA determined on each batch using both analytical methods. The results for iodine concentration found were within the working range obtained for both methods, with values between 15 and 25 ng. The resulting averages for SA values were 216 ± 13 Ci/mg for IG and 209 ± 12 Ci/mg for C-T, further comparison using unpaired t-test indicated that the statistical difference between both methods is not significant (P=0.1843). SA values found using chloramine-T as an oxidant were usually lower compared to the Iodogen® method (Figure 4), however this variability could be attributed to matrix effects since the calibration curve was performed in water and 124I- solutions have a matrix of sodium hydroxide 0.02M.
The fact that the average values of SA obtained are below the expected maximum theoretical value means that not all the Iodine atoms in the sample are radioactive. It is not surprising to obtain lower values since the theoretical value represents an upper limit in the range and also “cold” or non-radioactive iodine in the system is ubiquitous. The values obtained are notwithstanding very high, 86% (IG) and 83% (C-T) of the theoretical value of 251.6 Ci/mg, and demonstrate a valid raw material for use in the manufacture of radiotracers.
Determination of Te(IV) content
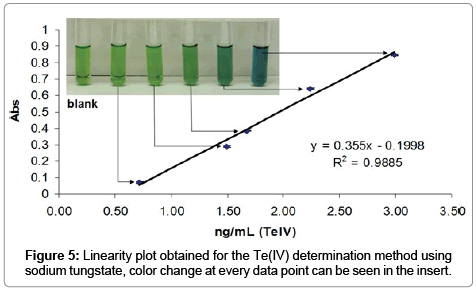
The determination of Te(IV) in 124I- solution batches was possible due to the formation of an ion association complex Te(IV)-WO4-nile blue in acidic solutions stabilized with poly(vinyl alcohol). We have scaled down this method in order to be used for radioactive samples and prepared a calibration curve using standard solutions of TeO2. The method was validated in the range 3.10 to 0.70 ng/mL since the change in color depending on Te(IV) concentration can be followed visually (Figure 5). The resulting values for the quality attributes evaluated are summarized in Table 3.
| Quality Attribute | Result |
|---|---|
| Specificity | Specific for Te(IV) |
| Linearity | R2=0.9885 |
| Range | 0.70-3.1 ng/mL |
| LOQ | 1 ng/mL |
| LOD | 0.7 ng/mL |
| Accuracy | 90-110% |
| Precision (Repeatability) | RSD<15% |
| Intermediate Precision | RSD<20% |
RSD: relative standard deviation
Table 3: Summary of quality attributes obtained from the validation of the method for Te(IV) using sodium tungstate.
Briefly the method was found to be linear in the expected working range, and under matrix effects coming from the basic 124I- solutions. The typical specification for Te(IV) content as a heavy metal is less than 1 μg/mL or 1 ppm. Twelve 124I- batches were tested using this procedure and the average concentration found was 0.40 ± 0.12 ppm, thus confirming that the Te(IV) content present in 124I- batches produced by the 124Te(p,n)124I reaction was within specifications. The color change was monitored via UV-vis at 580 nm.
Conclusion
As a conclusion, two highly sensitive methods for SA and Te(IV) determination in 124I- batches were validated and applied successfully. In addition, 124I- batches produced by the COSTIS target and the Reetz GmBH quartz thermochromatographic furnace possess high SA and low Te(IV) concentration. Given the small amount of sample needed for the SA and Te(IV) determination methods a workflow was developed in which only a small amount of 124I- solution was needed (<20 μL) to perform both quality control tests. This is important since given the typical activity concentration present on these solutions (ca. 1 mCi/ mL) the exposure to quality control personnel can be high. Decrease in radiation exposure is of main importance when considering that 124Isolutions also exhibit high energy gamma radiation (0.6 and 1.7 MeV) and have the potential for airborne contamination.
References
- Mittra E, Quon A (2009) Positron emission tomography/computed tomography: the current technology and applications. Radiol Clin North Am 47: 147-160.
- Miller PW, Long NJ, Vilar R, Gee AD (2008) Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed Engl 47: 8998-9033.
- Rice SL, Roney CA, Daumar P, Lewis JS (2011) The next generation of positron emission tomography radiopharmaceuticals in oncology. Semin Nucl Med 41: 265-282.
- Glaser M, Luthra SK, Brady F (2003) Applications of positron-emitting halogens in PET oncology (Review). Int J Oncol 22: 253-267.
- Koehler L, Gagnon K, McQuarrie S, Wuest F (2010) Iodine-124: a promising positron emitter for organic PET chemistry. Molecules 15: 2686-2718.
- Fonslet J, Koziorowski J (2013) Dry distillation of radioiodine from TeO2 targets. Appl Sci 3: 675-683.
- Lubberink M, Herzog H (2011) Quantitative imaging of 124I and 86Y with PET. Eur J Nucl Med Mol Imaging 38 Suppl 1: S10-18.
- Braghirolli AM, Waissmann W, da Silva JB, dos Santos GR (2014) Production of iodine-124 and its applications in nuclear medicine. Appl Radiat Isot 90: 138-148.
- Cascini GL, Niccoli Asabella A, Notaristefano A, Restuccia A, Ferrari C, et al. (2014) 124 Iodine: a longer-life positron emitter isotope-new opportunities in molecular imaging. Biomed Res Int 2014: 672094.
- Sgouros G, Hobbs RF, Atkins FB, Van Nostrand D, Ladenson PW, et al. (2011) Three-dimensional radiobiological dosimetry (3D-RD) with 124I PET for 131I therapy of thyroid cancer. Eur J Nucl Med Mol Imaging 38 Suppl 1: S41-47.
- Van Nostrand D, Moreau S, Bandaru VV, Atkins F, Chennupati S, et al. (2010) 124I positron emission tomography versus 131I planar imaging in the identification of residual thyroid tissue and/or metastasis in patients who have well-differentiated thyroid cancer. Thyroid 20: 879-883.
- Moroz MA, Serganova I, Zanzonico P, Ageyeva L, Beresten T, et al. (2007) Imaging hNET reporter gene expression with 124I-MIBG. J Nucl Med 48: 827-836.
- Simões MV, Miyagawa M, Reder S, Städele C, Haubner R, et al. (2005) Myocardial kinetics of reporter probe 124I-FIAU in isolated perfused rat hearts after in vivo adenoviral transfer of herpes simplex virus type 1 thymidine kinase reporter gene. J Nucl Med 46: 98-105.
- Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, Mathieu C, Guérard F et al. (2015) Tumor immunotargeting using innovative radionuclides. Int J Mol Sci 16: 3932-3954.
- van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR (2007) Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist 12: 1379-1389.
- Qiu-e C, Zhide H, Zubi L, Jialin W, Qiheng, X (1998) Highly sensitive spectrophotometric determination of trace amounts of tellurium(IV) with the tungstate-basic dyes-poly(vinyl alcohol) system. Analyst 123: 695-698.
- Alvarenga L, Ferreira D, Altekruse D, Menezes JC, Lochmann D (2008) Tablet identification using near-infrared spectroscopy (NIRS) for pharmaceutical quality control. J Pharm Biomed Anal 48: 62-69.
- FDA (2015) Analytical Procedures and Methods Validation for Drugs and Biologics. Guidance for Industry. Food and Drug Administration.
- Wilbur DS (1992) Radiohalogenation of proteins: an overview of radionuclides, labeling methods, and reagents for conjugate labeling. Bioconjug Chem 3: 433-470.
- Kloster G, Laufer P (1983) Determination of specific activity of radiohalide preparations (75Br, 77Br, 123I, 131I) by HPLC-UV detection following chemical derivatization to 1-halonaphthol-2. J Labelled Comp Radiopharm 20: 1305-1315.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12009
- [From(publication date):
specialissue-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11061
- PDF downloads : 948