Research Article Open Access
A Validated High Performance Liquid Chromatography Method for the Determination of Saxagliptin and Metformin in Bulk, a Stability Indicating Study
Sena Caglar* and Ali Rahmi AlpFaculty of Pharmacy, Department of Analytical Chemistry, Istanbul University, Istanbul, Turkey
- *Corresponding Author:
- Sena Caglar
Faculty of Pharmacy
Department of Analytical Chemistry
Istanbul University
34116, Beyazit
Istanbul, Turkey
Tel: +902124400000
Fax: +902124400252
E-mail: senacaglar@yahoo.com, sena@istanbul.edu.tr
Received date: March 24, 2014; Accepted date: April 18, 2014; Published date: April 22, 2014
Citation: Caglar S, Alp AR (2014) A Validated High Performance Liquid Chromatography Method for the Determination of Saxagliptin and Metformin in Bulk, a Stability Indicating Study. J Anal Bioanal Tech S12:010 doi: 10.4172/2155-9872.S12-010
Copyright: © 2014 Caglar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In this paper a simple, precise and rapid high performance liquid chromatography method with UV detection has been developed for the determination of saxagliptin and metformin in bulk. An Agilent, Zorbax CN (250 × 4.6 mm I.D., 5 μm) column was used with a mobile phase mixture of methanol-50 mM phosphate buffer (pH 2.7) in a gradient elution mode at a flow rate of 1.0 ml min-1. The analytes were detected at 225 nm and total run time for the method was 7 min. The calibration graphs were linear in the range of 5.00-125.00 μg ml-1 for saxagliptin and 2.50-62.50 μg ml-1 for metformin. For stability indicating study, saxagliptin was subjected to acid, neutral and alkali hydrolysis, oxidation and heat stress. The developed method could be used for quality control assay for SAX in tablets and for stability studies as the method separates SAX from its degradation products and tablet excipients.
Keywords
Saxagliptin; Stress testing; Stability-indicating; HPLC; Tablet
Introduction
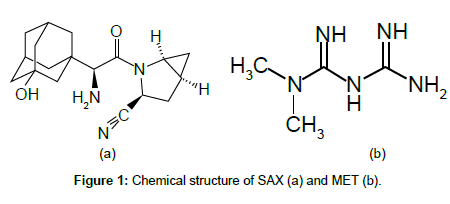
Saxagliptin (SAX) (1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-1- adamantyl)acetyl]-2 azabicyclo [3.1.0]hexane-3-carbonitrile)(Figure 1), with C18H25N3O2 chemical formula and 315.41 g/mol molecular weight, is an orally active, potent and selective inhibitor of dipeptidyl peptidase- IV (DPP-4) for the treatment of Type 2 diabetes, is marketed as OnglyzaTM tablet, and also in a fixed dose combination SAX/ metformin (MET) as KombiglyzeTM tablet in USA, Europe and some other countries [1]. DPP-4 inhibitors enhance the body’s own ability to control blood glucose by increasing the active levels of incretin hormones in the body. Their mechanism of action is distinct from any existing class of oral glucose-lowering agents. They control elevated blood glucose by triggering pancreatic insulin secretion, suppressing pancreatic glucagon secretion, and signaling the liver to reduce glucose production [2].
In literature two spectrophotometric methods have been reported for the determination of SAX in pharmaceutical preparations [3,4] and three other high performance liquid chromatography (HPLC) methods were developed for analysis of SAX and MET simultaneously [5-8]. In this study a new stability indicating HPLC method was developed for determination of SAX and MET in bulk drug and validated. The developed method could be used for quality control assay for SAX in tablets and for stability studies as the method separates SAX from its degradation products and tablet excipients.
Experimental
Chemicals
Saxagliptin was purchased from Richem International Co., Ltd. (Shanghai, China). Metformin was kindly supplied by Abdi Ibrahim Ilac (Istanbul, Turkey). Commercially available Onglyza® tablets were purchased from local drug store.
Sodium dihydrogen phosphate (NaH2PO4), analytical grade ortho-phosphoric acid, HPLC grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Deionized water up to a resistivity of 18.2 MΩ was purified with an Elga water purification system (London, UK).
Instrumentation and analytical conditions
A Shimadzu (Kyoto, Japan) LC 20A liquid chromatograph, consisting of a model LC 20 AT solvent delivery system, a DGU-20A5 degasser and a SIL-20AC auto sampler (set at ambient temperature) and a diode array detector SPD-M20A was used. During method development different wavelengths were scanned with PDA detector in one run and 225nm was found to be the ideal for this study. Data acquisition was performed using LC Solution 1.21 SP1 system software. Separations were performed on an Agilent, Zorbax CN (250 × 4.6 mm I.D., 5 μm) column with an Agilent guard column (4 × 3 mm I.D.) packed with the same material. Chromatographic separation was achieved at room temperature by using methanol-50 mM phosphate buffer (pH 2.7) mixture as mobile phase in gradient elution mode at a flow rate of 1.0 ml min-1. The gradient elution program (Table 1) was run for 7 min. A typical injection volume was 20 μL.
| Time (min) | A % | B % |
|---|---|---|
| 0.01 5 5.01 7 |
30 90 30 30 |
70 10 70 70 |
Where, A: Methanol B: Buffer (50mM phosphate buffer, pH 2.7)
Table 1: Gradient elution program.
Preparation of solutions
Stock solutions were prepared by dissolving SAX and MET in acetonitrile and water respectively to give a concentration of 1 mg ml-1. Standard solutions with a concentration of 100 μg ml-1.were obtained by diluting of the stock solutions with mobile phase mixture (10% methanol, 90% buffer). The final concentrations for SAX and MET solutions were 5.00-125.00 μg ml-1. and 2.50-62.50 μg ml-1., respectively. The chromatograms were evaluated on the basis of the peak areas of SAX and MET. Buffer solution was prepared by dissolving 6.0 g (NaH2PO4) in 1000 mL of water, pH is adjusted to 2.7 by adding orthophosphoric acid drop wise. The prepared solution was filtrated through 45μm filter and sonicated prior to HPLC analysis.
Stress testing
Stress testing was carried out according to the ICH stability testing guidance [9]. SAX was stressed under various conditions until to facilitate approximate 5-20 % degradation [10].
Acidic, neutral and basic hydrolysis: A solution of SAX 10 mg ml-1 concentration was prepared in acetonitrile, 1 ml of this solution was transferred to three different 10 mL volumetric flasks and diluted up to volume with water, 0.1 HCl and 0.1 NaOH solutions to give a concentration of 1 mg ml-1. Samples of 0.25 mL were transferred to 5 mL volumetric flask, neutralized and diluted to the volume with mobile phase to a final concentration of 50.00 μg ml-1. The samples were kept at room temperature and analyzed starting from first hour up to 6 hours. According to the degradation amount, the samples were further subjected to heat 70°C for an hour
Oxidation: A solution of SAX 10 mgml-1 concentration was prepared in acetonitrile, 1 ml of this solution was transferred to a 10 mL volumetric flask and diluted up to volume with 6 % H2O2. The prepared solution was kept at room temperature and was analyzed as the hydrolysis samples.
Thermal degradation: Bulk drug of SAX was exposed to 50°C dry heat for 1 h, 70°C for 1 h and 105°C for 6 hours.
Validation of the method
The methods were validated according to the ICH guidelines.
The calibration curves were obtained between the concentrations of 5.00-125.00 μg ml-1 and 2.50-62.50 μg ml-1, for SAX and MET respectively. The solutions were prepared in triplicate. Linear regression equations (intercepts and slopes) for mixtures of SAX and MET were established and calculated by the least square regression method.
Precision of the analytical method was tested by analyzing six replicate determinations of three different concentrations (high, medium and low) of SAX and MET both on within-day and day-to-day.
The accuracy was determined by recovery test using standard addition method. This experiment was performed at three different concentration levels, in which sample stock solutions (25.00 μg ml-1 and 12.50 μg ml-1 for SAX and MET, respectively) were spiked with standard drug solutions of 25.00, 50.00 and 75.00 μg ml-1 for SAX and 12.50, 25.00 and 37.50 μg ml-1 for MET. The mixtures were then analyzed by the proposed methods. Five replicate samples of each concentration level were prepared. The recovery tests were performed using tablet solution containing various amounts of SAX and MET.
Sensitivity of the method was decided by determination of limit of detection (LOD) and limit of quantification (LOQ). LOD is the lowest detectable concentration of the analytes by the method while LOQ is the minimum quantifiable concentration. LOD and LOQ were calculated by using the values of slopes and intercepts of the calibration curves for both of the drugs.
Assay for the tablets
Onglyza tablet which contains 5 mg saxagliptin was used for the tablet assay. Ten tablets were separately weighed and finely powdered. About 25 ml of methanol and 50 mL of buffer solution was added to accurately weighed amount of the powder equivalent to the median mass of one tablet in a 100 ml calibrated flask. The mixture was shaken mechanically and sonicated in ultrasonic bath totally 30 min and diluted to volume with buffer solution and then filtered. The filtrate was diluted with the mobile phase (90% buffer: 10% methanol) to give a final concentration of 20.00 μg ml-1 of SAX and analysed with HPLC. The quantity of the SAX was calculated from the regression equation constructed for the SAX assay.
Results and Discussion
Method development and optimization
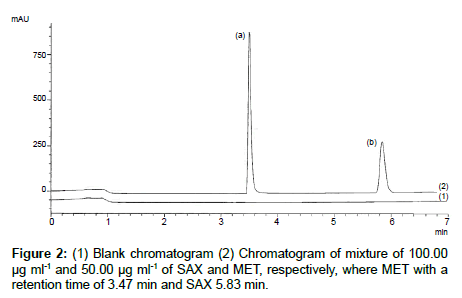
To optimize the chromatographic conditions a reversed phase cyano HPLC column and a phosphate buffer at a pH of 2.7 and methanol mixture were found to be the best stationary phases and mobile phase combinations to have two symmetrical and well-resolved peaks for SAX and MET. An optimized gradient program provided sufficient selectivity in a short separation time. The total runtime for the analysis is 7 min and the retention time for MET and SAX 3.47 and 5.83 min, respectively. Blank chromatogram overlapped with mixture of SAX and MET was presented in Figure 2 and single chromatograms of SAX and MET could be found in Figures 3-5, respectively. The system suitability parameters were found to be within the suitable range Rs (resolution) > 15 between two peaks (range Rs ≥ 2); T (peak tailing factor) 1.21 and 1.40 and N (theoretical plate number) 100485 and 103918 (range > 2000) for SAX and MET, respectively. and 1.40 and N (theoretical plate number) 100485 and 103918 (range > 2000) for SAX and MET, respectively.
Stress testing
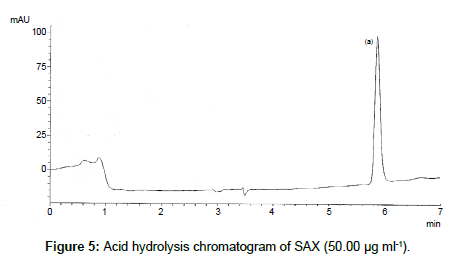
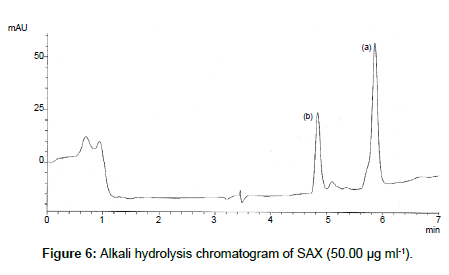
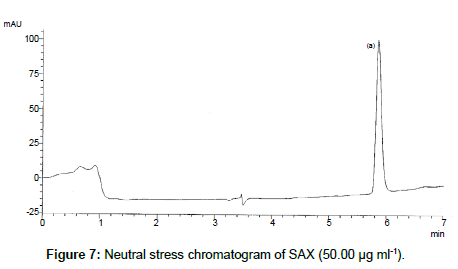
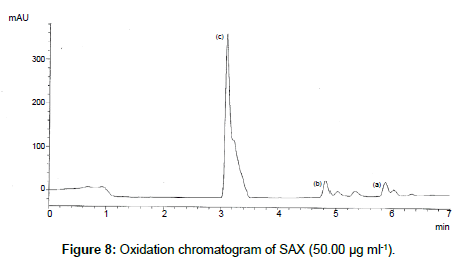
SAX was forced to degradation acid and alkali hydrolysis. The rate of acid hydrolysis was slower as compared to that alkali. Acid hydrolysis has almost no degradation product but just decreases in amount. The solution was stable up to 6 hours at room temperature, after this the solution was subjected to heat of 70°C for an hour and 17.03% of the solution was decomposed (Figure 5). In alkali hydrolysis there was degradation product in the beginning and the drug was decomposed of 14.36 % (Figure 6). The solution was stable in water first two hours but it was decomposed up to 15.41% without any degradation by-product (Figure 7). As per the oxidative hydrolysis the drug was decomposed immediately 24.75%, degradation products were shown in Figure 8. The drug powder was exposed to 50°C for 1 h and found to be stable, the temperature was increased to 70°C and the drug is degraded 16.23% in 1 h. SAX exposed to the heat for 6 hours at 105°C and the bulk drug substance was melted completely and decomposed at the end of this treatment. The percent amount of drug degraded after degradation studies are given in Table 2.
| Condition | Time (h) | Recovery (%) |
|---|---|---|
| Acid, 0.1 N HCl 6 h (at room temperature) + at 70°C 1 h | 6 +1 | 82.97 |
| Base, 0.1 N HCl (at room temperature) | 1 | 85.64 |
| Neutral hydrolysis (at room temperature) | 2 | 84.59 |
| H2O2, 6% (at room temperature) | 1 | 75.25 |
Table 2: Results of forced degradation study.
The results obtained from the stress testing showed that SAX is unstable under hydrolysis, oxidation and thermal stress. Therefore, care should be taken in the manufacturing process and during the storage of the product and the developed method could be used for stability studies of SAX.
Validation of the method
The equations of the calibration curves were y= 14859x+103450, r2=0.9976 and y=69798x-70654, r2=0.994 for SAX and MET, respectively. The result shows a good correlation existed between peak area and concentration of each drug within the concentration range tested. The analytic and chromatographic data for the developed methods were given in Table 3.
| Parameters | SAX | MET | |||
|---|---|---|---|---|---|
| Linearity range (µg ml-1) | 5.00-125.00 | 2.50-62.50 | |||
| Intercept (mean ± SD*) | 103533.7 ± 2236.07 | 70662 ± 4644.91 | |||
| Slope (mean ± SD*) | 14859.3 ± 70.29 | 69799.67 ± 244.8 | |||
| Correlation coefficient (mean ± SD*) | 0.9975 ± 3.5 × 104 | 0.9993 ± 3.8 × 104 | |||
| LOD (µg ml-1) | 0.45 | 0.19 | |||
| LOQ (µg ml-1) | 1.50 | 0.66 | |||
| Retention time (min) | Theoretical plate (N) | Tailing factor (T) | Resolution (Rs) | ||
| SAX | 5.83 | 103918 | 1.21 | 15.61 | |
| MET | 3.47 | 100485 | 1.40 | 17.90 | |
*Standard deviation
Table 3: Quantitative and chromatographic parameters for SAX and MET (n=6).
The precision of the methods were assessed by carrying out six replicate determinations of three different concentrations of SAX and MET both on within-day and day-to-day (Table 4). RSD values were less than 1.07 and 1.52% indicating good precision and there was no significant difference between the assays tested on the same day or different days.
| Actual concentration (µg mL-1) |
Intra-day (n=5) | Inter-day* (n=5) | |||
|---|---|---|---|---|---|
| Foundconcentration (µg mL-1) |
RSD (%) | Foundconcentration (µg mL-1) |
RSD (%) | ||
| 5.00 | 4.96 | 0.50 | 4.95 | 0.54 | |
| SAX | 50.00 | 50.10 | 0.18 | 49.92 | 0.22 |
| 100.00 | 99.62 | 1.07 | 98.86 | 0.94 | |
| MET | 2.5025.0050.00 | 2.4924.8949.88 | 1.520.120.78 | 2.5225.0150.12 | 0.270.481.03 |
*Results of five different days
Table 4: Results from intra-day and inter-day precision experiments for SAX and MET.
The accuracy of the developed method was determined by the standard addition technique which was done by adding three different concentrations of the standard drug solution to the tablet solution. The results obtained from the standard addition method were given in Table 5.
| Amount taken (µg mL-1) |
Amount added (µg mL-1) |
Total amount found (µg mL-1) (Mean ± SD) |
Recovery (%) | RSD (%) | |
|---|---|---|---|---|---|
| SAX | 25.00 | 25.00 | 50.75 ± 0.19 | 101.84 | 0.62 |
| 50.00 | 75.56 ± 1.41 | 99.85 | 2.03 | ||
| 75.00 | 99.56 ± 0.94 | 100.11 | 1.17 | ||
| MET | 0.00 | 12.50 | 12.75 ± 0.89 | 101.02 | 2.51 |
| 25.00 | 24.56 ± 0.70 | 99.27 | 1.55 | ||
| 37.50 | 37.06 ± 0.94 | 99.56 | 1.54 |
Table 5: Results of recovery studies by standard addition method
LOD and LOQ were calculated as 3σ/S and 10σ/S, respectively, where S is the slope of the calibration curve and σ is the standard deviation of y-intercept of regression equation [11].
Assay for the tablets
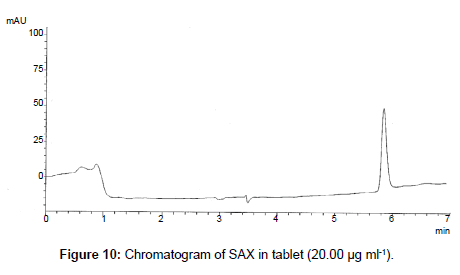
The proposed method was successfully applied to the analysis of marketed product (Onglyza Tablet®). The obtained results are satisfactorily accurate and precise as indicated by the good % recovery and SD<2 (Table 6). The related chromatograms were given in Figures 9 and 10.
| Onglyza Tablet®a (5mg/per tablet | Recovery (%) ± S.D | RSD (%) |
|---|---|---|
| 5 | 98.92 ± 0.53 | 0.53 |
aMarketed by Brystol Myers Squibb
Table 6: Determination of SAX in tablets (n=5)
Conclusion
The developed and validated HPLC method is selective, rapid and precise for the determination saxagliptin and metformin from bulk and it could be applied to the tablets. Since the method separates SAX from its degradation products and tablet excipients it could also be used for quality control assay and for stability studies. The results obtained from the stress testing showed that saxagliptin is unstable under hydrolysis, oxidation and thermal stress. Therefore, care should be taken in the manufacturing process and during the storage of the product.
Acknowledgements
The authors would like to thank the Research Fund of Istanbul University for support of this work (Project number: 34685, 32490)
References
- Augeri DJ, Robl JA, Betebenner DA, Magnin DR, Khanna A, et al. (2005) Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48: 5025-5037.
- Deacon CF (2007) Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 9: 23-31.
- Kalaichelvi R, Jayachandran E (2011) Validated spectroscopic method for estimation of saxagliptin in pure and from tablet formulation. International Journal of Pharmacy and Pharmaceutical Sciences 3: 179-180.
- El-Bagary, Ramzia I. Elkady, Ehab F, Ayoub, Bassam M (2012) Spectrophotometric methods based on charge transfer complexation reactions for the determination of saxagliptin in bulk and pharmaceutical preparation. International Journal of Biomedical Science 8: 204-208.
- Cumar RP, Vasudevan M, Deecaraman (2012) A validated RP-HPLC method for simultaneous estimation of metformin and saxagliptin in tablets. Rasayan Journal of Chemistry 5: 137-114.
- Prakash PP, Kalkotwar RS, Vikas VP, Vijay BJ, Nilesh PP (2012) A new RP-HPLC method for determination of Metformin HCl and Saxagliptin in tablet dosage form. International Journal of Pharmacy and Biological Sciences 2: 161-167.
- Sarat M, Murali Krishna P, Rambabu C (2012) RP-HPLC method for simultaneous estimation of saxagliptin and pioglitazone in tablets. International Research Journal of Pharmacy 3: 399-402.
- Abdul-Azim MM, Elkady EF, Fouad MA (2012) Development and validation of a reversed-phase column liquid chromatographic method for simultaneous determination of two novel gliptins in their binary mixtures with metformin. European Journal of Chemistry 3: 152-155.
- ICH (2003) ICH Q1 A (R2) Stability Testing of New Drug Substances and Products. International Conference on Harmonization, Geneva, 2003.
- Baertschi SW, Alsante KM, Reed RA (2005) Pharmaceutical Stress Testing, Prediction Drug Degradation. Taylor & Francis Group, Boca Raton, 14.
- ICH (1996) The European Agency for the Evaluation of Medicinal Products. ICH Topic Q2B Note for Guideline on Validation of Analytical Procedures: Methodology. GPMP/ICH/281/95.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20462
- [From(publication date):
July-2014 - Jul 14, 2025] - Breakdown by view type
- HTML page views : 15611
- PDF downloads : 4851