Research Article Open Access
Isolation and Quantitative Determination of New Tuberculostatic 1,2,4-Triazole Derivative in Urine and Plasma Samples
Malgorzata Tatarczak-Michalewska1, Jolanta Flieger1*, Monika Wujec2 and Marta Swatko-Ossor31Department of Analytical Chemistry, Medical University, Chodzki 4a, 20-093 Lublin, Poland
2Department of Organic Chemistry, Medical University, Chodzki 4a, 20-093 Lublin, Poland
3Department of Biochemistry, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland
- *Corresponding Author:
- Jolanta Flieger
Department of Analytical Chemistry, Medical University
Chodzki 4a, 20-093 Lublin, Poland
Tel: +48 81535-73-50
E-mail: j.flieger@umlub.pl
Received date: September 09, 2014; Accepted date: September 25, 2014; Published date: September 29, 2014
Citation: Tatarczak-Michalewska M, Flieger J, Wujec M, Swatko-Ossor M (2014) Isolation and Quantitative Determination of New Tuberculostatic 1,2,4-Triazole Derivative in Urine and Plasma Samples. J Anal Bioanal Tech 5:206 doi: 10.4172/2155-9872.1000206
Copyright: © 2014 Tatarczak-Michalewska M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A novel series of 1,2,4-triazole derivatives, have been synthesised. All synthesised compounds were screened for their antimycobacterial activity against Mycobacterium tuberculosis and antimicrobial activities against various bacteria and fungi. Among studied derivatives, 4-methyl-1-morpholinmethyl-2,4-dihydro-3H-1,2,4-triazol-3-thione was found as the most active, demonstrating inhibition of mycobacterial growth of Mycobacterium H37Ra at the same concentration level as Streptomycin. The quantitative analysis of this compound in the urine and serum samples was elaborated on. Quantification was performed by reversed-phase high-performance liquid chromatography (RP–HPLC) using C18 column with a mobile phase consisting of methanol and water (5:95) at flow rate of 1 ml/min. The chromatograms were recorded at 245 nm at 21°C. The chromatographic peak identification was based on the comparison the retention times and UV spectra of isolated analyte and synthetized standard. Moreover, different sample pre-treatment methods such as deproteinization and solid-phase extraction (SPE) were compared. The highest analyte recoveries ranging from 78,7% to 90,7% were obtained for sample deproteinized by 6% HClO4.
Keywords
Antimycobacterial activity; Mycobacterium tuberculosis; 1,2,4-triazole
Introduction
Tuberculosis (TB) belongs to infectious disease caused by Mycobacterium tuberculosis. There is an estimated 2.5 billion people infected with tuberculosis worldwide and an annual mortality of approximately 1.5 million people [1,2]. Despite the availability of TB first-line drug therapy consisting of rifampicin (RIF), isoniazid (INH), pyrazinamide (PZA), streptomycin (STP) and ethambutol(EMB), the increase in the incidence of both multidrug-resistant (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are observed [3,4]. Furthermore, treatment requiring the use of combination therapy causes serious side effects such as: thrombocytopenia occurring mostly due to rifampicin (RIF) [5], neuropathy induced by INH and the biggest problem connected with TB treatment- drug-induced hepatitis [6]. Considering that, the searching for novel antituberculosis substances, appears currently to be a dire must. As it could be observed, the synthesis of new compounds with anti-tuberculosis activity has been the target of many medicinal chemistry projects.
So far, a number of stable metal complexes with INH [7,8], ciprofloxacin and phenanthroline [9] possessing anti-tubercular activity have been designed. However, a special interest has been focused on five membered heterocyclic compounds derivatives of pyrrole [10], oxadiazole [11], imidazole [12], N-aryl-C-nitroazole [13] and triazole [14,15].
Many previous studies have shown that 1,2,4-triazole derivatives exhibit many promising biological properties. The significant differences in their activity depend on structural modifications. According to the chemical nature of the substituents, 1,2,4-thiazole derivatives display diverse pharmacological activities such as anti-inflammatory, antifungal or antiviral ones. Besides that, there exists a number known of drugs containing the 1,2,4-triazole group such as psychoactive Triazolam, Alprazolam, Etiazolam or antifungal Fluconazole, Ravuconazole, Voriconazole. In the light of recent data, 1,2,4-triazole derivatives could be also considered as potential tuberculostatic agents [13-16].
In our research project 1,2,4-triazole derivatives were synthesised and screened for their antimycobacterial activity against Mycobacterium tuberculosis. This article presents the use of optimal chromatographic system to perform quantitative analysis of the most active derivative of 1,2,4-triazole in the biological material (serum, urine). Moreover, the presented study is devoted to comparison of the most widely used sample preparation methods such as deproteinization by mineral acids and organic solvents and solid-phase extraction: SPE, which allows choosing the method characterized by the highest recovery of the analyte.
Materials and Methods
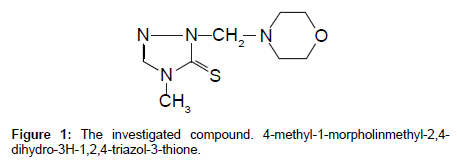
A new derivative of 1,2,4-triazole (Figure 1), was synthesized in the Department of Organic Chemistry in Medical University of Lublin [17].
Its structure was confirmed by GC-MS, 1H-NMR, 13C-NMR and IR analysis and the purity was assessed by elemental analysis. Quantitative determination of the examined compound in the serum and urine samples was performed by high performance liquid chromatography using an external standard approach. Analysis was performed using Merck-Hitachi Lachrom Elite HPLC chromatographic system with a gradient pump and photodiode array spectrophotometric (PAD) detection. The investigated samples were loaded on the Agilent Eclipse XDB C18 column (4.6 × 150 mm, 5 μm) using a Hamilton microsyringe and a Rheodyne dispenser equipped with a 20 μl sample loop. The mobile phase consisted of methanol and water (5:95). The flow rate was 1 mL min-1. The detection of the compound was set at 245 nm chosen accordingly with the recorded spectrum in the range of 200-900 nm. Identification of peaks in the chromatogram was performed based on the comparison the retention times and UV spectra of each analyte to the respective retention times and UV spectra of the standard. HPLC grade methanol (MeOH), was obtained from E.Merck (Darmstadt, Germany). Perchloric acid (70%, w/v) was purchased from J. T. Baker (Phillipsburg, NJ, USA). The mobile phase was filtered through a Nylon 66 membrane filter (0.45 μm) Whatman (Maidstone, England) by the use of a filtration apparatus. Statistical analyses were performed using the software: Excel 2003 (MS Office) and STATISTICA version 5.1 for Windows.
Calibration solutions
The stock solution at concentration of 1.0 mg mL-1 and the calibration solutions of investigated compound at the concentrations of 0.01; 0.025; 0.05; 0.75; 0.1 mg ml-1 were prepared gravimetrically and stored in darkness at 4°C in glass vials. The calibration curves representing the dependence of the peak area on the concentration were used to perform quantitative analysis.
Sample preparation procedures
All samples for the determination of an analyte in the biological material were prepared immediately before use by adding appropriate volumes of stock solutions prepared at concentrations of 0.025, 0.05 and 0.1 mg mL-1 to a blank serum or urine samples obtained from hospital laboratory. Solid-phase extraction (SPE) was performed using Baker SPE 12G system and Bakerbond SPE Silica Gel columns (J. T. Baker).
Serum samples
Deproteinization with 6% HClO4 : A 3 mL of 6% HClO4 was added to 1 mL of serum spiked with 1 mL of the investigated compound at the concentrations of 0.025, 0.05 and 0.1 mg mL-1, respectively, and shaken for about 15 min. After centrifugation (2,000 rev/min for 10 mins) the clear supernatant was evaporated to dryness and dissolved in 1 mL of eluent. The prepared samples were loaded directly on a chromatographic column. Typical injection volumes were 20 μL corresponding to the volume of the Rheodyne injector loop. Each sample injection was analyzed in three replicates.
Solid phase extraction (SPE)
Solid phase extraction was performed using Bakerbond SPE Silica Gel columns. The columns were conditioned prior to sample adsorption with 2 mL of methanol and 4 mL of hexane. 1 mL of serum containing 0.025, 0.05 and 0.1 mg mL-1of investigated derivative, deproteinized with 6% HClO4., was then applied to SPE columns. Analyte elution was accomplished with 1 mL of methanol. Three samples were prepared for each of the concentration levels (0.025, 0.05 and 0.1 mg mL-1).
Urine specimens
All urine specimens containing investigated compound were prepared for analysis using solid phase extraction procedure. SPE was performed with Bakerbond SPE Silica Gel (SiOH) columns. The columns were conditioned prior to sample adsorption with 2 ml of methanol and 4 mL of hexane, and 1 mL of urine diluted with phosphate buffer at pH 7.04 (1:1) was then applied. Analyte elution was accomplished with 1 mL of methanol.
Three samples were prepared for each concentration levels (0.05; 0.075 and 0.1 mg mL-1). Each sample injection was analyzed in three replicates.
Antibacterial Activity
Minimum inhibitory concentration (MIC), Minimum Bactericidal Concentrations (MBC), and Disk Diffusion against Mycobacterium smegmatis ATCC 20 (American Type Culture Collection), Mycobacterium Phlei and Mycobacterium H37Ra for investigated compound was carried out in Muller Hinton Broth (Oxoid, Anglia) medium applying broth dilution method. Disc diffusion assay was evaluated using Whatman filter paper discs prepared by pipetting 10 μl of solutions containing 250 μg of substance and placed on agar plates. After incubation for 24 hours at 37°C, a clear zone around a disc was an evidence of antimycobacterial activity. All biological tests were prepared together with Rifampicin and Streptomycin sulfate antibiotics as a control. A more extensive description of the methodology of the microbiological investigation is presented in the literature [18-21].
Results and Discussion
Chromatographic conditions
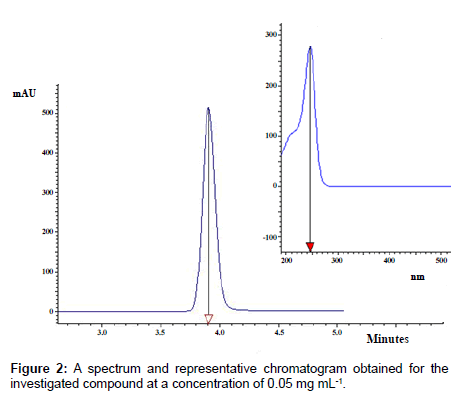
The best peak parameters regarding sensitivity, symmetry, retention time and chromatographic efficiency were obtained for the Agilent Eclipse XDB C18 column and the mobile phase consisted of methanol and water (5:95). Figure 2 shows a typical chromatogram obtained for the investigated derivative.
The proposed chromatographic system is characterized by short time analysis equaling 3.9 minutes. According to absorption spectrum elaborated on for examined compound in the range from 200 nm to 900 nm, the maximum wavelength was established. Further experiments with photodiode array detection were performed at analytical wavelength of 245 nm (Figure 2).
The calibration curve
The calibration curve was prepared using five concentrations in the range of 0.01-0.1 mg mL-1. A 20 μL of each dilution was injected in triplicate. The mean peak areas were taken for the construction of the calibration curves. The data were analyzed by linear regression least square model. The equation for the regression line of this relationship was found to be:
y = 308330102 (±7864299)x + 428257 (± 482870)
n = 5, R2 = 0.9981, se = 574147.7, F = 1537.13
Isolation method
Body fluids, including serum and urine, are the most accessible and investigated materials in clinical laboratories. The quantitative methods of substances assay in biological fluids should be elaborated on for the study of their pharmacokinetics as the potential drugs. When dealing with biological samples, endogenous matrix containing phospholipids and peptides is the main cause of both contamination of chromatographic columns causing instrument downtime and reduction sensitivity and accuracy of quantitative analysis [22-24].
This paper provides a comparison of the most widely-applied pretreatment methods that can be used for preparation of biological fluid sample for further HPLC analysis, such as deproteinization by mineral acid and organic solvents and solid-phase extraction. The identification of the investigated compound peak was based on the comparison of the UV spectra both isolated analyte and the standard. To check spectrum purity, the comparison of the UV spectra taken from the beginning, apex and end of the peak was performed.
Deproteinization
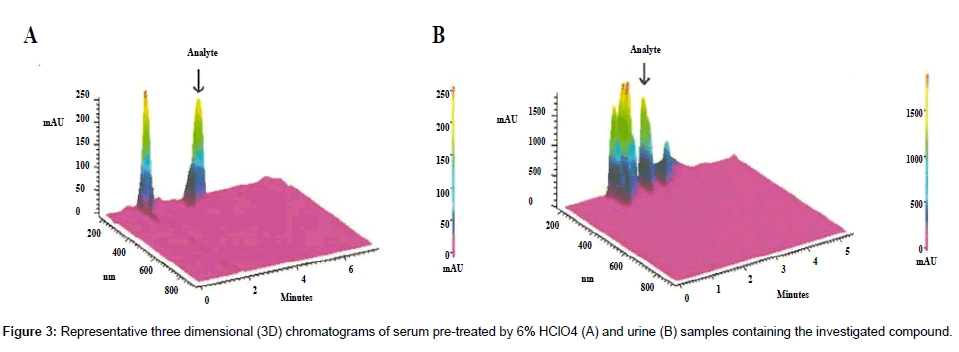
Deproteinization methods typically involve treatment one volume of serum (or plasma) with three volumes of water-soluble acid or organic solvent. Proper solvent selection significantly affects further analysis. For quantification of the investigated compound in biological fluids urine and serum samples, different precipitating reagents and their various proportions were tested. Using 6% CCl3COOH, a significant loss of investigated analyte was observed which may indicate a lack of stability of the compound in this acid. In turn, acetonitrile and methanol, used as precipitating agents, allowed obtaining very low recoveries of analyte in the range of 15.4% - 46.4%. The best recoveries ranging from 78.7% to 90.7% were obtained by the use of 6% HClO4. It can therefore be assumed that the investigated compound due to the basic character is highly bounded to plasma proteins, and only the use of a strong acid such as perchloric acid may release it. Figure 3A shows a chromatogram of a serum sample spiked with the investigated compound deproteinized by 6% HClO4.
SPE isolation
SPE analysis of the serum samples prepared for chromatographic analysis resulted in mean analyte recoveries ranging from 61.6% to 74.5%. Slightly higher analyte recoveries ranging from 64.3% to 83.6% were obtained, analyzing urine samples. Figure 3B shows chromatograms obtained for RP-HPLC analysis of urine sample after SPE.
Comparing the three dimensional chromatograms of serum pretreated by 6% HClO4 and urine samples prepared by SPE one could conclude that lower recovery obtained for urine samples is directly connected with insufficient sample cleaning step by SPE. Due to matrixinduced interferences visible on 3D graph, appearing as consequences of co-elution of matrix components with target analyte, reduction accuracy of determination is observed [24].
Statistical parameters describing the accuracy and precision of a measurement are summarized in Table 1.
| Statistic parameters | Serum | Urine | |||||||
| Depeptization by 6% HClO4 | SPE | SPE | |||||||
| 0.025 [mg/ml] |
0.05 [mg/ml] |
0.1 [mg/ml] |
0.025 [mg/ml] |
0.05 [mg/ml] |
0.1 [mg/ml] |
0.05 [mg/ml] |
0.075 [mg/ml] |
0.1 [mg/ml] |
|
| Mean amount fund (mg/ml) | 0.0197 | 0.045 | 0.091 | 0.0187 | 0.037 | 0.062 | 0.032 | 0.048 | 0.083 |
| Standard deviation (s) | 0.0039 | 0.007 | 0.014 | 0.0011 | 0.0024 | 0.0016 | 0.0022 | 0.0023 | 0.0077 |
| Variance (S2) | 1.5·10-5 | 4.9·10-5 | 2·10-4 | 1.2·10-6 | 5.8·10-6 | 2.6·10-6 | 4.8·10-6 | 5.3·10-6 | 5.9·10-5 |
| Relative standard deviation | 0.1969 | 0.1554 | 0.1595 | 0.0599 | 0.0652 | 0.0256 | 0.0674 | 0.0474 | 0.0923 |
| Coefficient of variation [%] | 19.69 | 15.54 | 15.95 | 5.99 | 6.52 | 2.56 | 6.74 | 4.74 | 9.23 |
| 95% confidence interval | 8.35·10-5 | 3.12·10-4 | 3.12·10-4 | 2.41·10-5 | 5.22·10-5 | 3.41·10-5 | 4.70·10-5 | 4.94·10-5 | 1.66·10-4 |
| Spread | 0.009 | 0.016 | 0.037 | 0.003 | 0.006 | 0.005 | 0.006 | 0.007 | 0.019 |
| Relative error [%] | 21.2 | 10 | 1 | 25.2 | 26 | 38 | 36 | 36 | 17 |
| Recovery [%] | 78.67 | 89.33 | 90.67 | 74.53 | 74.2 | 61.63 | 66.33 | 64.33 | 83.57 |
Table 1: Precision and accuracy assay results for investigated compound on spiked serum and urine samples analyzing using different pretreatment methods.
Biological tests
Disc diffusion assay showed promising inhibition activity of the investigated compound against Mycobacterium H37Ra strain comparable with Streptomycin used as a reference substance. Important results of these assays are disclosed in Table 2 together with the data for control drugs as Rifampicin and Streptomycin. MIC (Minimal Inhibitory Concentration) and MBC (Minimal Bactericidal Concentration) values of tested strains of microorganisms are presented in Table 3. Presented results suggest that the investigated compound is similar in inhibition activity against Mycobacterium H37Ra to Streptomycin. The values of MIC and MBC are exactly the same for both substances.
| Compound | Mycobacterium Phlei | Mycobacterium H37Ra | Mycobacterium smegmatisATCC 20 |
|---|---|---|---|
| Rifampicin | 28 | 30 | 26 |
| Streptomycin | 23 | 18 | 24 |
| Investigated compound | 15 | 16.5 | 16 |
Table 2: Inhibition zone diameters in millimeters for different Mycobacterium strains.
| Compound | Mycobacterium Phlei | Mycobacterium H37Ra | Mycobacterium smegmatis ATCC 20 |
|||
| MIC (µg/ml) |
MBC (µg/ml) |
MIC (µg/ml) |
MBC (µg/ml) |
MIC (µg/ml) |
MBC (µg/ml) |
|
| Rifampicin | 16 | 32 | 16 | 32 | 128 | 256 |
| Streptomycin | 128 | 256 | 512 | 1024 | 64 | 128 |
| Investigated compound | 512 | 1024 | 512 | 1024 | 254 | 512 |
Table 3: Antimicrobial activity of the investigated compound expressed as Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC).
Conclusion
The synthetized 1,2,4-triazole derivative compound, namely 4-methyl-1-morpholinmethyl-2,4-dihydro-3H-1,2,4-triazol-3- thione, demonstrates tuberculosis inhibition activity similar to the control drug - Streptomycin. The reported analytical method for its quantitative determination in body fluids exhibits good recovery in the range from 78.7 to 90.7% and precision expressed as RSD from 0.19 to 0.15 for the concentration ranging from 0.025 to 0.1 mg mL-1. The 6% perchloric acid appeared to be the most effective agent for biological samples pre-treatment for further HPLC analysis. The elaborated method can be applied for planned pharmacokinetic studies of this promising compound. This method can be recommended for analysis of 1,2,4-triazole derivatives in body fluids.
References
- Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, et al. (2010) Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet 375: 1814-1829.
- Jhamb SS, Goyal A, Singh PP (2014) Determination of the activity of standard anti-tuberculosis drugs against intramacrophage Mycobacterium tuberculosis, in vitro: MGIT 960 as a viable alternative for BACTEC 460. Braz J Infect Dis 18: 336-340.
- Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F, et al. (2006) Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet 368: 2142-2154.
- Balabanova Y, Ruddy M, Hubb J, Yates M, Malomanova N, et al. (2005) Multidrug-resistant tuberculosis in Russia: clinical characteristics, analysis of second-line drug resistance and development of standardized therapy. Eur J Clin Microbiol Infect Dis 24: 136-139.
- Yakar F, Yildiz N, Yakar A, Kiliçaslan Z (2013) Isoniazid- and rifampicin-induced thrombocytopenia. Multidiscip Respir Med 8: 13.
- Abideen PS (2013) Implementation of Self Reporting Pharmacovigilance in Anti Tubercular Therapy Using Knowledge Based Approach. Journal of Pharmacovigilance 1: 1-5.
- Bottari B, Maccari R, Monforte F, Ottanà R, Rotondo E, et al. (2000) Isoniazid-related copper(II) and nickel(II) complexes with antimycobacterial in vitro activity. Part 9. Bioorg Med Chem Lett 10: 657-660.
- Flieger J, Paneth P, Gielzak-Kocwin K, Tatarczak M (2009) Micropreparative isolation of Cu(II) complexes of isoniazid and ethambutol and determination of their structures. J Planar Chromatogr 22: 83-88.
- Saha DK, Sandbhor U, Shirisha K, Padhye S, Deobagkar D, et al. (2004) A novel mixed-ligand antimycobacterial dimeric copper complex of ciprofloxacin and phenanthroline. Bioorg Med Chem Lett 14: 3027-3032.
- Sbardella G, Mai A, Artico M, Loddo R, Setzu MG, et al. (2004) Synthesis and in vitro antimycobacterial activity of novel 3-(1H-pyrrol-1-yl)-2-oxazolidinone analogues of PNU-100480. Bioorg Med Chem Lett 14: 1537-1541.
- Küçükgüzel SG, Oruç EE, Rollas S, Sahin F, Ozbek A (2002) Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur J Med Chem 37: 197-206.
- Mamolo MG, Zampieri D, Falagiani V, Vio L, Banfi E (2003) Synthesis and antifungal activity of (+/-)-1-(5-aryl-3-pyridin-2-yl-4,5-dihydro-pyrazol-1-yl)-2-imidazol-1-yl-ethanone derivatives. Farmaco 58: 315-322.
- Walczak K, Gondela A, Suwiński J (2004) Synthesis and anti-tuberculosis activity of N-aryl-C-nitroazoles. Eur J Med Chem 39: 849-853.
- Klimesová V, Zahajská L, Waisser K, Kaustová J, Möllmann U (2004) Synthesis and antimycobacterial activity of 1,2,4-triazole 3-benzylsulfanyl derivatives. Farmaco 59: 279-288.
- Dabak K, Sezer O, Akar A, Anaç O (2003) Synthesis and investigation of tuberculosis inhibition activities of some 1,2,3-triazole derivatives. Eur J Med Chem 38: 215-218.
- Zamanii K, Faghihii K, Tofighii T, Shariatzadeh MR (2004) Synthesis and Antimicrobial Activity of Some Pyridyl and Naphthyl Substituted 1,2,4-Triazole and 1,3,4-Thiadiazole Derivatives. Turk J Chem 28: 95-100.
- Wujec M, Swatko-Ossor M, Mazur L, Raczynska Z, Siwek A (2008) Synthesis, structure and investigations of tuberculosis inhibition activities of new 4-methyl-1-substituted-4H-1,2,4-triazole-5-thione. J Heterocycl Chem 45: 1893-1896.
- Plech T, Wujec M, Siwek A, Kosikowska U, Malm A (2011) Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur J Med Chem 46: 241-248.
- Plech T, Wujec M, Majewska M, Kosikowska U, Malm A (2013) Microbiologically active Mannich bases derived from 1,2,4-triazoles. The effect of C-5 substituent on antibacterial activity. Med Chem Res 22: 2531-2537.
- Kusmierz E, Siwek A, Kosikowska U, Malm A, Stefanska J, et al. (2013) Antibacterial Activity and Structure-activity Relationship Studies of 4- substituted-5-(diphenylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Lett Drug Design Disc 10: 95-101.
- Plech T, Wujec M, Kosikowska U, Malm A (2012) Halogen substituents as an effective modulators of antibacterial activity of substituted 1,2,4-triazole-3-thiones. Lett Drug Design Disc 9: 947-952.
- Aurand CR (2013) Investigating matrix interference in analysis of antiarrhythmic cardiac drugs in plasma. Supelco Reporter 54: 13-16.
- Aurand CR, Bell DS, Wright M (2012) Highly selective isolation and separation of 25-hydroxyvitamin D and 3-epi-25-hydroxyvitamin D metabolites from serum. Bioanalysis 4: 2681-2691.
- Flieger J (2014) What are the advantages of RP-HPLC methods for the detection of drugs in plasma? Bioanalysis 6: 1151-1154.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13750
- [From(publication date):
September-2014 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 9361
- PDF downloads : 4389