Research Article Open Access
A Rapid and Sensitive UPLC��?ESI-MS/MS Method for Determination of Levonorgestrel by Chemical Derivatization in Human Plasma and its Application to Pharmacokinetic Study
Praveen Kumar V1,2*, Ashish Saxena1,3, Amol Pawar1, Sundara Moorthi Nainar M1, Ravikiran V1, Ravisekhar Kashibhatta1, Pratima Ashawat2and Ashawat MS41Bioanalytical Research Department, Lupin Bio-Research Center, Maharastra State, India
2Department of Science, Pacific University, Rajasthan State, India
3Department of Pharmacy, Pacific University, Rajasthan State, India
4Laureate Institute of Pharmacy, Himachal Pradesh, India
- *Corresponding Author:
- Praveen Kumar
Bioanalytical Research Department
Lupin Bio-Research Center
Pashan, Pune–411021,
Maharastra State, India
Tel: +91-020-66219242
Fax: +91-020-66219224
E-mail: praveenvittala@lupinpharma.com
Received date: June 06, 2014; Accepted date: June 23, 2014; Published date: June 25, 2014
Citation: Kumar VP, Saxena A, Pawar A, Nainar MSM, Ravikiran V, et al.(2014) A Rapid of Levonorgestrel by Chemical Derivatization in Human Plasma and its Application to Pharmacokinetic Study. J Anal Bioanal Techniques S6:003. doi: 10.4172/2155-9872.S6-003
Copyright: © 2014 Kumar VP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid and sensitive liquid chromatography–tandem mass spectrometry (LC–MS/MS) method in human plasma was developed for determination of levonorgestrel using ultra performance liquid chromatography aligned with tandem mass spectrometric detection. The analytes were extracted using solid–phase extraction technique followed by rapid derivatization. Levonorgestrel and levonorgestrel d6 were resolved on Kromasil C18 (50 × 4.6 mm) using gradient flow acetonitrile and 0.1% formic acid solution with a total run time of 5 minutes. The detection was achieved using Waters XEVO–TQ–S mass spectrometry system with a mass transition ion-pair of m/z 328.2 → 90.9 for levonorgestrel and m/z 334.1 → 91.0 for levonorgestrel D6. The method has been validated for a linear range of 100–30000 pg/ml with a correlation coefficient ≥ 0.99. The precision (%RSD) was less than 6.50% and accuracy (%RE) was within ± 5%. The overall recoveries for levonorgestrel and levonorgestrel D6 were 93.69% and 93.88% respectively. The validated method was applied for the pharmacokinetic study of levonorgestrel following a single oral dose of levonorgestrel/ethinyl estradiol (0.15 mg/0.03 mg) tablets in 36 healthy female volunteers.
Keywords
Levonorgestrel; Oxime–Derivatization; SPE; LC–MS/ MS; Human plasma
Introduction
Levonorgestrel is an active component of the racemic mixture of norgestrel is chemically identified as (–)–13–Ethyl–17–hydroxy–18, 19–dinor–17α-pregn–4–en–20–yn–3–one. Levonorgestrel is a second generation synthetic progestational hormone and has a clinical efficacy almost twice of its racemic mixture [1]. Levonorgestrel has been used singly as progesterone only pills (POPs) at very lower doses and used as monophasic or polyphasic in lower dose along with ethinyl estradiol to provide combined oral contraceptive formulations. Combination oral contraceptives (COCs) lower the risk of becoming pregnant primarily by suppressing ovulation. Other possible mechanisms may include cervical mucus changes that inhibit sperm penetration and endometrial changes that reduce the likelihood of implantation. As the use of oral contraceptives has drastically increased globally the need of monitoring the side effects and pharmacokinetic behavior as an individual drug and co-administered drug is gaining high importance [2].
Levonorgestrel is a major substrate of cytochrome P450(CYP)3A4 and is completely absorbed. It is metabolized by hydroxylation at carbons 2, 16 [3,4] and does not undergo first pass metabolism with peak plasma concentration in less than 2 h and half-life ranging between 17 and 20 h. Levonorgestrel has a protein binding of about 97-99%, majority of the binding is to sex hormone binding globulin (SHBG) and a lesser extent to serum albumin [5]. A sensitive bioanalytical method which can quantify levonorgestrel in picogram concentration with low sample volume and high throughput is required for the determination of pharmacokinetic parameters of levonorgestrel.
Steroid hormones consist of 3 hexagonal carbon rings and 1 pentagonal ring, arranged in 6,6,6,5 patterns. Levonorgestrel is structurally similar to other oxosteroid hormones like testosterone and progesterone. The ionization of the oxosteroid is comparatively low and the hydrophobic nature of levonorgestrel enhances the complication in quantitative analysis. Earlier, determination of racemic norgestrel was done using techniques like Radio Immuno Assay (RIA) [5], Enzyme Immuno Assay (EIA) [6], later a decade after analysis of Levonorgestrel in rat plasma [7], water samples [8] was done using HPLC. Luaritsen et al. [9] developed an analytical method using membrane inlet mass spectrometry using desorption chemical ionization technique. The introduction of LC–MS/MS for bioanalysis has introduced improvisations in the bioanalysis [10-12]. Extraction technique comprising LLE followed by online SPE for quantification of levonorgestrel in intrauterine devices (IUDs) for diagnostic purpose was done in sub–nanomolar concentrations were developed [13-15]. Later many analytical techniques were developed with betterment in the sample preparation with methods using liquid–liquid extraction (LLE) solid phase extraction (SPE), online SPE technique. To enhance the method sensitivity and recovery and to attenuate the matrix effect hybrid techniques using a combination of LLE and SPE were also established. Although these assays were sufficiently sensitive but the inclusion of liquid–liquid extraction with error-prone solvent evaporation and reconstitution steps in sample extraction increases the possibility of poor sample cleanup and longer run time. The keto (>C=O) functional group can be derivatized by oximation reaction thereby enhancing derivatives are used in oxosteroid estimation [16-18]. To date, for quantification of levonorgestrel with chemical derivatization has not been published and the current method would be an useful tool for optimal utilization of derivatization techniques for low detection requirement for the future low dossier formulations. The methods developed earlier for determination of levonorgestrel in human plasma at sub-nanomolar concentration were having a large sample volume or tedious extraction procedure with a longer run time and mostly had to compromise on one of these parameters.
Therefore, the current study aims to develop an ESI–LC–MS/MS method for the quantification of levonorgestrel in human plasma by chemical derivatization using hydroxylamine to enhance sensitivity, S/N and throughput with a lowest limit of quantification (LLOQ) 100 pg/mL and apply for bioequivalence study in healthy human volunteers.
Experimental
Chemicals and reagents
The working standards of levonorgestrel (99% purity) and levonorgestrel D6 (99.3% purity) were purchased from Clearsynth (Mumbai, India). High purity water was obtained from a Millipore water purification system (Bangalore, India). Gradient grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). LC/MS grade formic acid was purchased from fisher scientific (Geel, Belgium) and hydroxylamine hydrochloride (AR grade) was purchased from Spectrochem, India. 1cc SPE cartridges of HLB were purchased form Water, USA limited. Drug free (blank) human plasma was obtained from Drug Monitoring Research Institute (Mumbai, India) and was stored at –20°C prior to use.
Calibration curves
Stock solutions of levonorgestrel and internal standard levonorgestrel D6 were prepared in methanol at concentration of 250 μg/ml. Spiking solutions were prepared by diluting stock solutions with methanol:water (50:50%, v/v). A standard calibration curve for levonorgestrel was prepared by spiking 2% of levonorgestrel working standard solution in the blank plasma.
Considering the wider range a ten point calibration curve was prepared ranging from 100 to 35000 pg/ml. Quality control samples were prepared in a similar procedure using a independent stock at four concentration levels of lowest limit of quantification, low, middle and high concentration: 100, 290, 14000 and 27900 pg/ml.
Sample extraction and derivatization
To 300 μl of sample a 50 μl of internal standard working solution (0.1 μg/ml of levonorgestrel D6) and 300 μL of 0.1% formic acid solution was added and vortexed to mix for about 10s. The sample mixture was loaded into an Oasis HLB (1 cm3/30 mg), extraction cartridge which was pre-conditioned with 1 ml methanol and 1 ml water. The extraction cartridge was washed with 1 ml water followed by 1 ml 20% acetonitrile. Levonorgestrel and levonorgestrel D6 were eluted with 0.5 ml of methanol and evaporated under nitrogen stream in water bath (50°C) to dryness. The residue was reconstituted with 100 μL methanol to which equal volume of 0.7% Hydroxylamine and incubated at 50°C for 20 minutes. Another 100 μL of water added to stop the derivatization reaction. After vortexing for about 30 seconds the samples were transferred to conical HPLC vial and 10 μl was injected into the UPLC–ESI-MS system
Instrumentation
UPLC separation was carried out on Waters UPLC comprising of binary pump, column oven, autosampler aligned with Waters Xevo– TQMS mass spectrometer, a triple stage quadrupole mass analyzer with photomultiplier detector, equipped with electrospray ionization (ESI) source (Waters Ltd., UK). MassLynx 4.1 software was used acquisition and analysis. The liquid chromatography separation was performed on a Kromasil C18 (50 × 4.6 mm, 5 μm particle size) at 40°C. A mobile phase consisted of acetonitrile (A) and 0.1% formic acid solution (B) at a flow rate of 0.3 ml/min with a time and solvent gradient composition. Initial gradient at 50% A followed by a linear gradient to 90% A over 1.0 min, held at 90% A upto 2.0 min, shifted to initial gradient of 50% of A over 4.0 min and then held constant until the end of the run for column equilibration. The total run time was 5.0 min and sample injection volume was 10 μl. The mass spectrometer was operated in the multiple reaction–monitoring (MRM) mode. Sample introduction and ionization was ESI in the positive ion mode. The MS parameters for analytes are listed in Table 1.
| Step | Time | Flow rate (ml/min) | Mobile Phase A | Mobile Phase B |
|---|---|---|---|---|
| 0 | 0.00 | 0.300 | 50 | 50 |
| 1 | 1.00 | 0.300 | 90 | 10 |
| 2 | 2.00 | 0.300 | 90 | 10 |
| 3 | 4.00 | 0.300 | 50 | 50 |
| 4 | 5.00 | 0.300 | 50 | 50 |
Table 1a: Gradient elution program of chromatographic separation.
| Ion source | ||
| Capillary voltage | 3.40 kV | |
| Source temperature | 130°C | |
| Desolvation Temperature | 500°C | |
| Desolvation gas flow | 1100 L/Hr | |
| Polarity mode | Positive | |
| Analyte dependent parameters | ||
| Levonorgestrel | Levonorgestrel D6 | |
| Precursor ion (m/z) | 328.2 | 334.1 |
| Product ion (m/z) | 90.9 | 91.0 |
| Cone voltage (V) | 38 | 40 |
| Q1-HM/LM* (amu)a | 15.0 / 2.8 | 15.0 / 2.8 |
| Q3-HM/LM* (amu)b | 14.8 / 2.8 | 14.8 / 2.8 |
| Collision energy | 44 | 40 |
ªQuadrupole 1 High and Low Mass resolution parameters
bQuadrupole 3 High and Low Mass resolution parameters
Table 1b: Ion source and analyte-dependent parameters.
Validation
The validation was performed according to the Guidance to the Industry- Bioanalytical Method Validation recommended by USFDA and EMEA guidelines [19-20].
Specificity was evaluated by analyzing the blank plasma samples from eight different sources including lipemic and hemolysed plasma. Samples were compared with response of 50 pg/mL concentration of levonorgestrel to test no interference at the retention time of levonorgestrel and internal standard levonorgestrel D6. Selectivity was evaluated in the presence of most commonly used over-the-counter (OTC) which include ranitidine, paracetamol, ibuprofen and aspirin. Sensitivity was determined by analyzing six replicates of blank human plasma spiked with the analyte at the lowest level of the calibration curve.
Linearity was assessed by ten–point calibration curve from a range of 100–35000 pg/mL in human plasma in for 5 times in 4 different days. The curves were constructed by plotting the peak area ratio of levonorgestrel to the IS versus nominal concentration of levonorgestrel.
The intra–run and inter–run accuracy were determined by replicate (n=6) analysis of quality control samples and at LOQ that were extracted from the sample batch. The impact of co–administered drug ethinyl estradiol on the determination of levonorgestrel was evaluated by performing precision and accuracy (n=6) was determined at lowest limit of quantification, low, middle and high levels
Accuracy is defined as the percent of relative error (%RE) and was calculated using the formula %RE = (E −T)×(100 / T) where E is the experimentally determined concentration and T is the theoretical concentration. Assay precision was calculated by using the formula %RSD = (SD / MT)×100where %RSD is coefficient of variance, M is the mean of experimentally determined concentrations and SD is the standard deviation of M
The extraction efficiencies of levonorgestrel and levonorgestrel D6 were determined by comparing the peak area of extracted analytes to the peak area of non-extracted derivatized standards (analyte spiked post extraction in blank plasma). The recovery for levonorgestrel was also established in the presence of ethinyl estradiol.
The stability of levonorgestrel was evaluated under various storage and handling conditions using low, middle and high QC samples. The stability QC samples were compared with freshly prepared QC samples (time 0). The stability was evaluated by comparing the mean back–calculated concentrations of stability samples with that of freshly prepared QC samples.
Freeze–thaw stability was evaluated for five complete cycles at –30 & –75°C to 23°C. Short–term stability was evaluated by keeping the sample at room temperature for more than 8 h. In–injector stability was evaluated by re–injecting the samples kept in autosampler (at 10°C) for specific hours. Long–term stability of spiked human plasma samples for levonorgestrel and for levonorgestrel in the presence of ethinyl estradiol was evaluated by analyzing QC samples stored at –30°C and –75°C for longer duration with freshly prepared QC samples.
Stability was determined by calculating the %change and was calculated using the formula %Change = (S −C)×(100 / C)where S is the mean stability sample concentration and C is the mean of freshly prepared or comparison sample concentration. Analytes were considered stable if the %Change was within ± 15% of freshly prepared or comparison sample concentration.
Matrix effect was evaluated with eight different lots of female plasma containing K3EDTA as anticoagulant including hemolysed and lipemic plasma lot. Blank samples were prepared from each lot and were post–spiked with aqueous (derivatized) samples for levonorgestrel and levonorgestrel D6 at low, middle and high QC levels. The post spiked samples along with six replicate injections of aqueous spiked samples of LQC, MQC and HQC were analyzed. The matrix effect was evaluated by calculating the matrix factor for levonorgestrel and levonorgestrel D6, IS normalized matrix factor for levonorgestrel and matrix factor for mean ratio of levonorgestrel and levonorgestrel D6.
Matrix effect was performed with the aim to see the variability of plasma between different lots on the %RSD for mean area response and area ratio. It is considered there was no matrix effect if the %RSD for mean area and mean response ratio was less than 15%.
Results and Discussion
Tuning and chromatography optimization
Levonorgestrel, an oxosteroid has moderate ionization efficiency, the formation of a protonated ion [M+H+] would not be sufficient to be detected at lower concentrations. A solution of about 50 ng/mL was infused using the in-built infusion pump and the tuning was done with the underivatized sample where in the signal of the protonated ions was observed with a moderate response. The ionization was also verified in the negative mode and with APCI source; there was no significant enhancement in either of the ionization modes. The ionization was acceptable but to increase the sensitivity and detect further lower concentrations the ionization capacity was to be increased. Considering the drug being used mostly in female population the use of low sample volume for estimation of levonorgestrel was also equally important.
Derivatization
As discussed by Griffith et al. [16], ionization enhancement by simple derivatization using hydroxylamine hydrochloride, wherein the carboxyl functional group is substituted with N−OH moiety was considered for levonorgestrel. The formation of the levonorgestrel– oxime (L−ox) derivative has enhanced the ionization by more than 10 folds. The optimization of the concentration, temperature and time were the key factors which needed to be optimized. The concentration of about 0.7% hydroxylamine at 50°C and for duration of 20 minutes has shown complete and uniform derivatization, which was confirmed by the absence of underivatized compound in the sample.
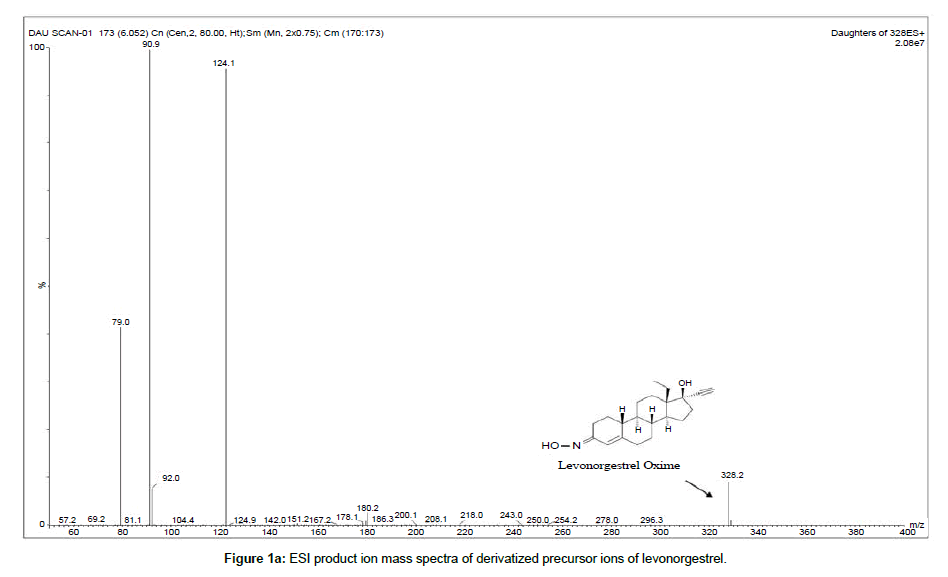
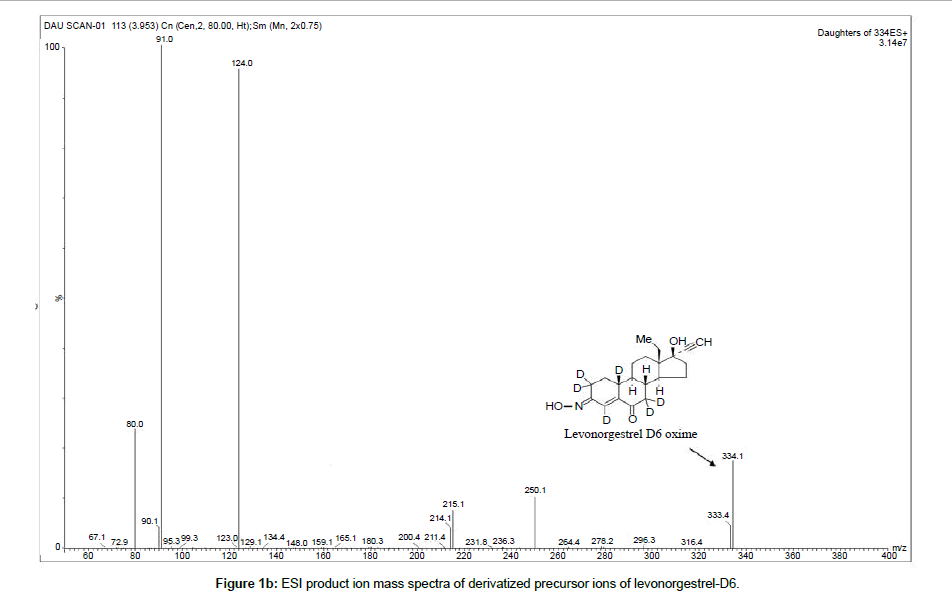
The compound and source parameters have been optimized, during tuning of the compound three major product ions (m/z) of 124.1, 91.0 and 79.0 were found to have profoundly significant response. Among the identified product m/z of 124.1 was found to have high signal but the ionization uniformity and consistency was found to be more reproducible for product ion with m/z of 91.0. The final MRM parameters of the L-ox and its internal standard are 328.2/90.9 and 334.1/91.0 respectively. The selected fragments of each compound, as product ion to be monitored are indicated in Figure 1.
The mobile phases at various pH from acidic to basic were tried and the optimal peak symmetry, low baseline noise with good signal to noise (S/N) were achieved in acidic conditions at pH about ~ 3.0 to 3.5. The mobile phase of acetonitrile-Pump-A and 0.1% Formic acid -Pump-B with gradient flow has shown the best chromatographic separation.
Columns including C8, C18, phenyl-hexyl and Cyano make, with varied column lengths ranging from 50 mm to 150 mm with a porosity of 5 μm, 3 μm and sub-2 micron columns were tried and the best results could be achieved in Kromasil C18 (50 × 4.6 mm) column. Pre-column chemical derivatization has enhanced the S/N ratio by more than 10 folds with good peak symmetry with a low peak width and shorter runtime.
The involvement of derivatization using hydroxylamine in the sample extraction protocol has added the requirement for better sample cleanup, hence traditional sample cleanup of precipitation and liquid–liquid extraction were not tried. Solid Phase Extraction trials with generic protocol were tried but there was significant interference and low recovery along with significant matrix suppression was observed in the sample. The initial sample cleanup to remove the possible phospholipids was done by SPE using hydrophillic–lipophillic balanced (HLB) cartridges. Post derivatization samples contained the excess hydroxylamine, hence and additional sample cleanup using mixed mode cation exchange (MCX) cartridges has been considered thereby ensuring complete removal of the traces of hydroxylamine from the sample. This extraction methodology had ensured better Signal-to-Noise (S/N), sensitivity, recovery with no matrix effect and interference being observed at the retention time of levonorgestrel and levonorgestrel D6.
Specificity and selectivity
Utilization of predominant product ions for each compound enhanced mass spectrometric specificity. The mass transition ion-pair selected were 328.2 → 90.9 for levonorgestrel and 334.1 → 91.0 for levonorgestrel-D6 and these transitions were found to be specific for both the analytes.
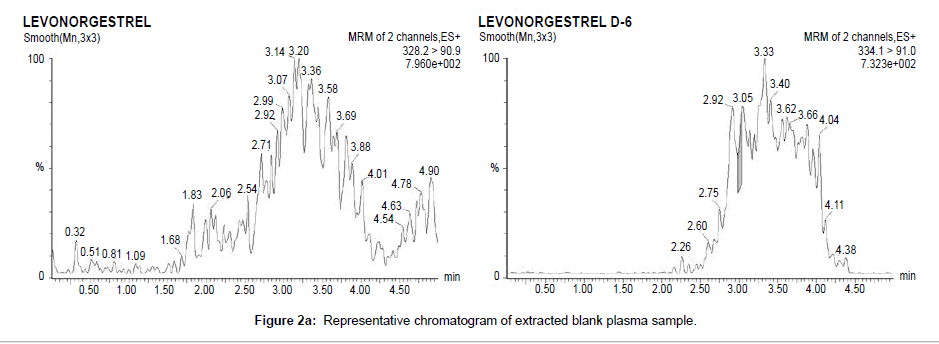
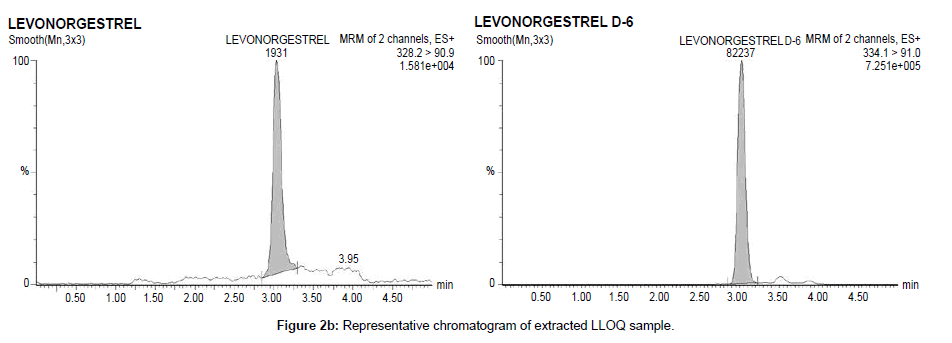
Chromatographic specificity of the method was demonstrated by the absence of endogenous interfering peaks at the retention times of levonorgestrel and levonorgestrel D6 in eight different lots of extracted blank plasma. Selectivity of the derivatized moieties has been demonstrated for levonorgestrel and levonorgestrel D6 wherein there was no significant response at the retention time of each compound individually and in the presence of concomitant drugs. Representative chromatograms of extracted blank plasma, extracted plasma samples containing 99.607 pg/ml levonorgestrel (LLOQ) and plasma samples from subjects are presented in Figure 2a–2c respectively.
Linearity
Linearity was assessed by analyzing six calibration curves in human plasma at ten levels on different days. The peak area ratios (area of levonorgestrel/area of levonorgestrel D6) of calibration standards were proportional to the concentration of analytes in each assay over the nominal concentration range of 100–35000 pg/ml for levonorgestrel. The calibration curves were constructed using a simple and linear weighted (1/concentration2) least square regression to achieve homogeneity of variance. The correlation coefficients were ≥ 0.9990 (n=6) for levonorgestrel. The mean (± SD) slopes of the calibration curves (n=6) for levonorgestrel were 0.000203939 (± 0.00004416).
Sensitivity (lower limit of quantification)
The LOQ is defined as the lowest concentration of the calibration standard yielding accuracy ± 20% and precision of ≤ 20%. The LOQ for levonorgestrel was 100 pg/ml. These data are tabulated in Table 2a for levonorgestrel. The intra-run precision (%RSD) at the LOQ plasma samples containing levonorgestrel was 6.44%. The intra-run accuracy (%RE) at the LOQ plasma samples containing levonorgestrel was –0.95%
| Analyte | QC Level | Spiked conc. (pg/ml) | Mean (± SD) calculated conc. (pg/ml) | %RSD | %RE |
|---|---|---|---|---|---|
| Levonorgestrel | LLOQQC | 99.611 | 98.662 (± 6.349) | 6.44 | -0.95 |
| LQC | 290.749 | 294.688 (± 8.943) | 3.03 | 1.35 | |
| MQC | 14537.450 | 13983.266 (± 198.507) | 1.42 | -3.81 | |
| HQC | 27956.635 | 27147.911 (± 206.752) | 0.76 | -2.89 |
Table 2a: Intra-run or within batch (n=6) precision and accuracy of levonorgestrel in human plasma.
Precision and Accuracy
The intra-run precision (n=6) was ≤ 6.44% and intra-run accuracy ranged from –3.81 to 1.35 for levonorgestrel (Table 2a). The inter-run precision and accuracy were determined by pooling all individual assay results of replicate (n=24) QC samples over the four separate batch runs.The inter-run precision (%RSD) was ≤ 4.30. The inter-run accuracy (%RE) ranged from –4.56 to –0.36 for levonorgestrel (Table 2b). The precision (%RSD) was ≤ 4.30 and accuracy (%RE) ranged from –8.16 to –0.35 for levonorgestrel in the presence of ethinyl estradiol (Table 2c).
| Analyte | QC Level | Spiked conc.(pg/ml) | Mean (± SD) calculated conc. (pg/ml) | %RSD | %RE |
|---|---|---|---|---|---|
| Levonorgestrel | LLOQQC | 99.611 | 98.504 (± 4.856) | 4.30 | -1.11 |
| LQC | 290.749 | 289.709 (± 10.092) | 3.48 | -0.36 | |
| MQC | 14537.450 | 13875.116 (± 336.590) | 2.43 | -4.56 | |
| HQC | 27956.635 | 27032.801 (± 811.777) | 3.00 | -3.30 |
Table 2b: Inter-run or between batch (n=24) precision and accuracy of levonorgestrel in human plasma.
| Analyte | QC Level | Spiked conc.(pg/ml)* | Mean (± SD) calculated conc. (pg/ml) | %RSD | %RE |
|---|---|---|---|---|---|
| Levonorgestrel | LLOQQC | 99.611 | 99.263 (± 5.906) | 4.30 | -0.35 |
| LQC | 290.749 | 284.350 (± 3.890) | 3.48 | -2.20 | |
| MQC | 14537.450 | 13351.197 (± 185.788) | 2.43 | -8.16 | |
| HQC | 27956.635 | 25973.737 (± 481.165) | 3.00 | -7.09 |
*About 400 pg/ml concentration of ethinyl estradiol concentration spiked in the QC samples
Table 2c: Precision and accuracy (n=6) of levonorgestrel (in the presence of ethinyl estradiol) in human plasma.
Recovery
Six replicates at low, medium and high quality control concentrations for levonorgestrel were prepared for recovery determination. The mean recovery for levonorgestrel and levonorgestrel D6 were 93.69% and 93.88% respectively. The mean recovery for levonorgestrel and levonorgestrel D6 in the presence of ethinyl estradiol were 93.83% and 94.96% respectively.
Stability
The results of the stability studies are enumerated in Table 3. The bench top stability results allowed us to conclude that levonorgestrel is stable for 10 h at room temperature. The autosampler stability results indicated that levonorgestrel is stable for 75 h at 10°C. The freeze-thaw stability results concluded that on even after five complete cycles of freezing and thawing there is no impact on the stability of levonorgestrel when stored at –30°C and –75°C. Long-term stability of levonorgestrel in plasma in storage at –30°C and –75°C was found to be stable for 116 days. Long-term stability in plasma of levonorgestrel in the presence of ethinyl estradiol at –30°C and –75°C was found to be stable for at least 73 days.
| Stability | Spiked conc. (pg/ml) | Mean (± SD) obtained concentration | % Change (Stability) | |
|---|---|---|---|---|
| Comparison samples (pg/ml) | Stability samples (pg/ml) | |||
| Bench topa | 27956.635 | 27428.770 (± 71.101) | 27557.790 (± 74.193) | 0.47 |
| 14537.450 | 13708.226 (± 125.746) | 13916.554 (± 53.782) | 1.52 | |
| 290.749 | 279.777 (± 4.203) | 284.292 (± 2.066) | 1.61 | |
| Process stabilityb | 27956.635 | 27466.251 (± 107.836) | 27348.265 (± 322.401) | –0.43 |
| 14537.450 | 14430.853 (± 107.114) | 14353.333 (± 131.016) | –0.54 | |
| 290.749 | 291.135 (± 3.740) | 289.277 (± 7.848) | –0.64 | |
| Freeze/thawc | 27956.635 | 27684.246 (± 72.812) | 27549.256 (± 132.294) | –0.49 |
| 14537.450 | 13914.170 (± 126.952) | 13768.270 (± 95.724) | –1.05 | |
| 290.749 | 272.259 (± 4.331) | 275.954 (± 4.053) | 1.36 | |
| Freeze/thawd | 27956.635 | 27684.246 (± 72.812) | 27556.702 (± 182.816) | –0.46 |
| 14537.450 | 13914.170 (± 126.952) | 13742.464 (± 74.795) | –1.23 | |
| 290.749 | 272.259 (± 4.331) | 275.627 (± 3.827) | 1.24 | |
| Long terme | 27956.635 | 29103.028 (± 276.083) | 29116.039 (± 376.954) | 0.04 |
| 14537.450 | 15159.266 (± 105.244) | 15144.490 (± 147.049) | –0.10 | |
| 290.749 | 293.156 (± 12.237) | 292.720 (± 12.506) | –0.15 | |
| Long termf | 27956.635 | 29103.028 (± 276.083) | 29266.360 (± 685.306) | 0.56 |
| 14537.450 | 15159.266 (± 105.244) | 15145.190 (± 94.743) | –0.09 | |
| 290.749 | 293.156 (± 12.237) | 293.062 (± 10.547) | –0.03 | |
| Long termg | 27956.635 | 29082.193 (± 323.626) | 28937.989 (± 291.306) | –0.50 |
| 14537.450 | 15091.547 (± 76.126) | 15036.159 (± 135.769) | –0.37 | |
| 290.749 | 307.782 (± 13.278) | 306.090 (± 13.228) | –0.55 | |
| Long termh | 27956.635 | 29082.193 (± 323.626) | 29009.723 (± 549.216) | –0.25 |
| 14537.450 | 15091.547 (± 76.126) | 15075.167 (± 94.549) | –0.11 | |
| 290.749 | 307.782 (± 13.278) | 310.764 (± 13.021) | 0.97 | |
* - % Change – Determined by calculating the percentage change of mean of stability QC concentration against mean of comparison QC concentrations
aAfter 6 hours at room temperature
bAfter 75 hours in autosampler at 10°C
cAfter five freeze/thaw cycles at –30°C
dAfter five freeze/thaw cycles at –75°C
eLong term matrix stability at –30°C for 72 days
fLong term matrix stability at –75°C for 72 days
gLong term matrix stability in presence of ethinyl estradiol at –30°C for 73 days
hLong term matrix stability in presence of ethinyl estradiol at –75°C for 73 days
Table 3: Stability results for levonorgestrel (n=6).
Matrix effect
The matrix factor for levonorgestrel and levonorgestrel D6 was calculated by comparing the area response observed in post spiked samples with that of unextracted derivatized samples at LQC, MQC and HQC level and the matrix effect was evaluated from the %RSD of matrix factor at each level. Three quality control samples at each level were analyzed and the mean of %bias of the samples analyzed was found less than 15% for each QC level for levonorgestrel and levonorgestrel D6 (Table 4).
| HQC | MQC | LQC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sr. no. | Parameters | Mean matrix factor | (± SD) | %RSD | Mean matrix factor | (± SD) | %RSD | Mean matrix factor | (± SD) | %RSD |
| 1 | Analyte area | 0.962 | 0.052 | 5.41 | 1.054 | 0.069 | 6.55 | 1.099 | 0.024 | 2.18 |
| 2 | IS area | 0.971 | 0.052 | 5.36 | 1.028 | 0.075 | 7.30 | 1.060 | 0.014 | 1.32 |
| 3 | IS normalized | 0.991 | 0.005 | 0.50 | 1.026 | 0.018 | 1.75 | 1.037 | 0.019 | 1.83 |
Table 4: Matrix effect (n=7) for levonorgestrel and levonorgestrel D6.
Hence this clearly proved that the elution of endogenous matrix peaks during the run has no effect on the quantification of levonorgestrel. Therefore the method of extraction of levonorgestrel from plasma was rugged enough and gave accurate and consistent results when applied to subject samples.
Dilution integrity
The dilution integrity experiment was intended to validate the dilution test to be carried out on account of high analyte concentrations (above ULOQ), which may be observed during real subject samples analysis. Dilution integrity experiment was performed at about 1.60 times the ULOQ concentration. Six replicates samples of ½ and ¼ dilution concentrations were prepared and the back calculated concentrations were derived by applying the dilution factor of 2 and 4 respectively against the freshly prepared calibration curve.
Application of method
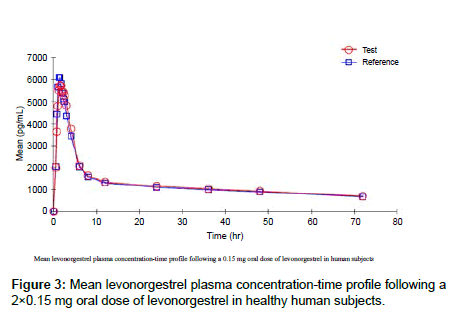
The analytical method validated was applied to evaluate bioequivalence of two formulations of for an oral contraceptive formulation. Plasma samples were periodically collected up to 72 h after a single oral dose administration of a 0.15 mg/0.03 mg tablet of levonorgestrel and ethinyl estradiol to 36 healthy female volunteers in each phase. A total of 19 samples for each period were collected at 0.00 (pre-dose), 0.50, 0.75, 1.00, 1.25, 1.50, 1.75, 2.00, 2.25, 2.50, 3.00, 4.00, 6.00, 8.00, 12.00, 24.00, 36.00, 48.00 and 72.00 h after oral dose administration of a 0.15 mg tablet. A total of 1368 human plasma samples from 36 healthy female volunteers were analyzed along with calibration standards and QC samples. Twenty calibration curves were processed for quantification of analysed samples including 150 samples for incurred sample reanalysis (ISR). No interference peak was found in pre-dose samples for all volunteers and the assay reproducibility was demonstrated by performing ISR in 8% of total subject samples with all samples found to be within ± 20% as compared to the initial concentrations. The mean (±SD) plasma maximum concentrations obtained were 6555.77 (± 2483.80) pg/ml and 6735.56 (± 2731.57) pg/ml, for the levonorgestrel of both test and reference formulations, respectively. The mean levonorgestrel plasma concentration–time profile following a 0.15 mg oral dose of levonorgestrel to human subjects is shown in Figure 3.
Conclusion
This article reports a novel and robust method for determination of levonorgestrel by oxime derivatization. The signal–to–noise of greater than 20 assures enables the method can be even detected to even lower concentrations for future requirements of low dose formulations. The SPE–Derivatization extraction is critical step in the sample preparation assures a better sample cleanup for the determination of levonorgestrel in human plasma with a limit of quantification of 100 pg/ml for levonorgestrel.
It is concluded that this method is sensitive, specific and reproducible for the quantitative determination of levonorgestrel in human plasma in pharmacokinetic and bioavailability studies.
Acknowledgements
Authors acknowledge the technical support provided by Lupin Bioresearch Center, India. This paper is part of research work for the grant of Doctorate in philosophy in science from Department of Science, Pacific University, Rajasthan, India.
References
- Croxatto HB, Díaz S, Salvatierra AM, Morales P, Ebensperger C, et al. (1987) Treatment with Norplant subdermal implants inhibits sperm penetration through cervical mucus in vitro. Contraception 36: 193-201.
- Falsetti L, Galbignani E (1990) Long-term treatment with the combination ethinylestradiol and cyproterone acetate in polycystic ovary syndrome. Contraception 42: 611-619.
- Moreno I, Quiñones L, Catalán J, Miranda C, Roco Á, et al. (2012) [Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study]. Biomedica 32: 570-577.
- Kook K, Gabelnick H, Duncan G (2002) Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception 66: 73-76.
- Watson TG, Stewart BJ (1988) A sensitive direct radioimmunoassay for assessing D-norgestrel levels in human plasma. Ann Clin Biochem 25 : 280-287.
- Munro CJ, Laughlin LS, VonSchalscha T, Baldwin DM, Lasley BL (1996) An enzyme immunoassay for serum and urinary levonorgestrel in human and non-human primates. Contraception 54: 43-53.
- Tang T, Li P, Luo L, Shi D, Li J, et al. (2010) Development and validation of a HPLC method for determination of levonorgestrel and quinestrol in rat plasma. Biomed Chromatogr 24: 706-710.
- Chang HF, Wang JQ, Wang B, Deng AP (2013) An immunochromatographic assay for rapid and simultaneous detection of levonorgestrel and methylprednisolone in water samples. Chinese Chemical Letters 24: 937-940.
- Lauritsen FR, Rose J (2000) Determination of steroid hormones by membrane inlet mass spectrometry and desorption chemical ionization. Analyst 125: 1577-1581.
- Zhao LZ, Zhong GP, Bi HC, Ding L, Deng Y, et al. (2008) Determination of levonorgestrel in human plasma by liquid chromatography-tandem mass spectrometry method: application to a bioequivalence study of two formulations in healthy volunteers. Biomed Chromatogr 22: 519-526.
- Wang QG, Wu ZP, Wang YM, Luo G, Wu ER et al. (2001) Determination of Levonorgestrel in human serum by Liquid Chromatographic-Electrospray Tandem Mass Spectrometry. Anal Lett 34: 103-112.
- Theron HB, Coetzee C, Sutherland FC, Wiesner JL, Swart KJ (2004) Selective and sensitive liquid chromatography-tandem mass spectrometry method for the determination of levonorgestrel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 813: 331-336.
- Moser C, Zoderer D, Luef G, Rauchenzauner M, Wildt L, et al. (2012) Simultaneous online SPE-LC-MS/MS quantification of six widely used synthetic progestins in human plasma. Anal Bioanal Chem 403: 961-972.
- Matejícek D, Kubán V (2007) High performance liquid chromatography/ion-trap mass spectrometry for separation and simultaneous determination of ethynylestradiol, gestodene, levonorgestrel, cyproterone acetate and desogestrel. Anal Chim Acta 588: 304-315.
- Bhargavi P, Lakshmi KS, Lakshmi S, Ravi Kumar (2011) Quantitative bioanalysis of Levonorgestrel in human plasma using LC-MS-MS. International Journal of Institutional Pharmacy and Life Sciences 1: 39-48.
- Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Owen WE, et al. (2006) Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem 52: 1559-1567.
- Liu S, Sjövall J, Griffiths WJ (2000) Analysis of oxosteroids by nano-electrospray mass spectrometry of their oximes. Rapid Commun Mass Spectrom 14: 390-400.
- Griffiths WJ (2003) Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom Rev 22: 81-152.
- US Department of Health and Human Services, Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Method Validation. Center for Drug Evaluation and Research (CDER).
- Committee for Medicinal Products for Human Use (CHMP) (2009) Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16667
- [From(publication date):
specialissue-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11909
- PDF downloads : 4758