Research Article Open Access
Determination of Ethambutol in Presence of Fixed Dose Combination Molecules from Human Plasma by Direct Injection to Liquid Chromatography Tandem Mass Spectrometry
| Chaitanya Krishna A1*, Saravanan RS2, Sathiyaraj M2, Jeevanantham S2 and Baskaran R2 | |
| 1Study Director, Bombay Bioresearch Centre, Mumbai, India | |
| 2Department of Bioanalaytical, Bombay Bioresearch Centre, Mumbai, India | |
| Corresponding Author : | Chaitanya Krishna A Study Director, Bombay Bioresearch Centre Govandi-400043, Mumbai, India E-mail: chaitanya@bbrc-cro.com |
| Received February 21, 2012; Accepted March 31, 2012; Published April 05, 2012 | |
| Citation: Chaitanya Krishna A, Saravanan RS, Sathiyaraj M, Jeevanantham S, Baskaran R (2012) Determination of Ethambutol in Presence of Fixed Dose Combination Molecules from Human Plasma by Direct Injection to Liquid Chromatography Tandem Mass Spectrometry. Clin Pharmacol Biopharm. 1:101. doi:10.4172/2167-065X.1000101 | |
| Copyright: © 2012 Chaitanya Krishna A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
A simple, rapid, specific and sensitive liquid chromatography tandem mass spectrometric method has been developed and validated for the estimation of Ethambutol from 100 μL of human plasma. Ethambutol is extracted from human plasma by Protein Precipitation Extraction. Glipizide was used as an internal standard. Detection was performed using TSQ Quantum Discovery max mass spectrometer with ESI source in positive polarity. The detection transition for Ethambutol is 205.230 → 116.090 and for Glipizide is 446.200 → 321.200. Chromatographic separation of analyte and internal standard were carried out using a reverse phase Agilent, Eclipse XDB-C18, 4.6 X 150 mm, 5 μ at a flow rate of 0.500 mL/min. The mobile phase is composed of methanol: 0.1 %TFA in 5 mM Ammonium Acetate (90:10) v/v. The assay of Ethambutol is linear over the range of 0.106 μg /mL to 7.006 μg/mL with a precision < 9.91% and the limit of quantification in plasma for Ethambutol was 0.106 μg/mL. Mean extraction recovery obtained was 98.70%. Samples are stable at room temperature for 6 hrs, processed samples were stable at least for 28 hrs and also stable at three freeze–thaw cycles. The method has been used to perform pharmacokinetic and bioequivalence studies in humans.
| Keywords |
| Ethambutol; LC-MS/MS; Validation; Human Plasma |
| Introduction |
| Ethambutol is an oral chemotherapeutic agent which is specifically effective against mycobacterium, which was the recent discovered (1961) first line treatment as anti-TB drug [1]. It has excellent activity in vitro and in vivo against M. tuberculosis. Ethambutol penetrates tissues rapidly and in high concentrations, including lung, liver and kidney in experimental tuberculosis. The absorption of ethambutol is rapid. Following a dosage of 25 mg/kg body weight, a peak serum concentration of 4 to 5 mg/L is achieved approximately two to four hours after administration [1]. Ethambutol is a white crystalline powder, soluble in chloroform, sparingly soluble in water, it has pKa 6.35 and 9.35, chemically called 2, 2’-(1, 2-Ethylenediimino) bis- 1-butanol with a molecular weight of 204.31 g/mol [2,3]. Glipizide is chemically called as N-[2-(4-{[(cyclohexylcarbamoyl) amino] sulfonyl} phenyl) ethyl]-5-methylpyrazine-2-carboxamide with a molecular weight of 445.178 g/mol. Glipizide is a hypoglycemic agent with molecular formula of C21H27N5O4S which was used as internal standard [4]. |
| According to the literatures available, methods found for the determination of Ethambutol in plasma were in High Performance Liquid Chromatography (HPLC) [5,6] where, extraction process involves more time consuming and tedious steps like derivatization. Though methods are available for Liquid Chromatography Mass Spectrometer tandem Mass Spectrometry (LC-MS/MS) [7-10], it is found that the chromatographic conditions and extraction processes were highly expensive as they use cartridges, and high solvent usages [10]. In addition, no literatures specify that, their methods can be used for four drug fixed dose combination formulations. The objective of the analyst has become more important i.e., to develop and validate a method for the determination of Ethambutol in human plasma by LC-MS/MS which should be simple in extraction process and chromatographic condition, precise, sensitive and should also applicable in BA-BE analysis for four drug fixed dose combination formulation. |
| Experimental |
| Materials and Reagents |
| Ethambutol is obtained from Maneesh Pharmaceuticals Ltd Mumbai. The Internal Standard Glipizide is purchased from Clearsynth Labs Pvt. Ltd Mumbai. Methanol (HPLC grade), Acetonitrile (HPLC grade), Potassium di-hydrogen acid (AR grade); Water (Ultra Pure grade), Ascorbic Acid, Ammonium Acetate (AR grade), Trifluroacetic used (AR grade). |
| Instruments |
| The Liquid chromatography coupled with tandem Mass Spectrometer (LC-MS/MS) system consists of a Finnigan Surveyor Auto sampler, Surveyor LC Pump Plus solvent delivery system and a column Oven (Thermo Electron Corporation) used for ion separations. The Mass spectrometer was Thermo Scientific TSQ Quantum discovery max Ultra triple stage quadrupole mass spectrometer used for ion detection. An Electron Spray Ionization (ESI) source was used. Data was collected and processed using LC Quan Version. 2.5.6 Data collection and integration software. |
| Chromatographic Condition: The Liquid Chromatographic separations were carried out by using EclipseXDB-C18, 4.6 X 150 mm, 5 μ (Agilent). Column temperature was held at 30°C. The auto sampler tray temperature was 10°C. The mobile phase is composed of methanol: 0.1 %TFA in 5 mM Ammonium Acetate (90:10) v/v with flow rate of 0.500 mL/min. A typical injection volume was 5.0 μL. |
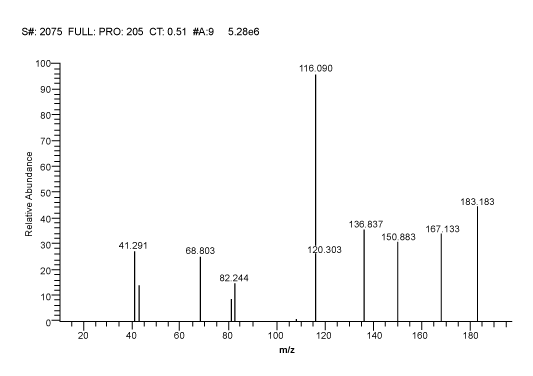
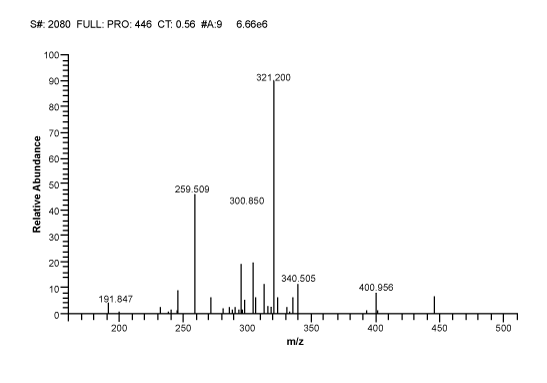
| MS/MS Detection: Precursor ions for analyte and internal standard were determined from mass spectra obtained by the TSQ mass spectrometer. TSQ mass Spectrometer includes an electronically-controlled, integrated syringe pump. The MS conditions for Ethambutol and Glipizde were optimized by separate infusion into the MS at a flow rate of 5 μL/min constantly, while adjusting MS parameters to achieve maximal intensity. Electro-spray ionization in positive ion mode (ESI+) was used for ionization and selective reaction monitoring (SRM) mode was chosen for detection. The optimized precursor ions pairs were m/z 205.230 → 116.090 for Ethambutol (Figure 1) and m/z 446.200 → 321.200 for Glipizide (Figure 2). The optimized MS parameters were as follows: Ion Spray voltage: 5000 volt, Sheath gas pressure: 30 psi, Auxiliary gas pressure: 5 psi, Capillary temperature: 350°C. Collision Pressure: 0.8 psi. Peak areas were automatically integrated using LC Quan Version 2.5.6 (Thermo Corporation). |
| Preparation of Calibration Standards and Quality Control Samples |
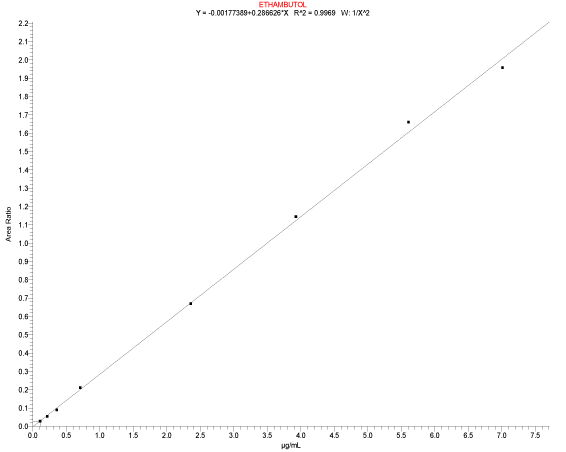
| The calibration standards and the quality control (QC) samples were prepared from separate stock standard solutions. The concentrations of stock solutions were 1000.871 μg/mL and 1000.812 μg/mL for calibration standard and quality control samples and methanol was used as diluent. The Spiking solutions for calibration standards and quality control concentrations were prepared in methanol:water (90:10) %v/v. The calibration standard human plasma samples were prepared by spiking corresponding spiking calibration standard solutions into blank human plasma (containing 0.1% Ascorbic acid) to provide concentrations range between 0.106 μg/ mL to 7.006 μg/mL (Figure 3). For quality control plasma samples preparation, the corresponding spiking quality control solutions is spiked to the human blank plasma to attain the concentration of 0.108 μg/mL, 0.318 μg/mL, 3.183 μg/mL and 6.005 μg/mL for LOQQC, LQC, MQC and HQC respectively. For the spiking, typically, the spiking solutions, volume of 20.000 μL were spiked into 0.980 mL of human blank plasma. Internal standard stock solution (1046.461 μg/mL) of Glipizide was prepared in methanol. Working internal standard solution (10.465 μg/mL) was prepared in methanol: water (90:10) %v/v. |
| Sample Extraction |
| A 100.0 μL aliquot of plasma samples was mixed with 50.0 μL of internal standard working solution (10.465 μg/mL) and vortex-mix the samples for approximately 1 min. Add 1.0 mL of methanol and vortex-mix the samples for 5 mins at 2000 rpm and centrifuge for 10.0 min in 4000 rpm at 4°C, Separate 0.200 mL of the supernatant in HPLC vial and subject 4 μL samples for chromatographic analysis. |
| Validation |
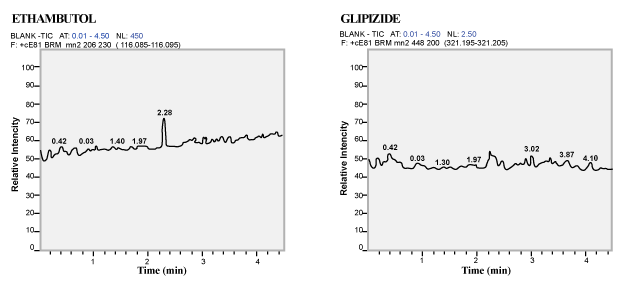
| Selectivity and Specificity: Blank human plasma from eight different lots (including one Haemolysed and one Lipimic) were processed without Analyte and internal standard. And with the same eight lots LLOQ level is processed to evaluate the presence of any interference at the retention time of Analyte and Internal standard [11]. |
| Matrix factor: Evaluate the matrix factor at low and high quality control concentrations, to ensure that the precision, selectivity and sensitivity are not compromised due to a change in matrix. Matrix factor can be termed as the quantitative measurement of the matrix effect. Prepare aqueous mixtures of internal standard and analyte at concentrations representing 100% extraction of internal standard and analyte at low and high QC concentrations. These shall serve as reference samples. Processed duplicate 8 different lots of blank matrices (from eight individuals, including, one Haemolysed and one Lipimic) without addition of IS. After drying step, reconstitute eight aliquots each with reference samples at low and high QC concentrations respectively. |
| Calibration Curve and Linearity: The eight-point calibration curve was constructed by plotting, peak area ratio of Ethambutol to their corresponding internal standard versus Ethambutol concentrations. A linear regression with weighing factor of linear 1/ x2 was applied. |
| Intra and inter-day assay accuracy and precision: Intra-day precision and accuracy were determined by analysis of six replicates of each QC sample (n = 6) at LOQQC, LQC, MQC and HQC concentration levels extracted with a set of standards in one batch. The same procedure was repeated on different day with new samples to determine inter-day precision and accuracy. |
| Recovery: Recovery is carried out to evaluate the loss of drugs and/or internal standards during sample extraction. The drugs and internal standards area counts from extracted QC samples were compared with corresponding QCs reference samples to evaluate any loss of either drugs or internal standards. It is preferable to observe consistent recovery for all three QC levels. |
| Stability: Stability of both drugs in different matrices and under different conditions was evaluated. The detailed tests are described below. Stability was assessed by comparing the mean concentration of the stored QC samples with the mean concentration of freshly prepared QC samples. Drug stability in pooled human blank plasma is a function of the storage conditions, the chemical properties of the drug and the matrix effect. The following tests were performed to evaluate the stability, Short-term and Long-term Stock solution stability, Bench top stability, Freeze and thaw stability, Auto sampler stability, Wet Extract Stability, Long Term stability In Matrix. |
| Result and Discussion |
| Method development |
| The method development of Ethambutol was performed with a concern to get a simple, rapid, selective method and it should also be compatible for the determination of ethambutol even in the presence of Rifampicin, Isoniazid, and Pyrazinamide, where in this method can be used for other fixed dose combination formulations. |
| In the optimization of chromatographic condition, there are two primary objectives: mobile phase and column selection, while achieving these primary objectives mobile phase should be very much compatible and simple and the column should also exhibit good chromatographic conditions. Mobile phase, Methanol: 0.1% TFA in 5 mM Ammonium Acetate (90:10)% v/v with agilent EclipseXDB-C18 column was found to be a suitable choice while optimising. It was found that the deutriated form of the molecule was being used as an internal standard in some articles [10], which is found to be an expensive alternative. In the current method Glipizide is used as an internal standard where, a good recovery of more than 87% was obtained with sharp peak shape and consistency under these chromatographic conditions. Though a deuterated standard was not used, major obstacles like matrix effects, relative matrix effects were not observed during the analysis. It has neither affected the extraction recovery also. |
| Some of the available processes of extraction [10] were reported, where those methods propose more number of extraction steps, thus process, solvent and time consumption. Thus, they were more tedious to perform. To avoid this situation authors contribute a new extraction process where the extraction steps and solvent usage are less, hence, less time consuming and simple. |
| Ethambutol is mostly analyzed in combination with other anti-TB products [12], where in molecules like rifampicin are not stable (photosensitive and prone to oxidative degradation) [13]. In such cases, all the combination drugs should be stored in plasma containing ascorbic acid for stability purpose. Owing to these complexities, authors suggest a method which can be used for analysis of Ethambutol in human plasma containing the stabilizing agent Ascorbic Acid. In addition, the same amount of ascorbic acid was added to the plasma, for all the validations experiments performed for Isoniazid and Pyrazinamide. From the validation data of all the analytes, it was found that there is no interference caused by ascorbic acid particularly for Isoniazid and Pyrazinamide. Hence, authors propose a simple method which is selective, compatible and specifically can be used for the determination of ethambutol in fixed dose combination formulations in BA-BE studies. |
| Validation |
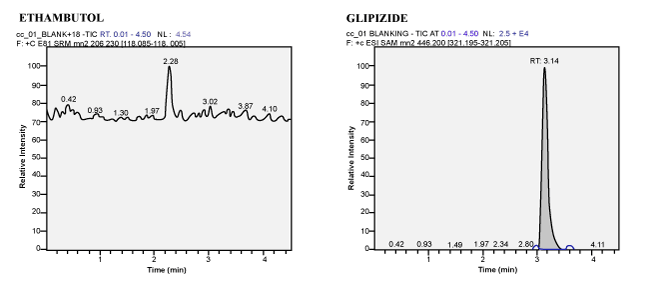
| Selectivity and Specificity: No interference from the blank plasma at the retention time of the analyte and Internal standard. |
| Matrix Factor: Observed % CV of matrix factor is 13.69% and 5.43% in LQC, 7.45% and 2.95% in HQC for Ethambutol and Internal standard respectively. All eight matrix lots showed very similar matrix effect for both analyte and their corresponding internal standard. |
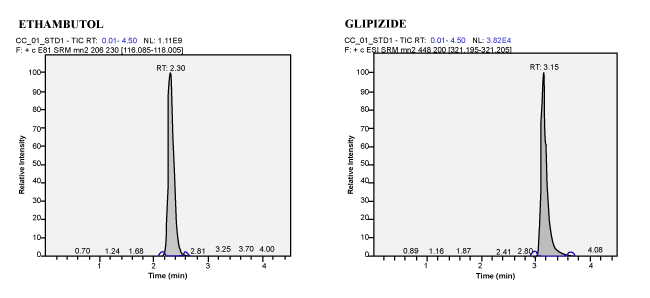
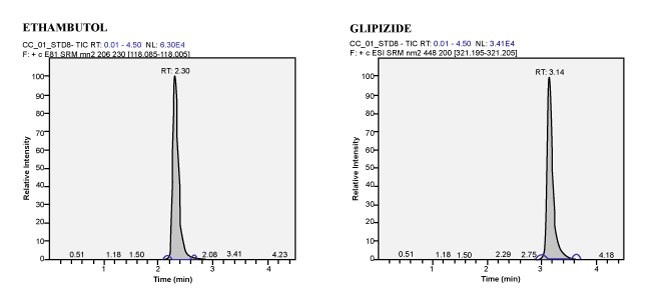
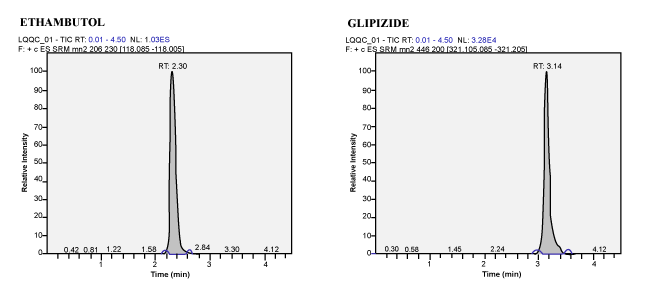
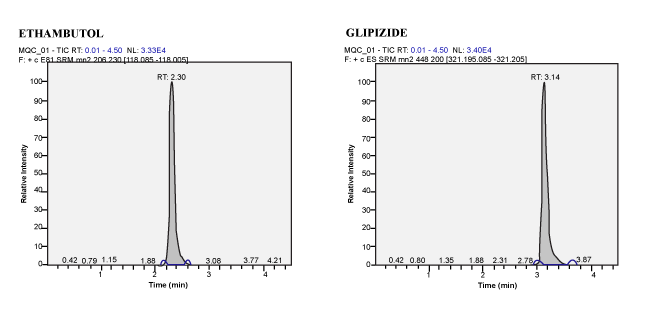
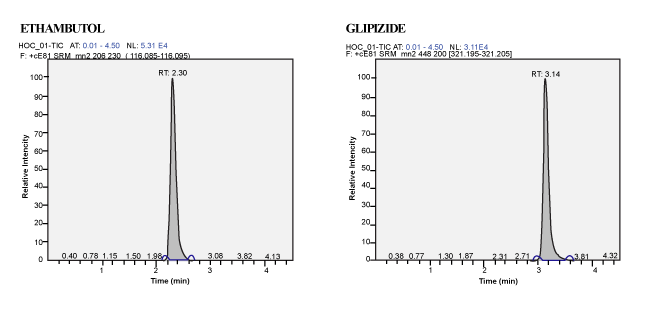
| Calibration Standard and Linearity: For three consecutive batches, the calibration curves showed an overall accuracy of 91.09%- 109.55% with %CV of 2.19%-13.19%. The calibration Standard Linearity has a regression Coefficient of > 0.9963 Calibration curve shown in Figure 3. The detailed results are shown in Table 1 and the chromatograms shown in Figure 4 to Figure 10. The Calibration Standards meets the acceptance criteria. |
| Accuracy and Precision: Table 2 shows inter and intra assay precision and accuracy. The method was found to be highly accurate and precise. For Ethambutol, accuracy of 96.84%-108.40% and precision of 3.80%-8.03% for intra-assay, and accuracy of 97.77%- 105.83% and precision of 6.23%-9.34% CV for inter-assay were obtained for all QC levels including LOQQC. |
| Recovery: Table 3 show the overall recovery of 98.70% for Ethambutol, and 87.76% for Glipizide were obtained. Both compounds show consistent recovery results for all three QC levels. |
| Stability: Stability of Ethambutol in human plasma under different conditions was evaluated. The detailed results are shown in Table 4. As seen from the table, three freeze/thaw cycles, 6 h room temperature storage, 83 days storage at -70°C and 28 h auto sampler stability has been established. In addition, 25 days stability for stock standard solutions and wet extract stability shown for 28 hrs were established. All of these demonstrate the ruggedness of the method. |
| Conclusion |
| Author’s proposed, a simple method in extraction and chromatographic condition, which is more precise, sensitive and also compatible for the analysis of Ethambutol in human plasma for fixed dose combination drugs by using LCMS/MS. The method was developed and validated, which was found to have good recovery and stability. In addition, during the subject sample analysis of ethambutol, there was no interference found in the subject sample due to the presence of Rifampicin, Isoniazid and Pyrazinamide (Figure 11). Hence, this method is a simple and rapid for the determination of Ethambutol in human plasma by LCMS/MS for four drug fixed dose combination formulation while conducting the Bioavailability and Bioequivalence studies. |
| Acknowledgements |
| We are thankful to Mrs. Gauri Sapte, Director, BBRC, the management of Maneesh Pharmaceuticals Ltd and Dr. Shedbalkar for their valuable support and contribution in carrying out this research work without which it will not be possible to publish the paper. |
| References |
References
- Hans LR (2002) Interventions for Tuberculosis Control and Elimination. International Union Against Tuberculosis and Lung Disease (IUATLD), March 2002.
- O'Neil MJ, Merck & Co (2001) The Merck index: an encyclopedia of chemicals, drugs, and biologicals. (13th edition) 663.
- http://www.drugbank.ca/drugs/DB00330
- http://www.drugbank.ca/drugs/DB01067
- Chenevier P, Massias L, Gueylard D, Farinotti R (1998) Determination of ethambutol in plasma by high-performance liquid chromatography after pre-column derivatization. J Chromatogr B Biomed Sci Appl 708: 310-315.
- Breda M, Marrari P, Pianezzole E, Strolin Benedetti M (1996) Determination of ethambutol in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 729: 301-307.
- Kumud S, James DT, Suresh K, Ramesh N, Sasijith SL (2011) Method Development and Validation of Ethambutol in Human Plasma by LCMS/MS. International Journal of Research in Pharmaceutical and Biosciences 1: 1-6.
- Conte JE Jr, Lin E, Zhao Y, Zurlinden E (2002) A high-pressure liquid chromatographic-tandem mass spectroscopic method for the determination of ethambutol in human plasma, bronchoalveolar Lavage fluid, and Alveolar cells. J Chromatogr Sci 40: 113-118.
- Chen X, Song B, Jiang H, Yu K, Zhong D (2005) A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of isoniazid and ethambutol in human plasma. Rapid Commun Mass Spectrom 19: 2591-2596.
- Gong Z, Basir Y, Chu D, McCort-Tipton M (2009) A rapid and robust liquid chromatography/tandem mass spectrometry method for simultaneous analysis of anti-tuberculosis drugs-Ethambutol and pyrazinamide in human plasma. J Chromatogr 877: 1698-1704.
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) (May 2001) BP Guidelines for Industry Bioanalytical Method Validation
- Immanuel C, Gurumurthy P, Ramachandran G, Venkatesan P, Chandrasekaran V, et al. (2003) Bioavailability of rifampicin following concomitant administration of ethambutol or isoniazid or pyrazinamide or a combination of the three drugs. Indian J Med Res 118:109-114.
- Swart KJ, Papgis M (1992) Automated high-performance liquid chromatographic method for the determination of rifampicin in plasma. J Chromatogr 593: 21-24.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
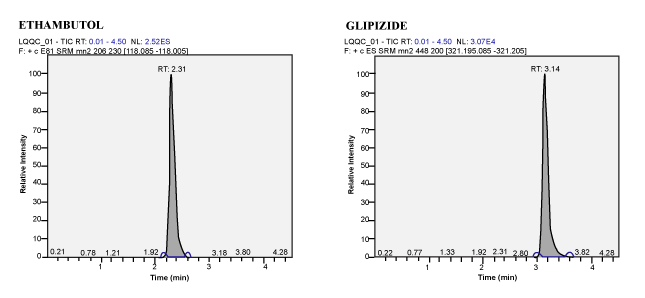 |
| Figure 5 | Figure 6 | Figure 7 | Figure 8 |
 |
 |
 |
| Figure 9 | Figure 10 | Figure 11 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 7163
- [From(publication date):
April-2012 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 2549
- PDF downloads : 4614
