Determinants of Hydroxychloroquine-Mediated Hemolytic Anemia in G6PD-Deficient COVID-19 Patients
Received: 05-Jun-2022 / Manuscript No. JIDT-22-65926 / Editor assigned: 08-Jun-2022 / PreQC No. JIDT-22-65926(PQ) / Reviewed: 22-Jun-2022 / QC No. JIDT-22-65926 / Revised: 29-Jun-2022 / Manuscript No. JIDT-22-65926(R) / Published Date: 06-Jul-2022
Abstract
Aim: To identify the factors contributing to Hydroxychloroquine (HCQS)-mediated hemolytic anemia in G6PD deficient COVID-19 patients.
Materials and methods: We have developed Multiple Linear Regression (MLR) and Classification and Regression Tree (CART) models based on the published data (n=13) and evaluated the impact of G6PD variants in exerting hemolysis.
Results: African Ancestry subjects had significant HCQS-mediated drop in hemoglobin (pre- vs. post-therapy Hb, g/dl: 12.75 ± 1.57 vs. 6.78 ± 0.62, p=0.0008) than those from non-African ancestry (pre- vs. post-therapy Hb, g/dl: 13.27 ± 1.51 vs. 9.97 ± 2.34, p=0.04). Diabetics had significant HCQS-mediated drop in hemoglobin (pre- vs. post-therapy Hb, g/dl: 12.96 ± 0.98 vs. 6.82 ± 0.76, p=0.0007) than non-diabetics (pre- vs. post-therapy Hb, g/dl: 12.88 ± 2.13 vs. 9.13 ± 2.51, p=0.05). MLR model explained 53.75% variability in HQCS-mediated hemoglobin drop when African Ancestry, diabetes, hypertension and azithromycin used as input variables. CART model efficiently explained HQCS-mediated hemolytic anemia by projecting African Ancestry and diabetes as the key predictors (R2=1.00). Although, African Ancestry G6PD A (-) variant had lesser ligand propensity than G6PD Mediterranean, G6PD Y70H and G6PD Union, it is thermolabile similar to G6PD Orissa, G6PD Y70H and G6PD L235F.
Conclusion: African Ancestry diabetic patients are more prone for HQCS-mediated hemolytic anemia in G6PD deficient COVID-19 patients. MLR and CART models explain 53.75% and 100% variability in HQCS-mediated hemolytic anemia. In view of similar in silico profile of Indian G6PD variants to G6PD A (-), HCQS should be used with caution in diabetics during COVID-19 therapy.
Keywords: Glucose-6-phosphate dehydrogenase; Diabetes; African ancestry; COVID-19 ; Hydroxychloroquine, Hemolytic anemia
Introduction
The G6PD (Glucose 6-Phosphate Dehydrogenase) regulates the pentose phosphate pathway. It reduces NADP+ to NADPH, thus regulating the regeneration of glutathione. G6PD deficiency causes hemolytic anemia following infection or the use of certain medications.
The most prominent medications that induce hemolysis are antimalarials and sulfonylureas. G6PD deficiency is an X-linked disorder. G6PD-deficient hemizygous men and homozygous women are prone for oxidative stress-induced hemolysis. Few studies showed 8-aminoquinololine-induced hemolysis even in G6PD heterozygous women [1,2]. The pharmacological relevance for G6PD deficiency is expanding. G6PD deficient subjects should avoid rasburicase, primaquine, dapsone, pegloticase, and methylene blue [3].
To tackle the COVID-19 pandemic, many hospitals have used drug repurposing. Hydroxychloroquine (HCQS) is one such drug that was effective in tackling this disease. But, many G6PD deficient COVID-19 patients experienced hemolysis following hydroxychloroquine therapy [4-12]. HCQS-therapy in COVID-19 resulted in an increased incidence of methemoglobinemia [13].
Few studies explored whether G6PD deficiency predisposes to COVID-19. The incidence of G6PD deficiency was 33% in Arab COVID-19 patients [14] while its incidence is <1% in the general population [15]. G6PD deficient COVID-19 patients exhibited the need for mechanical ventilation due to lower PaO2/FiO2 [16]. N-acetyl cysteine reversed the HCQS-induced hemolysis in G6PD deficient COVID-19 [17].
The mechanism of HCQS-mediated hemolysis in G6PD deficient COVID-19 patients is unclear. We have conducted a meta-analysis to delineate the causes of hemolysis. Besides, we explored the G6PD variants that prevalent in our population, which can contribute towards HCQS-mediated hemolytic anemia. We have assessed the impact of these variants using in silico approaches.
Materials and Methods
Review of literature
We did a literature review from Pubmed, Medline, and Google Scholar databases using the following key words: “G6PD deficiency”, “COVID-19”, “hydroxychloroquine” and, “hemolytic anemia”. We retrieved demographic, clinical and laboratory details from these studies.
Univariate analysis
We have carried out paired t-test to ascertain HCQS-mediated drop in hemoglobin in each group. The impact of co-morbidities mainly diabetes, hypertension, obesity, renal dysfunction; co-medications mainly azithromycin, sulfa drugs, methylene blue; and ethnic origin was assessed.
Multiple linear regression
We have used Free Statistics and Forecasting Software (FSFS) module to develop Multiple Linear Regression (MLR) model. The variables that are found to be relevant based on univariate analysis were included as inputs and HCQS-mediated hemoglobin drop was used as outputs. The contribution of each variable towards the drop in hemoglobin is assessed based on the slope value. The actual drop in hemoglobin is plotted against the MLR-predicted drop in hemoglobin. The ‘R2’ value depicts percentage variability explained by this model.
Classification and Regression Tree (CRT) model
We have used BigML module to develop CART model. This model was built with the key predictor at the apex of the tree and the subsequent predictors being the branches. This decision tree predicts the response variable by considering non-linear complex relationships.
Evaluation of G6PD variants prevalent in our population
We have recruited 1215 men in the age group of 18–72 year for this study from July 2012 to June 2021. These subjects were healthy and had no clinical implications. All the subjects consented to this study. Institutional Ethical Committee of Sandor Proteomic Pvt Ltd, Hyderabad (EC/SRP/008/2012, dated 26.6.12) approved the study protocol. We have collected whole blood samples in EDTA. None of them had Chronic Nonspherocytic Hemolytic Anemia (CNSHA).
Genotyping
We have used QIAamp DNA mini kit (Qiagen, USA) for DNA extraction and Infinium global screening array (GSA) v 1.0 for genotyping. The process involves Genome amplification, fragmentation, hybridization, enzymatic base extension, and fluorescent staining. The iScan system captured the bead chip images. The genome Studio V2011.1 facilitated the analysis and genotype calling. The sample dependent and sample independent controls ensured efficient quality control. We have excluded the markers with an SNP missing call rate of >0.03 from the analysis.
In silico analysis
We have used SiFT, Provean, SNAP2 and Position-specific Evolutionary Preservation time (PSEP) to assess the deleterious nature of the identified G6PD variants. The crystal structure of G6PD in complex with structural NADP was used as the template (PDB ID: 6E08) to predict thermal and denaturant stability of G6PD variants using Cologne University Protein Stability Analysis (CUPSAT). Ligand Protein Interaction Comparison and Analysis (LPIcom) computed the propensity score. This score is the probability of interaction of each residue of protein for NADP. The score ‘0’ indicates no probability. The score ‘9’ indicates the highest probability. Galaxy site explored ligand binding sites in the G6PD crystal structure. Besides, it modeled tertiary structures of variants. HHsearch facilitated the prediction of the binding ligands based on homology. LigDockCSA predicted the protein-ligand complex structures.
Results
Analysis of published data
We have included 12 case reports of hydroxychloroquine-mediated hemolytic anemia in G6PD-deficient COVID-19 patients published during the period of 2020–2021. All were men with the mean age of 49.5 ± 18.3 year. Out of these 12, seven patients were of African Ancestry (58.3%). Seven were diabetic (58.3%), two had hypertension (16.7%), two had cardiac problems (16.7%), three were obese (25%), two had renal problems (16.7%), five were using azithromycin (41.7%), two were on concomitant therapy with contra-indicated medications (sulfa drugs, methylene blue (16.7%). Two patients expired following the therapy. A 54 year old man from USA expired with Coomb’s negative hemolysis following co-administration of methylene blue along with hydroxychloroquine and azithromycin. A 60 year old African American man with acute kidney injury, electrolyte abnormalities, high anion gap, metabolic acidosis expired due to severe hemolytic crisis following HCQS therapy. N-acetyl cysteine was reported to be effective in reversing the hemolytic anemia induced by HCQS and sulfa in one patient. Folate was effective in reversing HCQS-mediated hemolysis in one patient (Table 1).
| Author name | Age (year) |
Gender | Comorbidities | G6PD U/g Hb |
Medications | Pre-treatment Hb (g/dl) |
Post-treatment Hb (g/dl) |
Study findings related to COVID-19 infection in G6PD deficient subjects |
|---|---|---|---|---|---|---|---|---|
| Maillart et al, 2020 [6] | 65 | M | Type 2 diabetes | 0.2 | Hydroxychloroquine, Azithromycin |
13.3 | 7.2 | Acute respiratory distress syndrome, acute hemolysis and acute renal failure |
| Beauverd et al, 2020 [5] | 68 | M | Type 2 diabetes | 2.5 | Hydroxychloroquine | 12 | 6.5 | Acute respiratory distress syndrome, acute hemolysis |
| Franceschi et al, 2020 [4] | 72 | M | Ischemic cardiomyopathy | --- | Lopinavir, Hydroxychloroquine | 15 | 12.5 | Acute hemolysis |
| Aguilar et al, 2020 [7] | 51 | M | Type 2 diabetes, hypertension, obesity | Deficient | Hydroxychloroquine | 14.5 | 5.9 | Acute hemolysis |
| Mastroianni et al, 2020 [8] | 32 | M | Obesity | <0.2 | Hydroxychloroquine | 10.2 | 7.7 | Hemolytic anemia. Cessation of HQCS followed by folate supplementation improved hemoglobin levels. |
| Naymagon et al, 2020 [10] | 54 | M | Diabetes | Deficient | Azithromycin, Hydroxychloroquine, Methylene blue |
….. | …… | Coomb’s negative hemolysis leading to death |
| Ibrahim et al, 2020 [17] | 44 | M | Obesity | 0.5 | H/o hemolytic reaction to sulfa drugs One dose of Hydroxychloroquine |
12.6 | 7.9 | Acute hemolysis following hydroxychloroquine therapy. N-acetyl cysteine was effective in blocking the hemolysis and bringing down liver enzymes, CRP and Ferritin in an adult |
| Palmer K et al, 2020 [13] | 62 | M | Type II diabetes, hypertension | 0.8 | Amoxicillin/clavulanic acid, heparin, amlodipine, metformin | 15.5 | 5.2 | Acute hemolysis. Two blood transfusions, oxygen therapy and folic acid supplementation improved the clinical condition. |
| Aljishi et al, 2021 [14] | 50 ± 24 | M:F 28:54 |
22 diabetics | 27 G6PD deficient | ………. | ….. | No different in the incidence of G6PD deficiency in symptomatic and asymptomatic patients (32.4% vs. 33.3%) | |
| Youssef et al, 2021 [16] | 53.5 | M:F 3:3 |
1 diabetic | 5.6 (1.8 – 9.3) |
…….. | 8.1 (6.6 -11.0) |
Low hemoglobin levels, lowest PaO2/FiO2 and prolonged duration of mechanical ventilation observed in G6PD-deficent COVID-19 patients compared to G6PD-normal COVID-19 patients. | |
| Aydemir et al, 2021 [9] | 44 | M | ------ | 0.91 | Favipiravir, convalescent plasma | Thrombocytopenia and lymphopenia following two doses of convalescent plasma. Methyl prednisolone treatment followed by Anakinra improved patient’s signs, symptoms and laboratory findings | ||
| Laslett N et al, 2021 [11] | 60 | M | Acute kidney injury, electrolyte abnormalities, high anion gap metabolic acidosis | 19.8 | Antibiotics, hydroxychloroquine | 14.1 | 6.8 | Severe hemolytic crisis followed by death during hydroxychloroquine therapy. |
| Chaney et al, 2020 [12] | 57 | M | NIDDM, hypertension, hypercholesterolemia, gastro-esophageal reflux disease and Glaucoma | 2.8 | Piperacillin tazobactam, azithromycin, hydroxychloroquine, ceftriaxone | 12.4 | 6.6 | Hemolytic anemia following hydroxychloroquine administration |
| Pelle MC et al, 2020 | 86 | F | Hypertension, anxiety-depressive syndrome | ----- | Hydroxychloroquine, Azithromycin | 10.6 | 8.3 | Autoimmune hemolytic anemia followed by myocardial infarction during hydroxychloroquine therapy |
Table 1: Studies depicting the association of G6PD deficiency with COVID-19 related complications.
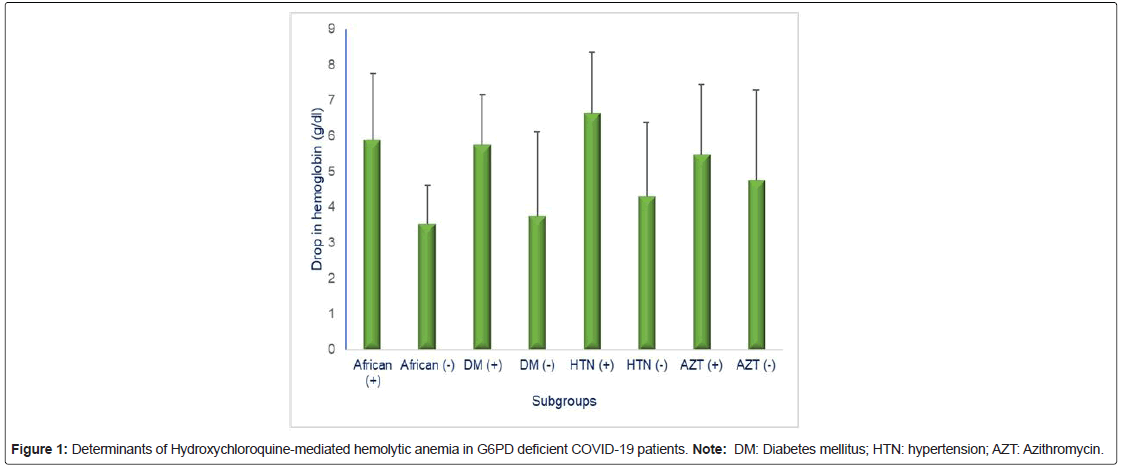
Subjects with African Ancestry exhibited significant lowering of hemoglobin following HCQS therapy (pre- vs. post-therapy Hb, g/dl: 12.75 ± 1.57 vs. 6.78 ± 0.62, p=0.0008) than those from those with non- African ancestry (pre- vs. post-therapy Hb, g/dl: 13.27 ± 1.51 vs. 9.97 ± 2.34, p=0.04). Diabetics exhibited significant lowering of hemoglobin following HCQS therapy (pre- vs. post-therapy Hb, g/dl: 12.96 ± 0.98 vs. 6.82 ± 0.76, p=0.0007) than non-diabetics (pre- vs. post-therapy Hb, g/dl: 12.88 ± 2.13 vs. 9.13 ± 2.51, p=0.05) (Figure 1).
Multiple linear regression
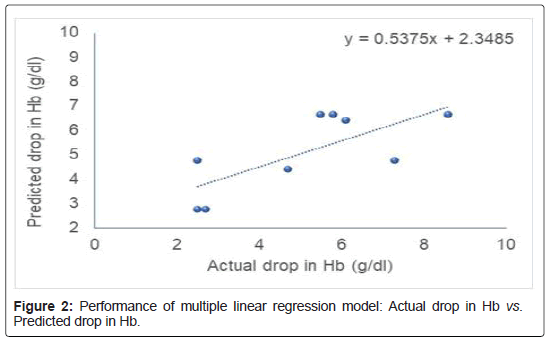
We have used African Ancestry, diabetes, hypertension, and use of azithromycin as the input variables and HCQS-induced hemolytic drop in Hemoglobin (Hb) as output variable. This additive model suggests that in G6PD-deficient subjects with African ancestry are more prone for developing hemolytic anemia with 2.04 g/dl drop in hemoglobin following HCQS therapy for COVID-19. Diabetics will have 1.51 g/dl drop in hemoglobin. Hypertension and azithromycin have only minor contribution towards inducing hemolytic anemia. MLR model based on these variables explained 53.75% variability in HCQS-induced hemolytic anemia (Figure 2). The mathematical representation of this model is shown below:
Drop in Hb (g/dl)=2.65+(2.04 × African Ancestry)+(1.51 × Diabetes)+(0.28 × Hypertension)+(0.47 × Azithromycin)
Classification and Regression Tree (CART) model
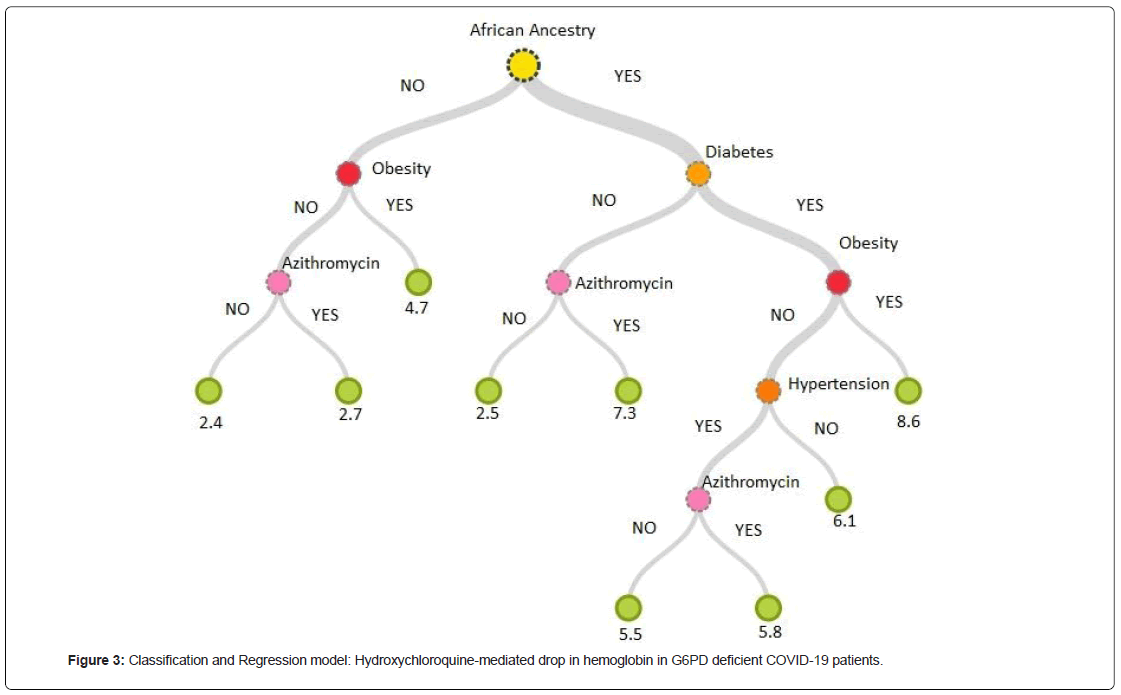
G6PD deficiency in African Ancestry subjects was found to be the key predictor of HCQS-mediated hemolytic anemia. The second predictor being the diabetes followed by obesity. Hypertension and azithromycin use being the mild contributors. These variables cumulatively explain all the contributors towards HCQS-mediated hemolytic anemia (R2: 1.00). G6PD deficient African Ancestry subjects with diabetes and obesity exhibited maximum drop in hemoglobin following HCQS-therapy (8.6 g/dl). G6PD deficient African Ancestry non-diabetic subjects exhibited maximum drop in hemoglobin when Azithromycin is co-administered concomitantly (7.3 g/dl). The drop in hemoglobin is lowest in subjects of non-African Ancestry without obesity and concomitant use of Azithromycin (2.4 g/dl) (Figure 3).
G6PD variant analysis in our population
We have used Infinium global screening array to identify the G6PD variants in our population, which are likely to be risk factors for HCQS- mediated hemolytic anemia. This array covers 56 G6PD variants. The SNP missing call rate was 0.0025 ± 0.005. Out of the 1215 men screened, 52 had G6PD deficiency. G6PD Mediterranean (n=19) and G6PD Kerala-Kalyan (n=18) are the most common variants identified. G6PD Orissa was observed in six subjects. G6PD Mahidol, G6PD Viangchan/Jammu, G6PD Chatham were observed in two subjects each. G6PD Y70H, G6PD L235F and G6PD Union were observed in one subject each. In silico studies (Table 2).
| Canonical | Variant | Provean | SIFT | SNAP2 | PSEP time (my) | Thermal stability (ΔG, Kcal/mol) | Ligand Propensity |
|---|---|---|---|---|---|---|---|
| G6PD Mediterranean | S188F | -4.22 | 0.017 | 87 | 220 | -0.62 | 9 |
| G6PD Kerala-Kalyan | E317K | -2.68 | 0.105 | 23 | 361 | 0.67 | 0 |
| G6PD Orissa | A44G | -2.93 | 0.001 | 76 | 4200 | -3.79 | 0 |
| G6PD Mahidol | G163S | -4.34 | 0.048 | 55 | 4200 | 0.69 | 7 |
| G6PD Viangchan | V291M | -2.58 | 0 | 92 | 4200 | 0.62 | 2 |
| G6PD Chatham | A335T | -0.84 | 0.003 | 89 | 324 | -0.65 | 2 |
| Y70H | -4.47 | 0 | 95 | 361 | -8.17 | 9 | |
| L235F | -2.44 | 0.075 | 37 | 455 | -3.04 | 2 | |
| G6PD Union | R454C | -7.13 | 0 | 87 | 4200 | -11 | 9 |
| G6PD A (-) | V68M | -1.56 | 0.007 | 73 | 1037 | -4.03 | 2 |
Table 2: Comparison of G6PD A(-) with G6PD variants prevalent in India using in silico tools.
G6PD wild
We have used the crystal structure of G6PD (PDB ID: 6E08) as a reference for the wild protein. The NADP interacts with 38G, 40S, 41G, 42D, 43L, 112Y,141A, 142L, 143P, 170E, 199I, 200D, 201H, 246R, 437Y residues. NADP forms 8 hydrogen bonds i.e., one each with 43L, 112Y, 199I; three with 171K, and two with 201H (Supplementary Fig 1).
G6PD mediterranean (S188F)
This is predicted to be deleterious by SIFT, Provean and SNAP2 scores. Its position specific evolutionary conservation (PSEP) time is 220 million years suggesting possibly damaging nature. The ligand propensity score is 9. In the S188F, the NADP binding site is devoid of 38G, 200D, and 246R residues. Hence, there is a disruption of the H-bond with 43L and the formation of new bonds with 40S, 72R, 141A, and 147Y.
G6PD Kerala-Kalyan (E317K)
This is predicted to be deleterious by Provean score. Its PSEP time is 361 million years suggesting possibly damaging nature. The ligand propensity score is 0. In the E317K variant, the NADP binding site is devoid of 200D and 246R. This results in the loss of H-bond with 43L and the formation of new H-bonds with 40S, 42D, and 72R.
G6PD Orissa (A44G)
This is predicted to be deleterious by SIFT, Provean and SNAP2 scores. Its PSEP time is 4200 million years suggesting probably damaging nature. It is found to be thermolabile (△G: -3.79 Kcal/mol). In the A44G variant, the NADP binding site is devoid of 199I, 200D, and 246R residues. This resulted in the loss of H-bonds with 43L, 112Y, 199I and the formation of new H-bonds with the 40S, 72R, 142L, and 147Y.
G6PD Y70H
This is predicted to be deleterious by SIFT, Provean and SNAP2. Its PSEP time is 361 million years suggesting possibly damaging nature. It has high thermolability (△G: -8.17 Kcal/mol). Its ligand propensity score is 9. In the Y70H variant, the catalytic binding site is devoid of 38G, 40S, 41G, 112Y, 141A, 142L, 143P, and 246R residues. This led to the loss of H-bonds with 43L, 112Y, and 199I and the formation of new H-bonds with 47K and 89K residues.
G6PD L235F
This is predicted as neutral variant by SIFT, Provean and SNAP2. However, its PSEP time is 455 million years suggesting probably damaging nature. It has thermolability ( △G: -3.04 Kcal/mol). Its ligand propensity score is 2. In the L235F variant, there is disruption of all the NADP binding sites except for 201H and 437Y residues. This results in the formation of New H-bonds with K47, 51T, 54W, and K205 residues.
G6PD Union (R454C)
This is predicted to be deleterious by SIFT, Provean and SNAP2. Its PSEP time is 4200 million years suggesting probably damaging nature. Its ligand propensity score is 9. In the R454C variant, the NADP binding site is devoid of 199I, 200D, 201H, 246R, and 437Y residues. This leads to disruption of H-bond with 199I and the formation of new H-bonds with 40S, 42D, 72R, 140L, and 142L.
Discussion
The meta-analysis of the published data on HCQS-mediated hemolytic anemia in G6PD deficient COVID-19 revealed African Ancestry and diabetes as the most significant determinants of hemolytic anemia. This is further confirmed by MLR and CART models. MLR model explained 53.75% variability in the drop of hemoglobin following HCQS therapy by considering African Ancestry, diabetes, hypertension and Azithromycin use as the predictors. CART model demonstrated maximum drop in hemoglobin in G6PD deficient African Ancestry diabetic subjects with obesity followed by G6PD deficient African Ancestry non-diabetic subjects with concomitant administration of Azithromycin. The drop in hemoglobin is lowest in subjects of non-African Ancestry without obesity and concomitant use of Azithromycin. Methylene blue is contraindicated medication in G6PD deficient subjects and hence its use along with HCQS might have triggered severe hemolytic crisis causing death in one patient. The other patient had acute renal failure with significant metabolic acidosis, which might have contributed to death in addition to HCQS-mediated hemolytic anemia.
In view of increased susceptibility of HCQS-mediated hemolytic anemia in African Ancestry G6PD deficient subjects, the possible G6PD variants causing this hemolysis should be explored. The rs1050828 (V68M) variant (A-) is one such variant with high frequency in Africans [18] and African Americans [19]. The allelic effect size of this variant for G6PD activity was -0.198 (-0.213 to -0.183) [20]. The Position- Specific Evolutionary Preservation (PSEP) time for this variant was 1037 millions of years (my) thus suggesting probably damaging nature of this variant consistent with SIFT and SNAP2 scores. This variant has thermolabile nature. This variant is also predominant in certain ethnic groups of India, specifically among Siddis of Karnataka, India [21].
A meta-analysis of 33 studies representing 16003 patients demonstrated increased COVID-19 severity (OR: 2.75, 95% CI: 2.09–3.62) and mortality (OR; 1.90, 95% CI: 1.37–2.64) in diabetic patients [22]. A meta-analysis of 189 studies representing 57563 COVID-19 patients revealed significant lowering of hemoglobin, red blood cell count, and higher ferritin in severe cases compared to moderate cases [23]. Lower hemoglobin levels were observed with older age, higher percentage of diabetes, hypertension and overall comorbidities, consistent with our observation [23]. Another meta-analysis representing 35879 veterans showed greater risk of hospitalization, ICU admission and mortality among diabetic subjects [24]. Diabetics using sulfonylurea had higher risk of hospitalization while those on insulin had higher risk of hospitalization and death [24]. In vitro studies depicted that thiazolidine derivatives inhibit SARS-CoV-2 protease at 0.01 µM [25]. Sitagliptin treatment at the time of hospitalization was reported to reduce mortality with improved clinical outcome in COVID-19 [26]. SGLT2 inhibitors were found to be effective in preventing vital organ damage in COVID-19 [27]. Sulfonyl ureas and pioglitazone may increase the risk for hemolysis in G6PD deficient subjects.
N-acetyl cysteine and folate were effective in minimizing HCQS- mediated hemolysis. N-acetyl cysteine therapy in sickle cell anemia was reported to decrease the percentage of dense cells, increase red cell glutathione and reduce risk for vaso-occlusion [28]. N-acetyl cysteine therapy in beta thalassemia was reported to reduce hemolysis and phagocytosis of RBC by macrophages, increase reduced glutathione content of RBC, platelets and neutrophils thus reducing the oxidative stress [29]. Oral administration of NAC in COVID-19 prevents the severity of COVID-19 by different mechanisms; i) blocking the angiotension-converting enzyme 2, which hampers the penetration of SARS-COV-2 into cells, ii) acting as an anti-oxidant; iii) serving as anti- inflammatory [30]. The efficacy of folate supplementation in reversing the hemolytic anemia in G6PD deficiency was demonstrated earlier also [31].
Our findings projecting S188F, E317K, and A44G as the most frequent G6PD variants in Indian population corroborated with a multi-ethnic study [32]. G6PD A(-) and G6PD Mediterranean variants are reported to cause structural changes in G6PD enzyme inducing conformational instability leading to the loss of binding of one or both substrates [33]. G6PD Mediterranean was reported to cause hemolytic anema in Palestinian children [34]. G6PD Viangchan and G6PD Viangchan+Mahidol were reported to exhibit 10- and 18-folds reduction in NADP+ catalytic efficiency [35]. Coinheritance of G6PD Kerala Kalyan with Chuvash polycythemia was shown to cause hemolytic erythrocytosis in a 32-year-old male [36]. The ligand propensity score is maximum in G6PD Mediterranean, G6PD Y70H and G6PD Union, hence likely to have significant reduction in NADP+ catalytic efficiency. G6PD Orissa, G6PD Y70H and G6PD L235F variants exhibit thermolabile nature.
Conclusion
African Ancestry G6PD deficient diabetic patients are more prone to developing HCQS-mediated hemolytic anemia following COVID-19. MLR and CART models explained the variability in HCQS-mediated hemoglobin drop by considering ethnic origin, diabetes, hypertension, obesity and azithromycin use. These models help in predicting the likelihood of hemolytic anemia so that early intervention strategies such as N-acetyl cysteine or folate supplementation can be initiated to minimize the life-threatening side effects. We have compared G6PD A (-) variant predominant in subjects with African Ancestry with other G6PD variants prevalent in India. G6PD Mediterranean is the most common G6PD variant that may induce hemolytic crisis in Indian subjects.
Acknowledgement
Funding Acknowledgement: Authors are grateful to The Deanship of Scientific Research, King Saud University for Funding through Vice Deanship of Scientific Research Chairs &COVID-19 Virus Research Chair.
Other Acknowledgements: The authors are grateful to the management of Yoda LifeLine Diagnostics Pvt Ltd, Hyderabad; Sandor Speciality Diagnostics Pvt LTd, Hyderabad.
Conflicts of Interest Statement
All the authors hereby declare no conflicts of interest.
Authors’ Contributions
The study was conceptualized and designed by SMN and VKK. The acquisition, analysis and interpretation of the data was carried out by SMN, VKK, TH and SAA. SMN and TH drafted this manuscript. VKK and SAA carried critical revision. The final version was approved by all the authors. All authors agreed to be accountable for all aspects of the work assuring accuracy and integrity of the work.
References
- Chu CS, Bancone G, Nosten F, White NJ, Luzzatto L (2018) Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J 17:101.
[Crossref] [Google Scholar] [PubMed]
- Chu CS, Freedman DO (2019) Tafenoquine and G6PD: A primer for clinicians. J Travel Med 26:23.
[Crossref] [Google Scholar] [PubMed]
- Belfield KD, Tichy EM (2018) Review and drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am J Health Syst Pharm 75:97-104.
[Crossref] [Google Scholar] [PubMed]
- de Franceschi L, Costa E, Dima F, Morandi M, Olivieri O (2020) Acute hemolysis by hydroxycloroquine was observed in G6PD-deficient patient with severe COVD-19 related lung injury. Eur J Intern Med 77:136.
[Crossref] [Google Scholar] [PubMed]
- Beauverd Y, Adam Y, Assouline B, Samii K (2020) COVID‐19 infection and treatment with hydroxychloroquine cause severe haemolysis crisis in a patient with glucose‐6‐phosphate dehydrogenase deficiency. Eur J Haematol 105:357-359.
[Crossref] [Google Scholar] [PubMed]
- Maillart E, Leemans S, van Noten H, Vandergraesen T, Mahadeb B, et al. (2020) A case report of serious haemolysis in a glucose-6-phosphate dehydrogenase-deficient COVID-19 patient receiving hydroxychloroquine. Infect Dis 52:659-661.
[Crossref] [Google Scholar] [PubMed]
- Aguilar J, Averbukh Y (2020) Hemolytic anemia in a glucose-6-phosphate dehydrogenase-deficient patient receiving hydroxychloroquine for COVID-19: A case report. Perm J 24:158.
[Crossref] [Google Scholar] [PubMed]
- Mastroianni F, Colombie V, Claes G, Gilles A, Vandergheynst F, et al. (2020) Hydroxychloroquine in a G6PD-Deficient patient with COVID-19 complicated by haemolytic anaemia: Culprit or innocent bystander? Eur J Case Rep Intern Med 7:1875.
[Crossref] [Google Scholar] [PubMed]
- Aydemir D, Dağlıoğlu G, Candevir A, Kurtaran B, Bozdogan ST, et al. (2021) COVID-19 may enhance risk of thrombosis and hemolysis in the G6PD deficient patients. Nucleosides Nucleotides Nucleic Acids 40:505-517.
[Crossref] [Google Scholar] [PubMed]
- Naymagon L, Berwick S, Kessler A, Lancman G, Gidwani U, et al. (2020) The emergence of methemoglobinemia amidst the COVID‐19 pandemic. Am J Hematol 95:196-197.
[Crossref] [Google Scholar] [PubMed]
- Laslett N, Hibbs J, Hallett M, Ghaneie A, Zemba-Palko V (2021) Glucose-6-phosphate dehydrogenase deficiency-associated hemolytic anemia and methemoglobinemia in a patient treated with hydroxychloroquine in the era of COVID-19. Cureus 13:15232.
[Crossref] [Google Scholar] [PubMed]
- Chaney S, Basirat A, McDermott R, Keenan N, Moloney E (2020) COVID-19 and Hydroxychloroquine side-effects: Glucose 6-Phosphate Dehydrogenase deficiency (G6PD) and acute haemolytic anaemia. QJM 113:890-891.
[Crossref] [Google Scholar] [PubMed]
- Palmer K, Dick J, French W, Floro L, Ford M (2020) Methemoglobinemia in patient with G6PD deficiency and SARS-CoV-2 infection. Emerg Infect Dis 26:2279-2281.
[Crossref] [Google Scholar] [PubMed]
- AlJishi JM, Alhajjaj AH, Alkhabbaz FL, AlAbduljabar TH, Alsaif A, et al. (2021) Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health 14:6-11.
[Crossref] [Google Scholar] [PubMed]
- Warsy AS, El-Hazmi MA (2001) G6PD deficiency, distribution and variants in Saudi Arabia: An overview. Ann Saudi Med 21:174-177.
[Crossref] [Google Scholar] [PubMed]
- Youssef JG, Zahiruddin F, Youssef G, Padmanabhan S, Ensor J, et al. (2021) G6PD deficiency and severity of COVID19 pneumonia and acute respiratory distress syndrome: Tip of the iceberg? Ann Hematol 100:667-673.
[Crossref] [Google Scholar] [PubMed]
- Ibrahim H, Perl A, Smith D, Lewis T, Kon Z, et al. (2020) Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol 219:108544.
[Crossref] [Google Scholar] [PubMed]
- da Rocha JE, Othman H, Tiemessen CT, Botha G, Ramsay M, et al. (2021) G6PD distribution in sub-Saharan Africa and potential risks of using chloroquine/hydroxychloroquine based treatments for COVID-19. Pharmacogenomics J 21:649-656.
[Crossref] [Google Scholar] [PubMed]
- Lo KS, Wilson JG, Lange LA, Folsom AR, Galarneau G, et al. (2011) Genetic association analysis highlights new loci that modulate hematological trait variation in Caucasians and African Americans. Hum Genet 129:307-317.
[Crossref] [Google Scholar] [PubMed]
- Shah SS, Macharia A, Makale J, Uyoga S, Kivinen K, et al. (2014) Genetic determinants of glucose-6-phosphate dehydrogenase activity in Kenya. BMC Med Genet 15:93.
[Crossref] [Google Scholar] [PubMed]
- Devendra R, Gupta V, Biradar SS, Bhat P, Hegde S, et al. (2020) G6PD A-is the major cause of G6PD deficiency among the Siddis of Karnataka, India. Ann Hum Biol 47:55-58.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, et al. (2020) Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 14:535-545.
[Crossref] [Google Scholar] [PubMed]
- Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, et al. (2020) Anemia and iron metabolism in COVID-19: A systematic review and meta-analysis. Eur J Epidemiol 35:763-773.
[Crossref] [Google Scholar] [PubMed]
- Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, et al. (2021) Risk factors for adverse outcomes among 35 879 veterans with and without diabetes after diagnosis with COVID-19. BMJ Open Diabetes Res Care 9:002252.
[Crossref] [Google Scholar] [PubMed]
- Petrou A, Zagaliotis P, Theodoroula NF, Mystridis GA, Vizirianakis IS, et al. (2022) Thiazole/thiadiazole/benzothiazole based thiazolidin-4-one derivatives as potential inhibitors of main protease of SARS-CoV-2. Molecules 27:2180.
[Crossref] [Google Scholar] [PubMed]
- Solerte SB, D’Addio F, Trevisan R, Lovati E, Rossi A, et al. (2020) Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: A multicenter, case-control, retrospective, observational study. Diabetes Care 43:2999-3006.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Fernandez B, D’Marco L, Górriz JL, Jacobs-Cacha C, Kanbay M, et al. (2020) Exploring Sodium Glucose Co-Transporter-2 (SGLT2) inhibitors for organ protection in COVID-19. J Clin Med 9(7):2030.
[Crossref] [Google Scholar] [PubMed]
- Pace BS, Shartava A, Pack‐Mabien A, Mulekar M, Ardia A, et al. (2003) Effects of N‐acetylcysteine on dense cell formation in sickle cell disease. Am J Hematol 73(1):26-32.
[Crossref] [Google Scholar] [PubMed]
- Amer J, Atlas D, Fibach E (2008) N-Acetylcysteine Amide (AD4) attenuates oxidative stress in beta-thalassemia blood cells. Biochim Biophys Acta 1780:249-255.
[Crossref] [Google Scholar] [PubMed]
- De Flora S, Balansky R, La Maestra S (2020) Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19. FASEB J 34:13185-13193.
[Crossref] [Google Scholar] [PubMed]
- Pamuk GE, Celik AD, Uyanık MS (2009) Brucellosis triggering hemolytic anemia in glucose-6-phosphate dehydrogenase deficiency. Med Princ Pract 18:329-31.
[Crossref] [Google Scholar] [PubMed]
- Sukumar S, Mukherjee MB, Colah RB, Mohanty D (2004) Molecular basis of G6PD deficiency in India. Blood Cells Mol Dis 33:141-145.
[Crossref] [Google Scholar] [PubMed]
- Sirdah M, Reading NS, Vankayalapati H, Prchal JT (2021) A computational study of structural differences of binding of NADP+ and G6P substrates to G6PD Mediterraneanc. 563T, G6PD A− c. 202A/c. 376G, G6PD Cairoc. 404C and G6PD Gazac. 536A mutations. Blood Cells Mol Dis 89:102572.
[Crossref] [Google Scholar] [PubMed]
- Sirdah M, Reading NS, Perkins SL, Shubair M, Aboud L, et al. (2012) Hemolysis and Mediterranean G6PD mutation (c. 563 C> T) and c. 1311 C> T polymorphism among Palestinians at Gaza Strip. Blood Cells Mol Dis 48:203-208.
[Crossref] [Google Scholar] [PubMed]
- Boonyuen U, Chamchoy K, Swangsri T, Saralamba N, Day NP, et al. (2016) Detailed functional analysis of two clinical glucose-6-phosphate dehydrogenase (G6PD) variants, G6PDViangchan and G6PDViangchan+ Mahidol: Decreased stability and catalytic efficiency contribute to the clinical phenotype. Mol Genet Metab 118:84-91.
[Crossref] [Google Scholar] [PubMed]
- Jamwal M, Mallik N, Aravindan AV, Jain A, Sharma P, et al. (2021) Hemolytic erythrocytosis: an amalgamated phenotype from coinherited Chuvash polycythemia and G6PD Kerala-Kalyan with acquired transient stomatocytosis. Ann Hematol 100:2107-2109.
[Crossref] [Google Scholar] [PubMed]
Citation: Naushad SM, Hussain T, Almajhdi FN, Kutala VK (2022) Determinants of Hydroxychloroquine-Mediated Hemolytic Anemia in G6PD-Deficient COVID-19 Patients. J Infect Dis Ther S4:004.
Copyright: © 2022 Naushad SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 3329
- [From(publication date): 0-2022 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 2850
- PDF downloads: 479