Research Article Open Access
Detection of Transcripts and an Infectious Dose of Murine Gammaherpesvirus 68 in Dermacentor reticulatus Ticks
Kudelova M1*, Janosova M2, Vrbova M2, Matuskova R1, Slovak M3 and Belvoncíkova P1
1Department of Molecular Pathogenesis of Viruses, Institute of Virology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
2Department of Microbiology and Virology, Faculty of Natural Science, Comenius University, Bratislava, Slovakia
3Institute of Zoology, Slovak Academy of Sciences, Bratislava, Slovakia
- *Corresponding Author:
- Marcela Kúdelová
Institute of Virology, Biomedical Research Center
Slovak Academy of Sciences, 845 05 Bratislava, Slovakia
Tel: 00421 02 59302434
Fax: 00421 02 54774284
E-mail: virukude@savba.sk
Received date: July 24, 2017; Accepted date: August 09, 2017; Published date: August 14, 2017
Citation: Kudelova M, Janosova M, Vrbova M, Matuskova R, Slovak M, et al. (2017) Detection of Transcripts and an Infectious Dose of Murine Gammaherpesvirus 68 in Dermacentor reticulatus Ticks. J Infect Dis Ther 5:330. doi: 10.4172/2332-0877.1000330
Copyright: © 2017 Kudelova M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: Murine gammaherpesvirus 68 is assumed to be a natural pathogen of murid rodents. Previous investigations of MHV68 in field-collected Dermacentor reticulatus, Ixodes ricinus, and Haemaphysalis concinna ticks support the idea that ticks acquire the virus from feeding on infected hosts. Based on our previous finding of a live MHV-68 capable to replicate in mammalian cells, we aimed to investigate if transcripts of MHV-68 are present in D. reticulatus ticks and to determine the amount of MHV-68 in these ticks.
Methods: This study utilized a sensitive nested RT-PCR method to detect transcripts of the early-late M3 gene of MHV-68, then nested PCR to screen MHV-68 presence and real-time PCR to quantify virus infectious dose in ticks.
Results: Transcripts of MHV-68 M3 gene were detected in 10 out of 11 questing ticks. MHV-68 was detected in 38 out of 48 questing ticks, in which an infectious dose of MHV-68 varies from 2.2 × 104–8.6 × 106 copies of the virus genome .
Conclusion: We report the first evidence of MHV-68 transcription and infectious dose of MHV-68 in field collected D. reticulatus ticks. Results provide unique evidence that ticks could act as a reservoir of gammaherpesvirus, which could be capable of replication.
Keywords
Murine gammaherpesvirus 68; Dermacentor reticulatus tick; Infectious dose; MHV-68 M3 gene transcripts
Introduction
Murid herpesvirus 4 strain 68 (MHV-68 or γHV68; genus Rhadinovirus, subfamily Gammaherpesvirinae) and the other four murine gammaherpesviruses were originally isolated from bank voles (Myodes glareolus) and yellow-necked field mice (Apodemus flavicollis) trapped in West Slovakia [1,2]. Epidemiological surveys in the UK, Germany, France, and Peru found several other gammaherpesviruses in free-living rodents [3-6]. Although it has been documented that MHV-68 is mainly transmitted in the rodent population via intranasal routes and through body fluids, such as saliva, urine, tears, and breast milk, it is not yet fully understood how this virus spreads in nature. After the clearing of an acute respiratory infection, the virus spreads via the bloodstream to the host body, and, similarly to other gammaherpesviruses, MHV-68 causes a life-long latent infection in host B-lymphocytes that may lead to lymphoproliferative disorders and tumor development. During latent infection, virus reactivation may occur, resulting in repeated lytic infection and further spreading of the virus [2,7-9].
Apodemus spp mice and M. glareolus, from which some murine herpesviruses were isolated, were found displaying the infection along with numerous tick borne pathogens from the ticks which fed on them. Various pathogens, including viruses, bacteria, protozoa and helminthes, are transmitted from the ticks to vertebrates, of which most have a life cycle that requires passage through the vertebrate host [10]. Tick-borne viruses are different from other viruses in their ability to replicate in both vertebrate and invertebrate cells. They are causative agents of several important human diseases. With a single exception, all arboviruses are RNA viruses. Currently, the only DNA arbovirus is the African swine fever virus, which is maintained in the sylvatic transmission cycle of ticks in Africa [11]. Less than 10% of known tick species were identified to act as virus vectors. They are found mainly in the genera Ixodes, Haemaphysalis, Hyalomma, Amblyomma, Dermacentor, Rhipicephalus and Boophilus [12].
In Europe, there are two important hard tick species, Dermacentor and Ixodes (Acari: Ixodidae), which act both as important arthropod vectors and reservoirs for a series of zoonotic pathogens affecting wildlife such as bacteria (e.g. Rickettsia spp., Coxiella burnetii, Anaplasma phagocytophilum, Ehrlichia spp., Borrelia burgdorferi sensu lato, Francisella tularensis), protozoa (e.g. Babesia spp.) [13] and viruses (e.g. tick-borne meningoencephalitis virus, Colorado tick fever virus, Crimean-Congo haemorrhagic fever virus) [14]. Dermacentor reticulatus Fabricius 1794 is the three-host meadow tick that parasitizes primarily wild and domestic mammals and, infrequently, humans. Recent comparative analyses have revealed changes in the distribution and abundance (almost doubled) of D. reticulatus ticks in some European countries, implying a higher risk of the transmission of tick-borne diseases [15-17]. The development of molecular diagnostic tools has not only enhanced opportunities for collecting important data on known tick borne pathogens but also created the opportunity to uncover previously undescribed pathogens.
The first evidence of MHV-68 in ticks was found in immature I. ricinus ticks infecting Lacerta viridis green lizards in which 15 of 799 nymphs and larvae (1.8%) were identified as virus-positive [18]. Later on, Kúdelová et al. [19] has shown MHV-68 positivity in approximately 23.3% and 40% of D. reticulatus adults collected in South-western Slovakia, near Dunaj River, in Gabčíkovo and Vojka nad Dunajom by nested PCR, respectively. An examination of the salivary glands, intestines and ovaries of some questing D. reticulatus female ticks identified live MHV-68 capable of replication in mammalian cells, suggesting that MHV-68 might replicate also in tick body [19]. Recently, MHV-68 was documented in H. concinna ticks with an incidence of 38.3% (18/47) and an infectious dose of 2.0 × 102–9.6 × 103 [20].
This current research was conducted to assess whether the transcription of MHV-68 genome is present in field collected adult D. reticulatus ticks by detecting transcripts of the M3 gene, which is known to be expressed in both acute and latent infection with MHV-68. We used a real-time quantitative PCR (qPCR) assay to determine the amount of MHV-68 genome copies in the body of infected D. reticulatus ticks.
Materials and Methods
Ticks and study siteal
A total of 59 adult D. reticulatus ticks were collected on the vegetation in Vojka nad Dunajom), situated in South-western Slovakia (47° 58′ 35″ N, 17° 22′ 50″ E) in spring 2013. Prior to examination, the ticks were divided to two groups. Eleven ticks of the first group were randomly selected and individually stored in 1.5 ml microcentrifuge tubes containing 300 µl RNA later stored (Sigma) at -80°C, and other ticks (n=48) were individually transferred into tubes and maintained alive at 4°C.
RNA isolation and virus transcripts detection by nested RT-PCR
Total RNA was isolated from 11 ticks using the Direct-zol™ RNA MiniPrep kit (Zymo Research, USA) and then treated with RNase-free DNAse I (Invitrogen, Germany) according to the manufacturer’s instructions. The total RNA isolated from sure known uninfected tick from the breeding station served as a negative control. Equivalent amounts of RNA were reverse–transcribed as previously described [19]. Specific primers targeting latent-lytic M3 gene of MHV68 (GenBank AccNo U97553, coordinates 6,060–7,277) were used in nested RT-PCR as previously described, except for using 50 ng of tick RNA as a template of the first round PCR [19]. We used outer primers: M3PF1: 5‘-ACT CCA GCC TGT ACT GTT GC-3‘; M3PR1: 5‘-TCT GCC CCA CAA CCA AGT TT-3‘and inner primers: M3PF2: 5‘-ACT GGC CCT CAA CCA GTC TA-3‘; M3PR2: 5‘-TAC AAG TAC AGC GTG AGC CC-3‘to amplify 520-bp and 241-bp PCR product, respectively. Nested PCR amplicons were resolved on a 1.5% agarose gel, and samples yielding PCR products of the expected size were determined to be positive for MHV-68 transcripts.
Genomic DNA isolation and viral DNA detection and quantification
Total genomic DNA was isolated from 48 ticks using the alkaline lysis method as previously described [20]. Equivalents of tick DNA samples were first screened for the presence of MHV-68 DNA by nested PCR targeting the ORF 50 gene of MHV-68, as previously described [19]. Viral genome loads in virus-positive ticks were identified by a real-time PCR method targeting ORF 65 gene using the Step One Real-Time PCR System (Applied Biosystems) as previously described [20]. qPCR standard curve were established on 10-fold serial dilutions of the MHV68 BAC DNA, and 106 to 100 copies were used as templates in PCR mixtures that were amplified in parallel. Primers ORF65F: 5‘-GTC AGG GCC CAG TCC GTA-3‘and ORF65R: 5‘-TGG CCC TCT ACC TTC TGT TGA-3‘were used to amplify a 65-bp fragment. Virus copy number in the triplicate samples was calculated by comparison of the sample data with a standard curve. Viral genome copies in the whole body of each tick were re-calculated from the DNA yield extracted from the tick.
All PCR work performed in this study complied with generally known strict protocols to control cross-contamination, such as pipetting the template in a separate PCR box in a dedicated room and using a PCR mixture without template as a negative control.
Results
Ticks contain MHV-68 M3 gene transcripts
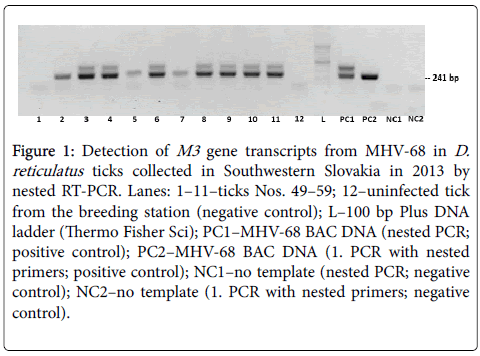
To determine whether D. reticulatus adult ticks collected on the vegetation eleven cDNA samples from the first group of inspected ticks we tested by nested RT-PCR. Before reverse transcription, RNA samples of all ticks were confirmed to be free of contaminant DNA by performing nested RT-PCR under the same conditions (data not shown). As shown on Figure 1, transcripts of the MHV-68 M3 gene were detected in samples of ten ticks, when the amount of viral transcripts found in ticks varied. One tick (No. 49, lane 1) tested negative, as its results were similar to an uninfected tick serving as a negative control (lane 12).
Figure 1: Detection of M3 gene transcripts from MHV-68 in D. reticulatus ticks collected in Southwestern Slovakia in 2013 by nested RT-PCR. Lanes: 1–11–ticks Nos. 49–59; 12–uninfected tick from the breeding station (negative control); L–100 bp Plus DNA ladder (Thermo Fisher Sci); PC1–MHV-68 BAC DNA (nested PCR; positive control); PC2–MHV-68 BAC DNA (1. PCR with nested primers; positive control); NC1–no template (nested PCR; negative control); NC2–no template (1. PCR with nested primers; negative control).
Infectious dose of MHV-68 in ticks quantified by qPCR
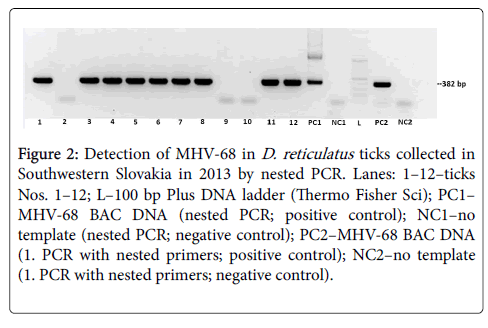
To determine the amount of MHV-68 genome copies in ticks we first examined 48 DNA samples of the second group of inspected ticks. Thirty eight of these ticks (79.1%) carried MHV-68 DNA, as we confirmed by nested PCR (Figure 2). The samples of all 38 MHV-68 positive samples we examined in triplicate by qPCR and showed a specific signal. As shown on Table 1, the copy numbers of virus genome copies per tick varied from 2.2 × 104 to 8.6 x 106 when accept a single tick (No 23), which carried as much as 8.6 × 106 virus genome copies. Except for this tick, an infectious dose of MHV-68 in ticks varied from approximately 104 to 105 MHV-68 genome copies.
Figure 2: Detection of MHV-68 in D. reticulatus ticks collected in Southwestern Slovakia in 2013 by nested PCR. Lanes: 1–12–ticks Nos. 1–12; L–100 bp Plus DNA ladder (Thermo Fisher Sci); PC1– MHV-68 BAC DNA (nested PCR; positive control); NC1–no template (nested PCR; negative control); PC2–MHV-68 BAC DNA (1. PCR with nested primers; positive control); NC2–no template (1. PCR with nested primers; negative control).
| Tick No. | MHV-68 copy number × 105 | Tick No. | MHV-68 copy number × 105 | Tick No. | MHV-68 copy number × 105 |
|---|---|---|---|---|---|
| 1 | 0.42 | 19 | 2.68 | 36 | 5.81 |
| 3 | 0.49 | 20 | 3.39 | 37 | 4.08 |
| 4 | 0.35 | 22 | 1.15 | 38 | 2.61 |
| 5 | 0.57 | 23 | 86.24 | 40 | 2.11 |
| 6 | 0.59 | 24 | 0.41 | 41 | 2.53 |
| 7 | 1.29 | 26 | 0.31 | 42 | 2.39 |
| 8 | 0.52 | 29 | 0.44 | 43 | 1.93 |
| 11 | 0.45 | 30 | 0.29 | 44 | 2.18 |
| 12 | 0.49 | 31 | 1.09 | 45 | 1.82 |
| 13 | 0.22 | 32 | 1.16 | 46 | 1.16 |
| 15 | 0.36 | 33 | 0.81 | 47 | 2.51 |
| 17 | 0.73 | 34 | 0.92 | 48 | 1.93 |
| 18 | 0.63 | 35 | 0.43 | ||
| Note: Samples were evaluated in triplicates. | |||||
Table 1: Determination of MHV-68 dose in D. reticulatus ticks by real-time PCR.
Discussion
As mentioned above, after primary/acute infection, the MHV-68 spreads to host organs via blood. As with other gammaherpesviruses, it causes lifelong latent infection of its host and can reactivate to a state of repeated lytic infection and reappear in host blood. This suggests that MHV-68 can exist for a relatively long time in the blood of murid rodents, which undoubtedly feed hard ticks, including spp. Dermacentor and Ixodes. Both tick species mentioned, the most common in Slovakia, were identified as vectors of many tick-borne pathogens. Most recently, three tick species living in Vojka were described as co-feeding on free-living rodents and being currently infected with several tick-borne pathogens. The dominant species was D. reticulatus (67.7 %), followed by I. ricinus (31.8 %) and H. concinna (0.5 %) [21]. In this study, we examined field collected D. reticulatus adult ticks from the same locality for the presence of MHV-68 infection as well as the transcripts of MHV-68 genome.
We have found that MHV-68 might replicate in D. reticulatus ticks because we found transcripts of the M3 gene in their bodies. The M3 gene is known to be expressed during both the acute (lytic) phase and latent infection of the animal host, although it is expressed at different levels. As many as ten out of eleven questing ticks were identified to have different amounts of M3 gene transcripts. Thus, we found transcription of MHV-68, which has not been previously described in D. reticulatus ticks, even in any other tick species. To the best of our knowledge, this study provides the first evidence of viral transcripts in field collected ticks that were previously limited to evidence from tick organs after virus propagation in vitro. Earlier studies identified live MHV-68 capable of replication in mammalian cells in the salivary glands, intestines, and ovaries of D. reticulatus ticks using an explanation and co-cultivation procedure, respectively [19]. In a very early study, neutralizing antibodies to the murine herpesvirus were found in the sera of rodents, fallow deer (Dama dama), wild boar (Sus scrofa), and red deer (Cervus elaphus) what gave rise to a hypothesis that MHV-68 can be transmitted via ticks from rodents to other animals living in the same biotope [22].
Our results reveal that as much as 79% of the ticks collected in Vojka in spring 2013 were infected with MHV-68. Prior studies of a much larger group of D. reticulatus ticks (n=312) collected in this locality during 2011–2014 identified approximately 40% incidence of the virus. We demonstrated the certain dependence of virus incidence in ticks on the size of the study group but also the collection season, locality, and tick sex [19]. However, MHV-68 infections were found each year during this period, although the incidence undoubtedly depends on the density of potential tick hosts that were infected. Here, we have shown that relatively a large dose of MHV-68 can be present in the tick. We documented that it varied from approximately 104-105 MHV-68 genome copies, except for one tick. By contrast, a smaller amount of the virus, varying from 102 to 104, has been recently identified in H. concinna ticks collected in Gabčíkovo from 2013 to 2014 [20]. The main reason for this difference may be much smaller size of H. concinna tick. As mentioned above, this work represents the first evidence of infectious dose of MHV-68 in field D. reticulatus ticks collected only in one time point and one region. Further studies to test the ticks collected at different time points and from other regions are necessary to provide unbiased statistical data on the infectious dose of MHV-68 in D. reticulatus ticks. Evidence of size infectious dose of MHV-68 in next tick species, D. reticulatus, provided by this study, supports the idea that infected ticks feeding on vertebrates hosts living in the same biotope could lead to infection of the vertebrate hosts with this virus [23]. This proposal is consistent with the finding that a relatively small dose of MHV-68 (1 or 40 PFU per mouse) can result in the establishment of long-lived infection in experimentally infected mice, although it resulted in a prolonged time of acute infection and delayed establishment of latent infection [24]. Based on the known data that 1 PFU of herpesviruses contains about 40 copies of virus genome, 104-105 MHV-68 genome copies detected in this study in field D. reticulatus ticks corresponds to 250-2500 PFU. It is a dose that goes well beyond the dose required for infection of natural host with MHV-68. In addition, finding of MHV-68 transcription in ticks also reinforces arguments about the possible infection of vertebrates with virus via ticks.
Conclusion
We provided data on the MHV-68 dose in D. reticulatus ticks, and we obtained data on virus transcripts in ticks. To-date evidence of MHV-68 infection of three tick species (I. ricinus, D. reticulatus and H. concinna), previous findings of a viable virus in salivary glands and other organs of D. reticulatus ticks and finally the evidence of MHV-68 transcripts in D. reticulatus ticks reported in this study represents the fulfillment of at least some specific requirements for recognizing MHV-68 as a potential arbovirus. Further experimental examination seeks to yield answers as to the mechanisms of MHV-68 transmission between ticks and hosts, and vice versa. Further work is needed to determine whether MHV-68 could co-infect ticks with known tick-borne pathogens and could be transmitted to humans via ticks.
Acknowledgements
This work was supported by the joint grant agency of the Slovak Ministry of Education and Slovak Academy of Sciences VEGA (#2/0087/17) and by the Slovak Research and Development Agency (#APVV-0621-12). The authors also acknowledge Prof. U. Koszinowski for providing the MHV-68 BAC.
References
- Blaškovič D, Stančeková M, Svobodová J, Mistríková J (1980) Isolation of five strains of herpesviruses from two species of free-living small rodents. Acta Virol 24: 468.
- Rajčáni J, Kúdelová M (2007) Murid herpesvirus 4 (MHV-4): an animal model for human gammaherpesvirus research. In “Latency strategies of herpesviruses” (J Minarovits, T Gonczol E. Valyi-Nagy, eds), Springer, Berlin, pp. 102-136.
- Blasdell K, McCracken C, Morris A, Nash AA, Begon M, et al. (2003) The wood mouse is a natural host for Murid herpesvirus 4. J Gen Virol 84: 111-113.
- Ehlers B, Kuchler J, Yasmum N, Dural D, Voigt S, et al. (2007) Identification of novel rodent herpesviruses including the first gammaherpesvirus of Mus musculus. J Virol 81: 8091-8100.
- Hughes DJ, Kipar A, Milligan SG, Cunningham C, Sanders M (2010) Characterization of a novel wood mouse virus related to murid herpesvirus 4. J Gen Virol 9: 867-879.
- Loh J, Zhao G, Nelson CA, Coder P, Droit L, et al. (2011) Identification and sequencing of a novel rodent gammaherpesvirus that establishes acute and latent infection in laboratory mice. J Virol 85: 2642-2656.
- Rajčáni J, Blaškovič D, Svobodová J, �?iampor F, Hučková D, et al. (1985) Pathogenesis of acute and persistent murine herpesvirus infection in mice. Acta Virol 29: 51-60.
- Nash AA, Dutia BM, Stewart JP, Davison AJ (2001) Natural history of murine gamma-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci 356: 569-579.
- Kúdelová M, Rajčáni J (2009) Gammaherpesviruses and Oncogenesis. In: “Herpesviridae: Viral Structure, Life Cycle and Infections” (Gluckman TR, ed), Book Series: Virology Research Progress, Nova Science Publishers, Inc., USA, pp. 187-226.
- Nuttall PA (2009) Molecular characterization of tick-virus interactions. Front Biosci 14: 2466-2483.
- Jori F, Vial L, Penrith ML, Pérez-Sánchez R, Etter E, et al. (2013) Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res 173: 212-227.
- Magnarelli LA (2009) Global Importance of Ticks and Associated Infectious Disease Agents. Clin Microb Newsletter 31: 5.
- Estrada-Peña A, Gray JS, Kahl O, Lane RS, Nijhof AM (2013) Research on the ecology of ticks and tick-borne pathogens—methodological principles and caveats. Front Cell Inf Microbiol 3: 29.
- Labuda M, Nuttall P (2004) Tick-borne viruses. Parasitology 129: S221-S245.
- Bullova E, Lukan M, Stanko M, Pet'ko B (2009) Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet Parasitol 165: 357-360.
- Reye AL, Stegniy V, Mishaeva NP, Velhin S, Hübschen JM, et al. (2013) Prevalence of Tick-Borne Pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One 8: e54476.
- Buczek A, Bartosik K, Wi�?niowski Ł, Tomasiewicz K (2013) Changes in population abundance of adult Dermacentor reticulatus (Acari: Amblyommidae) in long-term investigations in eastern Poland. Ann Agric Environ Med 20: 269-272.
- Ficová M, Betáková T, Pančík P, Václav R, Prokop P, et al. (2011) Molecular detection of murine herpesvirus 68 in ticks feeding on free-living reptiles. Microb Ecol 62: 862-867.
- Kúdelová M, Belvončíková P, Vrbová M, Kovaľová A, Štibrániová I, et al. (2015) Detection of Murine Herpesvirus 68 (MHV-68) in Dermacentor reticulatus Ticks. Microb Ecol 70: 785-794.
- Vrbová M, Belvončíková P, Kovaľová A, Matúšková R, Slovák M, et al. (2016) Molecular detection of murine gammaherpesvirus 68 (MHV-68) in Haemaphysalis concinna ticks collected in Slovakia. Acta Virol 60: 426-428.
- Švehlová A, Berthová L, Sallay B, Boldiš V, Olivier A, et al. (2014) Sympatric occurrence of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks and their pathogens Rickettsia and Babesia species in Slovakia. Ticks Tick-Borne Dis 5: 600-605.
- Mistríková J, Kožuch O, Klempa B, Kontseková E, Labuda M, et al. (2000) New findings on the ecology and epidemiology of murine herpes virus isolated in Slovakia. Bratisl Lek Listy 101: 157-162.
- Wágnerová M, Chalupková A, Hrabovská Z, Ančicová L, Mistríková J (2015) Possible role of different animal species in maintenance and spread of murine gammaherpesvirus 68 in the nature. Acta Virol 59: 14-19.
- Tibbetts SA, Joy Loh J, van Berkel, McClellan JS, Jacoby MA, et al. (2003) Establishment and Maintenance of Gammaherpesvirus Latency Are Independent of Infective Dose and Route of Infection. J Virol 77: 7696-7701.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 2324
- [From(publication date):
August-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 1720
- PDF downloads : 604