Research Article Open Access
Detection of Toxoplasma DNA in the Peripheral Blood of HIV-Positive Patients with Neuro-opportunistic Infections by a Real-Time PCR Assay
Nestor Cardona1, Natalia Basto2, Beatriz Parra2, Andres Felipe Zea2, Carlos A. Pardo3, Anilza Bonelo2, and Jorge Enrique G´omez-Marin1*
1GEPAMOL (Group of Molecular Parasitology), Biomedical Research Centre, Faculty of Health Sciences, University of Quindio, AA 460 Armenia, Quindio, Colombia
2Virology Research Group, School of Basic Sciences, Faculty of Health, University of Valle, Cali, Colombia
3Department of Neurology, School of Medicine, Johns Hopkins University, Baltimore, MD 21287, USA
- *Corresponding Author:
- Jorge Enrique G´omez-Marin
GEPAMOL (Group of Molecular Parasitology), Biomedical Research Centre
Faculty of Health Sciences, University of Quindio, AA 460 Armenia, Quindio, Colombia
E-mail: gepamol2@uniquindio.edu.co
Received Date: 10 April 2011; Revised Date: 28 April 2011; Accepted Date: 29 April 2011
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
We determine if peripheral blood sample could be used for diagnosis of cerebral toxoplasmosis by a real-time PCR assay and we analyzed the clinical and laboratory findings in 22 patients with confirmed cerebral toxoplasmosis compared to 27 patients with other neuro-opportunistic infections. We compared two gene targets (B1 and RE) in the Taqman-PCR real-time assay. Efficiency values were also calculated. We found that 18.8% (4/22) of cases with cerebral toxoplasmosis and 7.4% (2/27) of patients with other neuroinfections had positive results with the Taqman PCR assay. The mean number of parasites was 67.7 (SD 69.0) tachyzoites/mL in patients with cerebral toxoplasmosis and of 31.8 (SD 6.3) tachyzoites/mL in other neuroinfections. Clinical symptoms as headache were significantly less frequent and focal neurological symptoms were significantly more frequent in cerebral toxoplasmosis than in other neuroinfections. In our conditions, real-time PCR on peripheral blood samples was not useful for diagnosis of cerebral toxoplasmosis.
Keywords
toxoplasma; cerebral toxoplasmosis; HIV; Colombia; real-time PCR
Introduction
Neurological opportunistic infections are an important indicator of infection by the human immunodeficiency virus (HIV). Contrary to the recent reports in Europe and United States where a significant reduction of neurological oppor-tunistic infections has been observed, in Latin America countries, there is still an increase in opportunistic infections and frequently are the first manifestation and the first motive for consultation in a person with HIV infection [13]. In spite of the introduction of the highly active antiretroviral therapy (HAART), that reduces the morbidity and mortality, the barriers for the patients to get access to these medications in Latin American countries explain the continuous presence of these manifestations [15]. In Colombia, a report of 131 cases with opportunistic infections of the central nervous system, seen during the years 1995 to 2005 at the city of Cucuta, found that cerebral toxoplasmosis was the etiology in 56% of cases [12]. Notoriously, most of the cases (79%) were diagnosed since the year 2000. In another region of Colombia, in a case series at the Hospital of Quindio Department, in 21 patients seen during 2006, toxoplasmosis was the etiology in 62% of cases [4]. In both Colombian series, cryptococosis was the second most frequent opportunistic infection in central nervous system (38% in Cucuta and 23% in Quindio) [4,12].
An accurate diagnosis of these neurological complica-tions is crucial for HIV infected patients, since most such complications are likely to be treated, and a prompt effective intervention may eventually yield to longer survival or better quality of living. Several studies have demonstrated the usefulness of PCR on cerebrospinal fluid (CSF) samples for the diagnosis of cerebral toxoplasmosis [1,15,19]. However, a lumbar puncture could be contraindicated in a subgroup of patients with expansive cerebral lesions [6]. In this setting, peripheral blood samples present an additional advantage. A previous study in Brazil on 64 patients with cerebral toxoplasmosis and 128 controls reported a sensitivity of 80% and a specificity of 98% by using a conventional polymerase chain reaction (PCR) targeting the B1 gene on peripheral blood samples [7]. But the use of a variation of the PCR method, the real-time PCR, has grown considerably over the recent years; the technique has proven to be useful for the early and accurate diagnosis of toxoplasmosis and for guiding pre-emptive therapy in patients at high risk of devel-oping invasive disease [14]. This method has the advantage of automatic detection of amplified products, thus avoiding potential contamination and reducing the risk of false positive results. We report here our results obtained during the standardization of a quick real-time method to detect toxoplasmic DNA and we analyze his performance to detect parasitemias on peripheral blood of patients with neurologi-cal opportunistic infections. Additionally, we examined and compared the clinical and laboratory findings in patients with cerebral toxoplasmosis compared to other etiologies.
Material and Methods
Study design, patients and setting
This study was a descriptive clinical study, based on prospectively collected information and a standardization and clinical validation of a laboratory assay. The project was approved by the research and ethics committees of the institutions involved (Universidad del Valle and Hospital Universitario del Valle). Patients were informed about the study and written informed consent was obtained from each one of them or their closest relative. Clinical, radiological and laboratory data were collected from clinical charts at the University Hospital, and the immunovirological markers were determined for the specific purpose of the study at Universidad del Valle- in Cali-Colombia, between April 2007 and April 2008. The study enrolled 49 HIV/AIDS adult patients with advanced disease and central nervous symptoms. Diagnosis was established following the CDC [17] and American Academy of Neurology AIDS Task Force criteria [5]. Blood samples were collected in tubes with EDTA using a standard venipuncture technique before initiation of any anti-Toxoplasma treatment. One mL of whole blood was fractioned in 100 µL aliquot immediately after collection and stored at −70 °C until used. In this group, 22 subjects were HIV-positive patients with suspected cerebral toxoplasmosis according to the CDC criteria which include the following clinical and radiological features: (i) a recent onset of a consistent focal neurological abnormality with intracranial disease or reduced level of consciousness, (ii) a lesion having a mass effect evidenced by cerebral tomography imaging and (iii) a serum antibody to T. gondii or successful response to treatment of toxoplasmosis. Response to specific treatment was exhibited two weeks later. Twenty-seven subjects were HIV-infected patients who displayed neurological signs by other diseases (9 with cryptococal meningitis, 5 with cerebral mass, 5 with tuberculous meningitis, 3 with CMV encephalitis, 4 patients with HIV-dementia and one with a cerebral hemorrhagic episode).
Toxoplasma strain and DNA extraction from parasites and blood samples
Tachyzoites of the Toxoplasma gondii RH strain were obtained from ascitic fluid from Swiss mice intraperitonally inoculated. The tachizoites were counted in a hemocytome-ter. DNA extraction was performed on 300 µL of harvested tachyzoites from mice or from whole blood samples by using Wizard Genomics Kit (Promega, Madison, WI, USA) according to the manufacturer’s instruction. Then, 100 µL of DNA rehydration solution was used to elute the DNA and 9 µL of the DNA was used for each PCR. Purified DNA of tachyzoite was quantified with Quant-iT dsDNA HS assay kit on a Qubit flurometer (Invitrogen, Carlsbad, CA, USA).
The Wizard Genomic DNA Purification Kit (Promega, USA) was used for processing the blood samples and pro-cedures were performed as recommended by the manufac-turer. Briefly, DNA from white blood cells was obtained by incubating sample for 10 min at room temperature with the cell lysis solution. After centrifuging at 13,000 g for 20 s at room temperature, supernatant was discarded and pellet recovered. The cellular proteins were then removed by a salt precipitation step and genomic DNA was concentrated and desalted by isopropanol precipitation.
PCR assays
A real-time PCR TaqMan probe-based assay was used for this study. This test amplifies an 87-bp fragment of the B1 gene of Toxoplasma, which is the locus most often routinely used for PCR detection and is tandemly arrayed 35-fold-repetitive, and a 77-bp of a 529-bp repetitive fragment (RE) that is reported to be repeated 300 times in the genome of T. gondii (Genebank accession numbers AF179871 and AF146527) [3]. Human GAPDH gene was co-amplified and detected as the internal control for DNA isolation and PCR amplification from whole blood samples. The primers for B1, F-GAGACACAGCGTGTTATGAACAAAT & R-GCACGTCTCTTGTTCTTCTTCTGTA, with the TaqMan probe CCTCTTCGCGAAACCT, and the primers for (RE) F-CTTGGAGGAGAGATATCAGGACTGT & R-CTCGTCGCTTCCCAACCA, with the TaqMan probe CACCCTCGCCTTCATC, were designed using Primer Express software (Applied Biosystems). The TaqMan probe was labeled at the 5 with 6-carboxyfluorescein (FAM) and at the 3 with non-fluorescent quencher. Real-time PCR was performed on Applied Biosystems 7500 PCR system using TaqMan Universal PCR Master Mix, No AmpErase with uracyl-DNA-glycosylase 2X (Applied Biosystems, Foster City, CA, USA). The amplification protocol consisted of 2 initial stages of 2 min for 50 °C and 10 min for 95 °C, after 45 cycles of 15 s of denaturation for 95 °C followed by 1 min of annealing and extension for 60 °C. For quantification, 5-fold dilutions of T. gondii DNA were included. Results of the PCR (Cycle thresholds or Ct values) were extrapolated into numbers of tachyzoites per mL of blood.
PCR analytical sensitivity and specificity
Amplification efficiency from the slope of one calculated standard curve (10−1/slope) was obtained from purified T. gondii DNA in a five-fold serial dilution standard curve (0.25 to 1 × 106 tachyzoites). Slopes between −3.1 and −3.6, giving reaction efficiencies between 90% and 110%,are typically acceptable. In order to determine the real-time PCR sensitivity and to estimate the loss of T. gondii DNA during the extraction procedure from blood, seronegative human blood spiked with five-fold serial dilutions with tachyzoites ranging from 0.25 to 5 × 105 tachyzoites/mL was prepared. After this procedure, DNA extraction was performed as described previously. Samples were tested in triplicate in each independent experiment (inter-assay variation) and three to seven different experiments in different dates were performed to estimate the intra-assay variation. A percent of the coefficient of variation (CV) was calculated as a measure of the experimental variation using the formula: CV = standard deviation (replicates)/mean Ct (replicates) ×100. To determine the specificity of the experiment, a real-time PCR was performed using DNA by testing DNA extracted from mouse and human and the following purified or concentrated parasites and fungi: Giardia, Blastocystis, and Candida.
Statistical analysis
Differences in proportions were compared by the Fischer’s exact test. The Kruskal-Wallis test was applied to continuous variables. Odds ratios (ORs) with a 95% confidence interval were calculated for all variables. We considered P < .005 as a significant value. The statistical analysis was carried out with the Epi-info software version 3.5.1 (CDC, Atlanta). Sensitivity, specificity, positive predictive value, and nega-tive predictive value were calculated according to applicable tables.
Results
Standardization and optimization of the real-time PCR assay
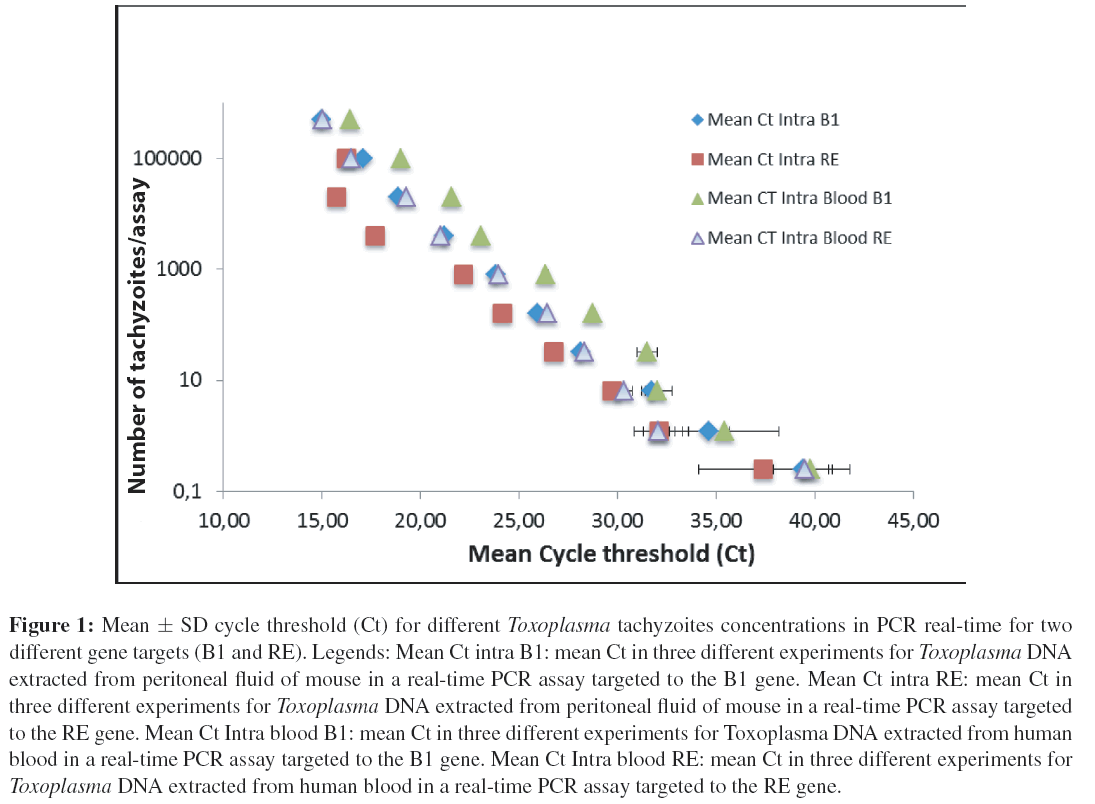
The efficiency of the assay was measured by a regression curve of the Ct values versus the decimal logarithm of the equivalent number of tachyzoites per PCR reaction. The coefficient of correlation (r2) was of 0.998891 and the slope was of −3.67 for B1 and a (r 2) of 0.997345 and a slope of −3.45 was obtained for RE. Therefore, efficiency was of 94.8% for primers detecting B1 gene and of 87.2% for primers detecting RE gene. The limit of detection with an acceptable intraassay and interassay coefficient variation (less than 3%) was of 6 tachyzoites for both primers, and the lower mean Ct values were obtained with the primers targeted to RE gene (Figure 1). The greater Ct values were observed in the blood samples (Figure 1). T. gondii DNA amount detected in blood samples was sixfold less, compared to purified Toxoplasma DNA extracted from peritoneal mouse fluid. No amplification was found when using DNA from different organisms (human, mice, Candida or Giardia).
Figure 1: Mean ± SD cycle threshold (Ct) for different Toxoplasma tachyzoites concentrations in PCR real-time for two different gene targets (B1 and RE). Legends: Mean Ct intra B1: mean Ct in three different experiments for Toxoplasma DNA extracted from peritoneal fluid of mouse in a real-time PCR assay targeted to the B1 gene. Mean Ct intra RE: mean Ct in three different experiments for Toxoplasma DNA extracted from peritoneal fluid of mouse in a real-time PCR assay targeted to the RE gene. Mean Ct Intra blood B1: mean Ct in three different experiments for Toxoplasma DNA extracted from human blood in a real-time PCR assay targeted to the B1 gene. Mean Ct Intra blood RE: mean Ct in three different experiments for Toxoplasma DNA extracted from human blood in a real-time PCR assay targeted to the RE gene.
Results of real-time PCR assay for B1 Toxoplasma gene on clinical samples
For clinical samples only B1 target was assayed, due to the lower efficiency of the RE target. In 18.8% (4/22) of cases of cerebral toxoplasmosis, the parasite B1 DNA real-time PCR assay was positive in blood, compared with 7.4% (2/27) of the patients with different opportunist infections or HIV-associated neurological complication; the differences were not statistically significant (Fisher’s test P : 0.38). In the two real-time PCR positive cases with other neuro-opportunistic infections, both patients had evidence of previous exposure or active systemic toxoplasmosis and were IgG anti-Toxoplasma antibodies positive. One of them was IgM anti-Toxoplasma negative, but IgA anti-Toxoplasma positive. In the other case, both specific anti-Toxoplasma immunoglobulins were positive (IgM and IgA). The average value in cases of cerebral toxoplasmosis was 67.7 (SD 69.0) tachyzoites/mL compared with 31.8 (SD 6.3) tachyzoites/mL for cases with other HIV-associated neurological complications.
Clinical and laboratory findings
The results of clinical and laboratory examinations performed in both groups of patients are summarized in Tables 1 and 2. Clinical symptoms as headache were significantly less frequent and focal neurological symptoms (e.g. hemiparesia) were significantly more frequent in cerebral toxoplasmosis than in other neuroinfections. Previous prophylaxis with trimetoprim/sulfa or previous HAART did not influence in a statistically significant manner the results of the quantitative PCR (Table 3).
| Variable | Median value in CT (n: 22) | Range | Median value in non-CT (n: 27) | Range | P (Kruskal-Wallis test) |
| Age in years | 36 | 18–66 | 36 | 23–63 | .34 |
| IgG anti-Toxoplasma levels (IU) | 769 (N = 14) | 49–2 764 | 451 (N = 9) | 2–16 320 | .81 |
| Number of Toxoplasma tachyzoites/mL by qPCR assay (N = 4) | 57.3 | 5.1–151 | N =2; 31.9 | 27.4–36.4 | .89 |
| Symptoms duration before | 14.5 | 2–65 | 21 | 3–70 | .19 |
| consultation (days) | |||||
| HIV viral load log | 5.3 | 3.2–6.3 | 5.1 | 3.3–6.7 | .97 |
| Blood CD4 number/mm3 | 64 | 14–118 | 66 | 13–296 | .20 |
| Blood CD8 number/mm3 | 468 | 74–1 006 | 369 | 54–14 443 | .23 |
| CD4/CD8 ratio | 0.15 | 0.05–0.35 | 0.1 | 0.05–0.98 | .10 |
| Hemoglobin levels | 11.4 | 5.6–14.9 | 10.8 | 7.6–14.3 | .84 |
| Platelet number/mm3 | 243 500 | 77 000–493 000 | 258 000 | 19 000–801 000 | .89 |
| Survival time (90 days | 90 | feb–90 | 16 | feb–90 | .14 |
| observation period) | |||||
| Karnofsky scale | 85 | 50–100 | 90 | 30–703 | .85 |
Table 1: ANOVA analysis of quantitative variables between cases with definitive clinical diagnosis of cerebral toxoplasmosis (CT) versus other neuro-opportunistic manifestations (non CT) in HIV infected patients in Cali (Colombia, 2008).
| Variable | % in CT | % in non-CT | Odds ratio | IC95% | P |
| Gender: male | 90.9 | 70 | |||
| Survival (at 90 days follow-up) | 40.9 | 59.3 | 0.47 | 0.15–1.49 | .16 |
| Previous HAART | 4.5 | 3.7 | 1.2 | 0.07–20.9 | .7 |
| Previous trimeotprim/sulfa prophylaxis | 36.4 | 14.8 | 3.8 | 0.8–12.9 | .07 |
| HIV de novo diagnosis | 31.8 | 48.1 | 0.5 | 0.15–1.6 | .19 |
| Behavioral changes | 45.5 | 42.3 | 1.13 | 0.3–3.5 | .52 |
| Headache | 40.9 | 73.1 | 0.2 | 0.07–0.8 | .02 |
| Seizures | 18.2 | 19.2 | 0.9 | 0.2–4 | .61 |
| Focal neurological deficit | 50 | 15.4 | 5.5 | 1.4–21 | .01 |
Table 2: Analysis of qualitative variables between cases with definitive clinical diagnosis of cerebral toxoplasmosis (CT, n: 22 patients) versus other neuro-opportunistic manifestations (non CT, n: 27 patients) in HIV infected patients in Cali (Colombia, 2008).
| Variable | % in positive qPCR | % in negative qPCR | Odds ratio | IC95% | P |
| Previous HAART | 0 | 4.7 | 0 | Undefined | .76 |
| Previous prophylaxis with trimetoprim/sulfa | 33.3 | 23.3 | 1.6 | 0.2–10 | .45 |
Table 3: Analysis of qualitative variables between cases with a positive test (n: 6) and a negative test (n: 42) at the qPCR for B1 Toxoplasma gene assay in HIV infected patients in Cali (Colombia, 2008).
Discussion
Among immunocompetent patients, toxoplasmosis diagno-sis can be usually attained either through direct parasite detection or differences in specific antibody titres in serology tests. In the case of AIDS patients, however, it is recommended to use an algorithm based on imaging examination criteria, that is, brain CT scan and/or MRI, along with therapeutic proof for around 14 days. Treatment failure usually leads to serious clinical involvement due to misdiagnosis.
We evaluated a PCR real-time assay for Toxoplasma DNA detection on clinical samples from blood. The targeting of B1 gene was more efficient than the RE genomic repeated element. The PCR efficiency has a major impact on the fluorescence history and the accuracy of the calculated expression result and is critically influenced by PCR reaction components [16]. Efficiency evaluation is an essential marker in gene quantification procedure. Constant amplification efficiency in all compared samples is one important criterion for reliable comparison between samples. This becomes crucially important when analyzing the relationship between unknown sequences versus a standard sequence, which is performed in all relative quantification models [16]. Therefore, in our conditions, B1 targeting was the more adequate to precisely quantify the number of parasites.
Despite a good analytical sensitivity and specificity, and that the coefficient of variation index of our assays was optimal to detect a concentration of parasites greater than 6 tachyzoites on blood samples artificially spiked with the parasite, few patients were positive for Toxoplasma parasitemia on blood samples. Previous prophylaxis with trimetroprim-sulfa did not influence this result and even there was a greater percent of previous history of this prophylaxis in patients positive for Toxoplasma in blood than in patients who were negative. The presence of parasitaemia in patients diagnosed with other neuro-opportunistic infections different to Toxoplasma is not surprising and this could not be attributed to false positive results, because both patients had specific anti-Toxoplasma antibodies. Our results are in agreement with a recent work performed in HIV Brazilian patients [8]. As in our case, a highly sensitive PCR real-time assay was positive in blood in only 1.5% of cases and CSF testing produced better results, with a sensitivity of 35.3% [8]. We think that our results can be explained by an inhibitory effect of blood on DNA detection as we could determine in our assays comparing curves of increasing tachyzoites concentration between DNA extracted from blood or from peritoneal exudates from mice. Real-time PCR has been shown to be useful in T. gondii detection on amniotic fluid samples [18], but in the context of the cerebral toxoplasmosis, the preliminary works [10], recent findings [8] and our results conclusively showed that it is not useful for use on peripheral blood samples in HIV patients. Regarding the discordance with published results by using conventional PCR [7], it should be mentioned that real-time PCR is not always more sensitive than conventional PCR [2]. An additional consideration for results of conventional PCR is that this assay may co-amplify human sequences [11]. This lack of specificity can be counter balanced by the use of conventional PCR with complementary techniques, such as nested PCR, restriction fragment length polymorphisms (RFLP) analysis of the PCR product or hybridization with a probe that anneals to an internal region of the amplified product [11]. Therefore, the results of conventional PCR should be taken with caution in absence of this kind of modifications.
Consequently, the reports of studies that included rigorous controls for PCR specificity and our own results indicate that PCR in blood is not useful because in most of patients during the clinical overt manifestation period there is not a significant systemic parasitaemia, instead most probably there exists a local reactivation. This assumption is supported by the report of a higher PCR positivity in cerebrospinal fluid than in blood [8] and the central nervous system inflammation and pathological changes in the brain during progression to AIDS that may lead to local reactivation of latent CT [9].
In conclusion, our work shows that blood examination by a highly sensitive real-time PCR assay did not detect Toxoplasma in peripheral blood samples from patients withproved toxoplasmic encephalitis, and therefore it is not a useful diagnostic tool in this specific situation.
Acknowledgment
This project was funded by a Colciencias grant: “Perfil de Marcadores de Neuroinflamacion en el LCR de pacientes con Infecciones oportunistas del SNC asociadas a VIH/SIDA” (Col-ciencias code: 110634319282).
References
- Y. Alfonso, J. Fraga, C. Fonseca, N. Jimenez,´ T. Pinillos, A. J. Dorta-Contreras, et al., Molecular diagnosis of Toxoplasma gondii infection in cerebrospinal fluid from AIDS patients,Cerebrospinal Fluid Res, 6 (2009), 2.
- P. Bastien, G. W. Procop, and U. Reischl, Quantitative real-time PCR is not more sensitive than “conventional” pcr, J ClinMicrobiol, 46 (2008), 1897–1900.
- S. Cassaing, M. H. Bessieres,` A. Berry, A. Berrebi, R. Fabre, and J. F. Magnaval, Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR, J ClinMicrobiol, 44 (2006), 720–724.
- J. C. Castano˜ Osorio, G. Sanchez´ Vallejo, D. Franco Andrew, M. M. Gonzalez´ de Schroeder, and A. M. Giraldo Garc´├?┬▒a, Determinacion´ de las caracter´├?┬▒sticas cl´├?┬▒nico-epidemiologicas´ de la neuroinfecccion´ en pacientes con diagnostico´ de VIH/sida en el departamento del quind´├?┬▒o, Infectio, 11 (2007), 173–182.
- K. G. Castro, J. W. Ward, L. Slutsker, J. W. Buehler, H. W. Jaffe, R. L. Berkelman, et al., 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults, MMWR Recomm Rep, 41(1992), 1–19.
- A. Cingolani, A. De Luca, A. Ammassari, A. Antinori, and Ortona, Minimally invasive diagnosis of acquired immun-odeficiency syndrome-related primary central nervous system lymphoma, J Natl Cancer Inst, 90 (1998), 364–369.
- F. A. Colombo, J. E. Vidal, A. C. Penalva de Oliveira, A. V. Hernandez, F. Bonasser-Filho, R. S. Nogueira, et al., Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples, J Clin Microbiol, 43 (2005), 5044–5047.
- C. C. Correia, H. R. Melo, and V. M. Costa, Influence of neurotoxoplasmosis characteristics on real-time PCR sensitivity among AIDS patients in brazil, Trans R Soc Trop Med Hyg, 104(2010), 24–28.
- F. Gonzalez´-Scarano and J. Mart´├?┬▒n-Garc´├?┬▒a, The neuropathogene-sis of AIDS, Nat Rev Immunol, 5 (2005), 69–81.
- T. Hierl, U. Reischl, P. Lang, H. Hebart, M. Stark, P. Kyme, et al., Preliminary evaluation of one conventional nested and two real-time PCR assays for the detection of Toxoplasma gondii in immunocompromised patients, J Med Microbiol, 53 (2004), 629–632.
- A. Kompalic-Cristo, S. A. Nogueira, A. L. Guedes, C. Frota, L. F. Gonzalez,´ A. Brandao,˜ et al., Lack of technical specificity in the molecular diagnosis of toxoplasmosis, Trans R Soc Trop MedHyg, 98 (2004), 92–95.
- J. Lizarazo, F. Castro, M. de Arco, O. Chaves, and Y. Pena,˜ Infec-ciones oportunistas del sistema nervioso central en pacientes con VIH atendidos en el Hospital Universitario Erasmo Meoz, Cucuta,´ 1995–2005, Infectio, 10 (2006), 226–231.
- A. Mamidi, J. A. DeSimone, and R. J. Pomerantz, Central nervous system infections in individuals with HIV-1 infection, JNeurovirol, 8 (2002), 158–167.
- R. Martino, S. Bretagne, H. Einsele, J. Maertens, A. J. Ullmann, R. Parody, et al., Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation, Clin InfectDis, 40 (2005), 67–78.
- F. L. Nogui, S. Mattas, G. Turcato Junior,´ and D. S. Lewi, Neurotoxoplasmosis diagnosis for HIV-1 patients by real-time PCR of cerebrospinal fluid, Braz J Infect Dis, 13 (2009), 18–23.
- M. W. Pfaffl, Quantification strategies in real-time PCR, in A-Z of Quantitative PCR, S. A. Bustin, ed., International University Line (IUL), La Jolla, CA, USA, 2004, ch. 3, 87–112.
- W. Pryse-Phillips, Companion to Clinical Neurology, Oxford University Press, New York, 3rd ed., 2009.
- S. Romand, M. Chosson, J. Franck, M. Wallon, F. Kieffer, K.Kaiser, et al., Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii, Am J Obstet Gynecol, 190(2004), 797–802.
- J. E. Vidal, F. A. Colombo, A. C. de Oliveira, R. Focaccia, and V.L. Pereira-Chioccola, PCR assay using cerebrospinal fluid for diagnosis of cerebral toxoplasmosis in Brazilian AIDS patients, JClin Microbiol, 42 (2004), 4765–4768.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 13323
- [From(publication date):
December-2011 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 8819
- PDF downloads : 4504