Detection of Tacrolimus in Saliva using a Lateral Flow Assay and Surface-Enhanced Resonance Raman Scattering
Received: 01-Mar-2022 / Manuscript No. jabt-22-55806 / Editor assigned: 03-Mar-2022 / PreQC No. jabt-22-55806(PQ) / Reviewed: 17-Mar-2022 / QC No. jabt-22-55806 / Revised: 22-Mar-2022 / Manuscript No. jabt-22-55806(R) / Published Date: 29-Mar-2022 DOI: 10.4172/2155-9872.1000447
Abstract
The lives of ~900,000 US citizens have been extended by organ, and more recently, limb transplants from 1988 to 2021. Currently, there are over 250,000 patients in the US with a functioning kidney transplant. Unfortunately, the success of these methods requires lifelong drug treatment that includes immunosuppression drugs, such as tacrolimus. Furthermore, the dosage and regiment must be monitored to ensure that concentrations are sufficient to avoid transplant rejection, yet not lead to toxicity. Patients must constantly visit a hospital or clinic to have the drugs in their blood measured. Unfortunately, this burden has led to non-adherence and consequently, transplant failure in over 20% of patients, most often resulting in death. In an effort to reduce this burden, we have developed a simple to use assay to measure tacrolimus in saliva at home in 10-20 minutes. The assay employs a lateral flow assay cassette to separate tacrolimus from saliva, surface-enhanced Resonance Raman scattering (SERRS) dye probes as reporters to increase sensitivity, and a simple handheld Raman spectrometer to measure the tacrolimus concentration. Using this assay, tacrolimus spiked in saliva was successfully measured from 1 to 25 ng/mL.
Keywords
Immunosuppressants; Tacrolimus; lateral flow; Saliva; SERRS; at-home; Raman spectroscopy
Introduction
Nearly 1 million transplants have been performed in the US since the first kidney transplant in 1954 [1]. Approximately 50% of the transplants are kidneys, 25% livers, and the remainder heart, lung, pancreas, liver [2], and more recently non-organ fingers, hands, feet, arms and legs [3-5]. The use of immunosuppressant drugs, such as tacrolimus, prednisone, sirolimus, and azathioprine [6], can extend patients’ lives for 20 years or more [7]. Of course these drugs must be taken for the rest of the patients’ lives to avoid transplant rejection [3]. Tacrolimus, formerly known as FK506 [8], has become the drug of choice to suppress the recipient’s immune system so that the transplant is not rejected. However, the dosage and regiment, like all immunosuppressant drugs, must be monitored to ensure that concentrations are sufficient to avoid rejection, yet not produce toxic effects, such as renal damage and neurotoxicity. This is challenging since the immune system and drug pharmacokinetics are recipient dependent. In the case of tacrolimus, the general protocol is trough drug levels at 10 to 15 ng/mL for the 1st 5 months, and 5 to 10 ng/ mL thereafter [9]. However, these concentrations have to be monitored every week post operation to set the patient dependent concentrations, and at least once a month for the rest of their lives. This requires patients to make frequent visits to hospitals or clinics to measure their drug-plasma concentrations. The analysis involves multiple steps: drawing blood, centrifugation to separate the plasma, and analysis by complex lab instruments, such as liquid chromatography to separate the drug from plasma, and tandem mass spectrometers to further isolate and detect the drug (LC-MS/MS) [10]. This technology is labor intensive and takes 3-6 hours to obtain results. The combination of potential transplant rejection, patient specific dosing, complex lab measurements, frequently visit medical clinics, hours at a time has led to medical non-adherence as high as 39% [11,12], which in turn is associated with very high transplant failures and death in greater than 90% of patients [13].
There is clearly a need for a fast, minimally-invasive, and simple method to monitor drug concentrations. Ideally this method could be performed by the patient at home. The last requirement can be best met using saliva as the sample medium. Recent studies have shown that saliva concentrations are similar to blood and can be used to monitor tacrolimus treatment [14, 15]. Tacrolimus does not ionize at physiological pH [16], and readily passes through salivary glands into the oral cavity [17]. This is ideal, since the unionized, i.e., the free form, is responsible for the therapeutic effect, as well as toxicity [18]. Finally, saliva is safe to handle, sampling is painless and can be performed by a non-expert, which makes it ideal for at-home testing. The concept of measuring drugs in saliva is not new, and there exist many commercial lateral flow assay (LFA) test kits [19-21].
However, these kits are not quantitative, providing only a visible “Test Line”, that indicates the presence of a drug, usually by class. For more than 15 years we have been investigating the ability of surfaceenhanced Raman spectroscopy (SERS) to measure drugs in saliva [22- 28]. SERS employs a laser to generate a plasmon field at the surface of gold or silver nanoparticles, and at the same time amplify the Raman scattering from molecules within that field by as much as 6 orders-ofmagnitude [29,30]. While SERS provided increased sensitivity, our previous measurements required the use of liquid or solid extraction methods, limiting the ability to perform the analysis at-home [19- 22]. Recently, we developed a rudimentary LFA that incorporated a SERS-active pad as the Test Line and measured fentanyl in saliva at 500 ng/mL [31]. However, tacrolimus requires quantitation from 5 to 15 ng/mL. Consequently, we investigated the ability to further amplify the Raman scattering by adding a dye molecule to generate surface enhanced Resonance Raman spectroscopy (SERRS), which has been used to detect single molecules in water [32,33]. Here we describe the development of a SERRS LFA. In use, a saliva sample is added to the LFA, the saliva flows through the conjugate pad of SERRS probes, that bind tacrolimus, which if present, bind to a second antibody at the Test Line, where a Raman spectrometer measures the SERRS probe dye intensity representative of the tacrolimus concentration.
Materials and Methods
Tacrolimus and all reagents were obtained from Sigma-Aldrich (Allentown, PA), while tacrolimus antibodies were obtained from Creative Diagnostics (Shirley, NY), and de-identified, pooled saliva was obtained from Lee Biosolutions (Maryland Heights, MO). The SERRS probes consisted of synthesized gold nanoparticles [21], coated with a dye, as a reporter molecule, and functionalized with antibodies specific to tacrolimus [25]. The LFA cassettes were of standard construction (nanoComposix, San Diego, CA) consisting of
1) A conjugate pad containing the SERRS probes,
2) A Test Line functionalized with the tacrolimus antibodies,
3) A Control Line functionalized with goat anti-mouse IgG,
4) A wicking pad, all on
5) A nitrocellulose support,
6) Enclosed in a plastic cassette containing a sample addition port and a viewing section.
The LFA cassettes were measured using a compact, 5-lb Raman spectrometer of in-house design. It employed a 785 nm diode laser and a room temperature 512 channel Si array detector. The cassette Control Line was visually examined after 10 minutes to confirm sample flow. The cassettes were inserted into a simple enclosure attached to the spectrometer that fixed the distance of the laser focal point on the cassettes. The cassettes were positioned manually on the Test Line for measurements. All measurements used 40 mW laser power and a 1-sec acquisition time. All sample preparations and measurements were performed in a Biosafety Level 2 cabinet following standard safety procedures.
Results
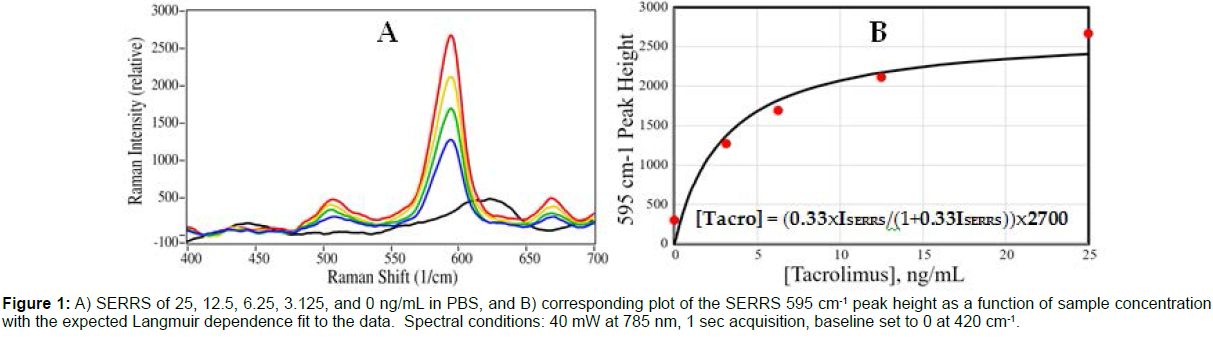
The capabilities of the LFAs were initially tested using tacrolimus in phosphate buffered saline (PBS) at 0 to 25 ng/mL. For each concentration, 0.1 mL of sample was added by pipette to a cassette and allowed to flow for 15 min, after which the Test Line was measured by the Raman spectrometer. The measured height of the dye SERRS peak at 595 cm-1 was used to develop a concentration plot (Figure 1).
Figure 1: A) SERRS of 25, 12.5, 6.25, 3.125, and 0 ng/mL in PBS, and B) corresponding plot of the SERRS 595 cm-1 peak height as a function of sample concentration with the expected Langmuir dependence fit to the data. Spectral conditions: 40 mW at 785 nm, 1 sec acquisition, baseline set to 0 at 420 cm-1.
The peak height intensity is a function of the tacrolimus concentration, which is limited by the concentration of the available antibodies on the Test Line, and follows a standard Langmuir equation [34] (Equation 1). [Tacro] = (kxISERRS/(1+kxISERRS))xS
Where [Tacro] is the tacrolimus concentration, k is a constant, ISERRS is the measured 595 cm-1 peak height, and S is a constant representing the available antibodies on the Test Line, which is equivalent to the maximum peak height.
Next measurements of tacrolimus added to de-identified, pooled saliva were performed. A stock solution of tacrolimus was prepared at 0.1 mg/mL in PBS, and serially diluted in saliva to obtain concentrations from 0 to 25 ng/mL. For measurements, 0.1 mL of each sample
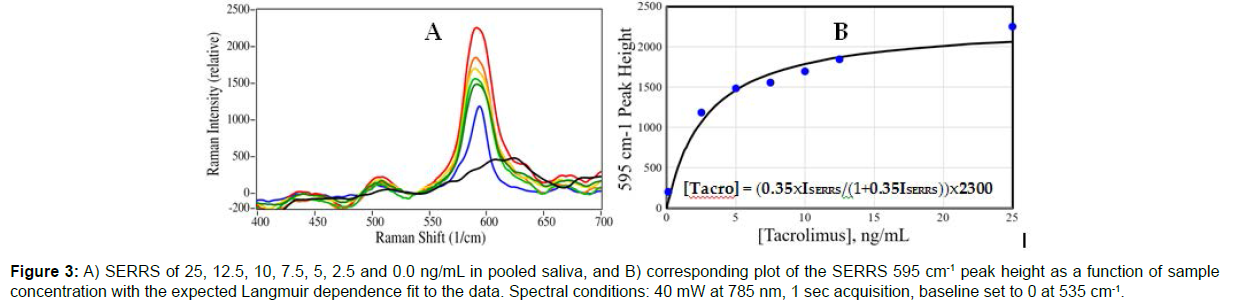
concentration was added to three cassettes (Figure 2). Each cassette was measured by the Raman spectrometer, and the spectra were averaged for each concentration (Figures 3A). The averaged measured height of the dye SERRS peak at 595 cm-1 for each concentration was used to develop a concentration plot, which was fit by a Langmuir equation (Figure 3B). For these saliva samples, the peak heights were lower in intensity by 13-17% compared to the PBS samples (Figure 2A). This intensity decrease was expected as the saliva mucins block some of the antibody binding sites, as reflected by the constant S (Figures 1-3).
The standard deviation generally increased with concentration, and ranged from 0.3 to 2.7 ng/mL, ignoring the sample with no tacrolimus added (Table 1).
| SERRS Peak Height 9595 (cm-1) | Tacrolimus (ng/ml) | ||||||
|---|---|---|---|---|---|---|---|
| Cassette 1 | Cassette 2 | Cassette 3 | Average | Stan Dev | Prepared ± Stan Dev | Calculated | Cllc Error |
| 2534 | 2136 | 2085 | 2251.7 | 245.8 | 2.5 ± 2.7 | 60 | 35 |
| 1723 | 2054 | 1763 | 1846.7 | 180.7 | 12.5 ± 1.2 | 11.5 | -1 |
| 1620 | 1522 | 1951 | 1698 | 224.6 | 10 ± 1.3 | 8.1 | -1.9 |
| 1659 | 1456 | 1559 | 1558 | 101.3 | 7.5 ± 0.5 | 6 | -1.5 |
| 1831 | 1263 | 1357 | 1483.4 | 133.4 | 5 ± 1.0 | 3 | 0.5 |
| 1167 | 1326 | 1061 | 1184.4 | 133.4 | 2.5 ± 0.3 | 3 | 0.5 |
| 125 | 189 | 296 | 203.3 | 86.4 | 0 ± 0 | 0.28 | 0.28 |
Table 1: Measured SERRS peak heights at Test Lines for 3 cassettes each at 25, 12.5, 10.0, 7.5, 5.0, 2.5, and 0 ng/mL Tacrolimus with average and standard deviation, as well as concentration standard deviation, and Langmuir calculated concentrations and error.
Figure 3: A) SERRS of 25, 12.5, 10, 7.5, 5, 2.5 and 0.0 ng/mL in pooled saliva, and B) corresponding plot of the SERRS 595 cm-1 peak height as a function of sample concentration with the expected Langmuir dependence fit to the data. Spectral conditions: 40 mW at 785 nm, 1 sec acquisition, baseline set to 0 at 535 cm-1.
The averaged peaks heights were also used to predict the concentrations using the Langmuir equation that was fit to the data, as would be performed by software in a product. The error for these predictions ranged from -1.9 to 0.5 ng/mL, ignoring the 25 ng/mL sample, which had a large error due to the imprecision of the equation at this higher concentration.
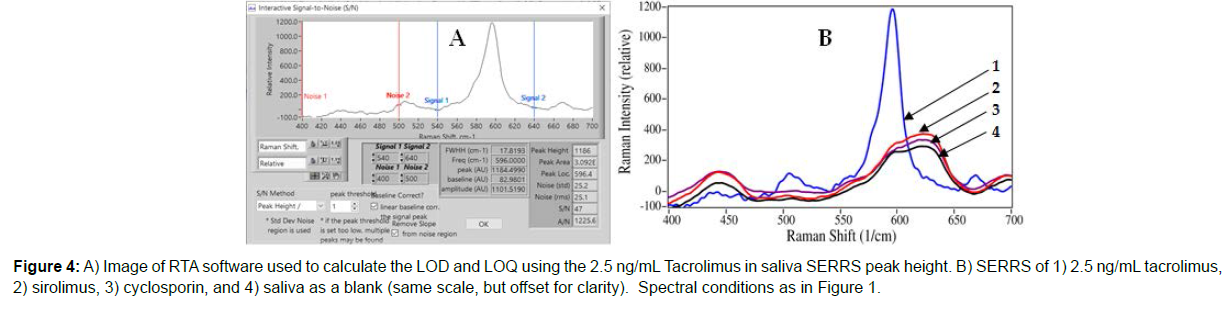
The lowest measured saliva sample, 2.5 ng/mL, was used to calculate a limit-of-detection (LOD) based on the signal-to noise ratio (S/N) of 3, and the limit-of-quantitation (LOQ) based on an S/N of 10, using RTA’s signal and noise determination software (Figure 4A). The signal is calculated as the baseline corrected peak height, while the noise is calculated as the root mean square (rms) noise of a baseline equivalent in width to the peak at the baseline. The LOD was determined to be 0.16 ng/mL (2.5 ng/mL x [3/47]), and the LOQ was determined to be 0.53 ng/mL (2.5 ng/mL x [10/47]) in saliva (Figure 4).
In an effort to demonstrate selectivity, sirolimus, another immunosuppressant drug with a structure very similar to tacrolimus; and cyclosporin, often used as a co-administered drug, were both measured using this assay. Spectral measurements of both samples prepared at 500 ng/mL, 50 times the normal tacrolimus dose, yielded no spectra of the SERRS Probes demonstrating excellent antibody specificity, even at this high concentration. In fact, the spectra were identical to that of a blank sample of PBS (Figure 4B).
Conclusion
A lateral flow assay cassette for Tacrolimus in saliva was developed that employed novel SERRS probes that bind tacrolimus, which were quantitatively measured using a Raman spectrometer. Successful measurements of tacrolimus in phosphate buffered saline were followed by measurements of tacrolimus in purchased de-identified, pooled saliva covering the treatment range of 5 to 15 ng/mL. The SERRS intensity as a function of tacrolimus concentration followed a standard Langmuir equation, which was fit to the data and used to predict the concentrations based on the SERRS peak heights. The average error for the samples from 2.5 to 12.5 ng/mL was ±1 ng/mL tacrolimus. In addition, measurements of sirolimus, another immunosuppressant drug with a very similar chemical structure, and cyclosporin, an often used co-drug, showed no antibody cross reactivity at concentrations 50 times those used during treatment. While these measurements represent a limited set of ideal samples, these results are in-line with the requirements for monitoring dosage. Future research will test the ability of the LFA cassettes and SERRS measurements to detect tacrolimus in actual human samples with the goal of developing a simple-to-use, athome immunoassay drug monitor. We believe that the elimination of the 1-4 monthly visits to hospitals or clinics for painful blood draws, will greatly reduce non-adherence, and thereby reduce organ rejection, and in many cases extend lives.
Acknowledgments
The authors are grateful for important guidance from Dr. Curtis Cetrulo, Jr. of Massachusetts General Hospital and Harvard Medical School. The authors appreciate the funding from the Department of Defense, USA Medical Research that made this research possible (W81XWH19-C-0079).
References
- , United Network of Organ Sharing, History of Transplantation.
- , Organ Procurement and Transplantation Network, managed by the Health Resources and Services Administration, U.S. Department of Health & Human Services
- Leonard DA, CR Gordon, DH Sachs, CL Cetrulo (2012) Immunobiology of Face Transplantation. J Craniofac Surg 23(1):268-271.
- Leonard DA, Kurtz JM, Cetrulo CL, Jr (2014) Achieving immune tolerance in hand and face transplantation: a realistic prospect? Immunother 6(5): 499-502.
- Ng ZY, Lellouch AG, Rosales IA, Geoghegan L, Gama A-R, et al. (2019) Graft vasculopathy of vascularized composite allografts in humans: a literature review and retrospective study. Transpl Int 32(8): 831-838.
- Cochrane ZR (2019) About Immunosuppressant Drugs. Healthline.
- Pérez-Sáez MJ, Pascual J (2015) Kidney Transplantation in the Diabetic Patient. J Clin Med 4(6): 1269-1280.
- Starzl T, Todo S, Fung J, Demetris AJ, Venkataramman R, et al. (1989) FK506 for human liver, kidney and pancreas transplantation. Lancet 2(8670): 1000-1004.
- Kershner R, Fitzsimmons E (1996) Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 62(7): 920-926.
- Kalt DA (2017) Tacrolimus: A Review of Laboratory Detection Methods and Indications for Use. Lab Medicine 48(4): e62-e65.
- Dew MA, DiMartini AF, De Vito Dabbs, Myaskovsky L, Steel J, et al. (2007) Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation 83(7): 858-873.
- Al-Sheyyab A, Binari L, Shwetar M, Ramos E, Kapp ME, et al. (2019) Association of medication non-adherence with short-term allograft loss after the treatment of severe acute kidney transplant rejection. BMC Nephrol 20(1): 373.
- Laederach-Hofmann K, Bunzel B (2000) Noncompliance in Organ Transplant Recipients: A Literature Review. Gen Hosp Psychiatry 22(6): 412-424.
- Hamadi S, Banna F, Al-Awwa I, Al-Ghazawi A, Idkaidek N (2018) Saliva versus blood therapeutic drug monitoring of tacrolimus in Jordanian kidney transplant patients. Nov Appro Drug Des Dev 4(2): 1-5.
- Ghareeb M, Gohh R, Akhlaghi F (2018) Tacrolimus concentration in saliva of kidney transplant recipients: factors influencing the relationship with whole blood concentrations. Clin Pharmacokinet 57(9): 1199-1210.
- , DrugBank. Tacrolimus, accessed 2/22/2022
- Haeckel R (1993) Factors influencing the saliva/plasma ratio of drugs. Ann N Y Acad Sci 694: 128-142.
- Zahir H, McCaughan G, Gleeson M, Nand R, McLachlan AJ (2004) Changes in tacrolimus distribution in blood and plasma protein binding following liver transplantation. Ther Drug Monit 26(5): 506-515.
- , T-Cube 6 panel oral fluid drug tests, Transmedeco
- , OralTox, Premier Biotech
- , 6-panel-oral-saliva-drug-test, Uritox
- Farquharson S, Shende C, Inscore F, Maksymiuk P, Gift A (2005) Analysis of 5-fluorouracil in saliva using surface-enhanced Raman spectroscopy. J Raman Spectrosc 36(3): 208-212.
- Inscore F, Shende C, Sengupta A, Huang H, Farquharson S (2011) Detection of drugs of abuse in saliva by surface-enhanced Raman spectroscopy (SERS). Appl Spectrosc 65(9) 1004-1008.
- Dana K, Shende C, Huang H, Farquharson S (2015) Rapid analysis of cocaine in saliva by surface-enhanced Raman spectroscopy. J Anal Bioanal Tech 6(6): 1-5.
- Farquharson S, Dana K, Shende C, Gladding Z, Newcomb J, et al. (2017) Rapid identification of buprenorphine in patient saliva. J Anal Bioanal Tech 8(3).
- Farquharson S, Brouillette C, Smith W, Shende C (2019) A surface-enhanced Raman spectral library of important drugs associated with point-of-care and field applications.Front Chem 7: 706-721.
- Shende C, Brouillette C, Smith W, Farquharson S (2019) Quantitative measurements of codeine and fentanyl on a surface-enhanced Raman-active pad. Mol 24(14): 2578-2585.
- Jing N, Lipert R, Dawson B, Porter M (1999) Immunoassay Readout Method Using Extrinsic Raman Labels Adsorbed on Immunogold Colloids. Anal Chem 71(21): 4903-4908.
- Jeanmaire DL, RP van Duyne (1977) Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem 84(1): 1-20.
- Weaver MJ, S Farquharson S, Tadayyoni MA (1985) Surface-enhancement factors for Raman scattering at silver electrodes. Role of adsorbate-surface interactions and electrode structure. J Chem Phys 82: 4867-4874.
- Shende C, Brouillette C, Farquharson S (2019) Detection of codeine and fentanyl in saliva, blood plasma and whole blood in 5 minutes using a SERS flow-separation strip. Analyst 144(18): 5449-5454.
- Kneipp K, Wang Y, Dasari RR, Feld MS (1995) Approach to Single-Molecule Detection Using Surface-Enhanced Resonance Raman Scattering (SERRS). Appl Spectrosc 49: 780-784.
- Nie S, Emory SR, (1997) Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Sci 275(5303):1102-1106.
- Langmuir I (1918) The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum. JACS 40(9): 1361-1402.
- , Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Farquharson S, Shende C (2022) Detection of Tacrolimus in Saliva using a Lateral Flow Assay and Surface-Enhanced Resonance Raman Scattering. J Anal Bioanal Tech 10: 446. DOI: 10.4172/2155-9872.1000447
Copyright: © 2022 Farquharson S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2133
- [From(publication date): 0-2022 - Nov 23, 2024]
- Breakdown by view type
- HTML page views: 1797
- PDF downloads: 336