Detection of Rota Virus with the Help of Nanomaterial Based Field Effect Transistor (BIO-FET)
Received: 29-Nov-2017 / Accepted Date: 13-Dec-2017 / Published Date: 20-Dec-2017 DOI: 10.4172/2090-4967.1000149
Abstract
Biosensors are analytical devices composed of a recognition element of biological origin and a physiochemical transducer which converts the reaction into readable signals. The biosensors are providing their application in medical diagnosis and clinical analysis from last fifty years. In this work we have synthesized reduced graphene oxide, with this nano-film of RGO a FET- biosensor was developed and tested for the rapid detection of Rota-virus. It is a sensitive, specific and label free detection protocol. In the present methodology graphene; which is a single monomer unit of graphite was used and fabricated between the 3 μm apart micro-fabricated Au-electrode over hot plate at 70°C on a crystal surface by drop casting method. This thin film of graphene on FET crystal provide a better platform for attachment of antibodies through pyrene-NHS (Linker) complex. Pyrene- NHS is identified more suitable for antibody immobilization on the graphene film to be used in nano-immunosensor for the detection of specific antigens. The Rota-virus interation to its corresponding antibody were studied with respect to changes in the conductance in the graphene channel. This chemi-resistive FET transducer based 1D nanostructure have attracted much attention because of its rapid, sensitive, and cost effective point of care device for quantitative detection of Rota-virus so that gastroenteritis in infants can be detected early. The graphene nanofilm has been characterized by UV-Vis Spectroscopy, FT-IR, EDS, and SEM.
Keywords: Graphene oxide (GO); Reduced Graphene oxide (RGO); Field effect transistor (FET); Fourier transform infrared spectroscopy (FT-IR); Energy dispersive spectroscopy (EDS); Scanning electron microscopy (SEM)
Introduction
A virus is a parasite which infects a living system or cell so that they can reproduce themselves. Although viruses shares many features similar with living being, like the presence of genetic material i.e. containing nucleotide sequence (DNA or RNA), but they are not considered to be living as they lack many machinery required for replication and cell division. Viruses are not only infecting the human but also infect animals, plants, bacteria and fungi. Viruses in present scenario are of great concern as they are potential agents for biological warfare and terrorism. Exposure to biological aerosols such as those from H1N1 influenza, severe acute respiratory diseases, bird flu and bioterrorism attacks has resulted in huge human and economic cost. Furthermore, the sustained growth in international travel multiplies the risk that an infectious disease may develop into a pandemic. In 1973 Ruth Bishop with his colleagues has discovered Rotavirus with the help of an electron micrograph image and accounts” 60% of hospitalizations for intense diarrhoea in babies and growing children’s. “A rotavirus has a characteristic wheel-like image. The word Rota-virus has been derived from Latin word Rota which means “wheel”. These viruses are not enveloped by any coat as other viruses do but are double shelled.”The genomic sequence of virus consists of eleven segments which are ds- RNA that code for six different structural protein“(VP1-VP4, VP6, VP7) and five non-structural proteins (NSP1-NSP5).”Scientists have described seven rotavirus groups (A to G). While only group A, B and C infects humans, out of them group a causes the majority of childhood infection. The Rotavirus is a member of Reoviridae family of viruses, it infects in bowels, causing a severe gastroenteritis results bowels and inflammation of the stomach”. The virus usually causes severe diarrhea among infants throughout the world, and causing death of about 500,000 children worldwide per year. From 2006, vaccination is available for the rotavirus infection. Previously almost all children became infected with rotavirus. Repeated infections with different viral strains are possible. After several episodes of infections children acquire immunity to rotavirus. Throughout the world, rotavirus infection is still a major cause of death in new born babies and children. This virus infects population in all society groups and equally exists in developed and developing countries, so the changing of sanitation practices and water supply is not likely to reduce the incidence of infection. In U.S rotavirus infections has high incidence during the fall months in the southwest and by spring covers the whole northeast, however, infection can happen anytime of the year. The time period of initial infection to occurrence of symptoms for rotavirus disease is typically around one to two days. The disease symptom carries fever, watery diarrhea and vomiting. Profuse watery diarrhea up to several times per day causes abdominal pain. The new born babies and children infected from viral infection suffer from severe case of dehydration and often lead to death. So it is important to recognize infection at its early incidence. The disease symptoms which arise due to Rota-virus are lethargy, dry and cold skin, absence of tears while crying, dry or sticky mouth, sunken eyes or sunken membranous spot and extreme thirst. In the presented work Rota-virus detection is the prime moto.
Biosensors are of paramount importance in the field of medical diagnosis [1] but also in forensic industries, national security and environmental monitoring. The significant increase in the interest and research in biosensor is for the utilization of high specificity and sensitivity of biomolecules and living biological system [2]. The uses of biosensors for sensitive and specific detection of various analytes are of great importance, and its success is often dictated by the nature of the detection element (the specific ligand) and the choice of target analysis [3]. All the traditional methods used before mainly work on chromophore or radioactive labeling, optical detection using fluorochrome-tagged oligonucleotide [4,5] or PCR-based amplification of target DNA [6]. A biosensor is defined as an analytical device, which converts the biochemical responses occurring in the bio-receptor into the quantifiable electronic signal with the help of the transducer [7,8]. A biosensor responds to an analyte present in the sample and thus tells about the concentration of analyte in an electronic signal with the help of appropriate combination of a biological recognition system and a transducer. “With the advancement in scientific and technological progress, these devices will play highly important roles in all sectors of human Endeavour. In particular, biosensor will form the basis of cheap, simple, sensitive, specific, less time consuming devices for knowing the chemical information, presence of analyte and bringing sophisticated analytical technique to the non-specialist and general public and is easy to use even by the layman. The market opportunities for the rapid exploitation of novel development in this sector are of considerable importance. The Biosensor research is also likely to have a significant impact on the development of modern electronics latest example of such biosensor is nano-material based Bio-FET.
The nanotechnology is the branch of technology which deals with dimensions less than 100 nm, here changes in atom and molecules is done to get the expected product size by ultra-sonication or other methods.“The binding of the biological species in the nano-biosensor is considered to perturb the electrical properties of nano-biosensor and thus yielding a quantitative detection of analyte [9]. Several necessary factors, like sensitivity, specificity, and real-time monitoring, became easy when the biosensor get fabricated with nano material like graphene [10,11] graphene oxide (GO), Silicon nanowire tube (SWT), carbon nanotubes (CNT) [12,13] nanoparticle, quantum dots, nanodiamonds. Out of them graphene shows a unique combination of properties that is ideal for next generation electronics, because of its mechanically flexible, high electrically conductive, and chemically stable nature. Nanomaterial’s can be replacement for natural materials which can function with the living systems. Research in the field of bio-nanomaterial has opened new doors in two important fields of life science and material science. Scientists have examined the graphene oxide. Biosensors like graphene-oxide based glucose biosensor have been developed.
Among all carbon-nanomaterial two compounds namely graphene and graphene oxide has acquired tremendous interest between physicist, chemists and materials scientists. Due to these specific types of bonds and electron arrangement, graphene has amazing properties. It has a large surface area, hall effect, a band gap which is tunable, high room-temperature, huge mechanical power, high thermal conductivity and elasticity respectively [14]. Graphite has carbon–carbon bond with sp2 hybridization and weak bond energy amongst the head-tohead layers and bond with in and out of plane of p orbital respectively [15]. Graphene and its derivatives such as graphene oxide, reduced graphene oxide have turn out to be best emerging nano building blocks for membrane parting in various tunable physicochemical properties and laminar processes. Graphene has molecular separation properties, so graphene membranes are used in the refining water and gases [16]. Among the wide variety of biosensor the Bio-FET is of keen interest nowadays. In Bio-FET, biosensor detect how electrical characteristics of the of a system changes due to closeness or contact with analyte. The most promising among these devices is the nanowires field effect transistor (NW-FET). The FET is devices that monitor the current flow in between the two electrical terminals or electrodes, called the source and the drain, embedded in a semiconductor. A FET biosensor is composed of a semiconductor which connects the source and the drain terminal. The charged bio-molecule gets absorbed in the semiconductor and thus produces an electric field that acts as the gate and changes the charge carrier density within the device. The limit of detection of this device is basically higher than 1 nm for most of the bio-molecules. This problem is partly overshadowed with the use of nano-scale objects so that the bridge can be created between the source and the drain. Recently use of carbon based biocompatible nanomaterials has been increasingly incorporated into biosensing application which increases efficacy and sensitivity of most of detecting devices [17]. The nano-scale objects used are e.g. graphene, carbon nanotubes, or lithographically defined nano-wires. Thus there is increase in surface to volume ratio and allow the electric field created due to absorbed material to spread in a higher portion of the channel, yielding the lower detection limit less enough to make the detection of single virus particle possible. Now a day’s metals, and graphene based nanomaterial have been extensively used in the biosensing devices to increase the effectiveness, sensitivity and selectivity of most of the diagnostic devices, like field effect transistor (FET) based devices. Among the variety of biosensors proposed so far, the integration of biologically active material onto the ion sensitive field effect transistor is one of the most fascinating approaches. The invention of ISFET was done by Bergveld in the year 1970 and the first mini sensor developed was silicon based chemical sensor. The BIO-FET is new generation miniaturized biosensor. They show a lot of potential advantage such as small size, weight, quick response, low output impedance, and the possibility of automatic package at wafer level. “Thus because of long list of advantages bio-FET can be used in field of medicine, biotechnology, environment monitoring, drug industries to defense and security. The major factor for which there is significant enhancement in research and interest in biosensors is to utilize the high specificity and sensitivity of biomolecules. Bio-FET can be classified [18] on the basis of bio-recognition element that is used for sensing purposes of analyte as enzyme FET, Immunologically modified FET, DNA modified FET or Gene modified FET, Cell based FET and ‘beetle/chip’ FET. Keeping in view the historic and the recent development in the field of biosensor, the present work focuses on fabrication of the FET (field effect transistor) with graphene and the detection of rotavirus through bio-FET. The present work comprises of Synthesis of graphene oxide (GO) followed by reduced graphene oxide synthesis (RGO). Biophysical characterization of GO as well as RGO. Fabrication of the FET (Field effect transistor) chip with graphene by drop casting method now attachment of linker (pyrene NHS) followed by immobilization of antibody onto the gate region of FET. Different concentration of antigen is placed for the sensitivity check of the apparatus followed by encapsulation of data and its interpretation.
Materials and Methods Reagents
The entire chemical used in the study were of analytical grade and were obtained from Himedia, SRL, SIGMA, Molychem, Titan, and Acros. Potassium chloride (SRL), Sodium chloride (Himedia), potassium dihydrogen phosphate (Himedia), Sodium hydrogen phosphate (Himedia) was used for preparing PBS. Phosphate buffer saline was made by mixing NaCl (0.8 gm), KCl (0.02 gm), KH2PO4 (0.024 gm), NaHPO4 (0.144 gm) in 100 ml double distilled water. Concentrated sulphuric H2SO4 (3 ml), Graphite powder (2 gm), Potassium persulphate (1 gm) Phosphorus pentaoxide (1 gm), concentrated Sulphuric acid (23 ml), Potassium permanganate (3 gm), and Hydrochloric acid leads to graphene oxide formation. The antigen and antibody were obtained from IVRI barely.
Synthesis of graphene oxide
Graphene oxide was synthesized by modified Hummer’s method.” Here the graphite powder was treated with highly oxidizing agents under ideal condition for graphene oxide synthesis. The GO dispersion of 1 mg/ml of black in color was made in which was bathing sonicated as well as ultrasonicated to obtain the smaller flakes of graphene oxide. This black color graphene oxide dispersion was then further centrifuged to obtain the yellow-brown aqueous suspension. GO suspension was stored at room temperature and was used further for the preparation of reduced graphene oxide RGO.
Synthesis of reduced graphene oxide
The reduced “graphene oxide is synthesized “by” chemical reduction of GO” using L-ascorbic acid and pouring it into the GO suspension and then ammonia solution is “added to adjust the pH of suspension to 9-10.” Then followed by sonication, heating and thus resulting in RGO formation. The produced RGO is designated as “LAA RGO,” which was then “centrifuged at 3000 rpm for 15 min”, followed by wash process with distilled water until pH comes neutral.
The Characterization of Graphene Oxide” (GO) and Reduced Graphene Oxide (RGO) was Done by Various Instrumentation
Double Beam UV-Visible Spectrophotometer and Fourier Transform Infrared spectroscopy (FT-IR) installed in the Membrane biophysics and Nanobiosensor Research Laboratory.
The Energy Dispersive Spectroscopy (EDS) system (BRUKER) was attached to JOEL SEM system that detects the X-ray emitted” from a sample during electron imaging.
Scanning electron microscopy (SEM) images (including high resolution images) was acquired using a JEOL JSM-6610/LV/A/LA scanning“electron microscope (JEOL Ltd., Japan) operated at 200 kV.”
TGA of “graphene oxide” and “reduced graphene oxide” was performed by instrument installed in “CSIR-CBRI Lab” at IITRoorkee. The available virus sample is detected with the help of Bio- FET by taking following steps to develop A Nano biosensor for the rapid detection of virus. The development of biosensor follows the following process described briefly.
Cleaning of the crystal surface
In the first step of cleaning the crystal was treated with methanol for 30 min. After that the crystal was rinsed with water successively and dried under the stream of nitrogen air.
Fabrication of graphene on the crystal surface
The pretreated crystal undergoes coating with graphene by using drop casting method. We fabricate the FET biosensor by simply dropcasting 100 μL of reduced graphene oxide (RGO) aqueous solution of concentration 0.5 mg/mL-1 in between the pair of gold electrodes over a hot plate at a temperature of 70°C.
Activation of crystal
The R-GO FET channel is activated with bilinker 1-Pyrenebutyric acid N-hydroxysuccinimide ester in methanol for 2 h at “room temperature, “followed by washed with methanol, and dried under N2 flow. After the reaction the FET crystal was mounted in the platform for the detection of fluctuation in current.
Blocking of non-specific binding sites
The gold microelectrodes crystal surface was then passivated by incubation with 6 mm, 6-mercapto-1-hexanol (MCH) in methanol for 1 h to block the nonspecific binding sites from the attachment of antibody or antigen.
Antibody immobilization
This was then covalent immobilization by 100 μg/mL-1 antibodies of rotavirus in “phosphate buffer solution (PBS; pH 7.4) at 4°C. The device was then repeatedly washed with PBS to remove the unbound protein molecules and dried under N2 flow. Now the crystal is mounted in the FET cell for measuring change in current flow.
BSA blocking layer
For avoiding non-specific interaction, the non covered surface is blocked with a 1% bovine serum albumin in phosphate buffer saline (PBS).
Antigen detection
To investigate the sensitivity of the hybrid device, it was exposed to varying concentration of target antigen (Rota-virus) in the PBS buffer (pH 7.4). The device was exposed to 2 μl aliquot of different concentration of Rota-virus for 10 min, at room temperature, followed by washing thoroughly with distilled water, dried with N2 gas. The normalized response of Rota virus-G-FET hybrid was observed by (RRο)/ Rο “where Rο and R is the resistance of the device measured before and after the exposure to Rota-virus in PBS, respectively as the function of Rota-virus concentration in PBS.
Results and Discussion
Characterization of graphene oxide and reduced graphene oxide
The GO and “RGO flakes” and suspension was characterized using microscopic and spectroscopic techniques; Like UV-Vis spectroscopy, FT-IR, SEM, EDX, TGA.
UV-VIS spectroscopy
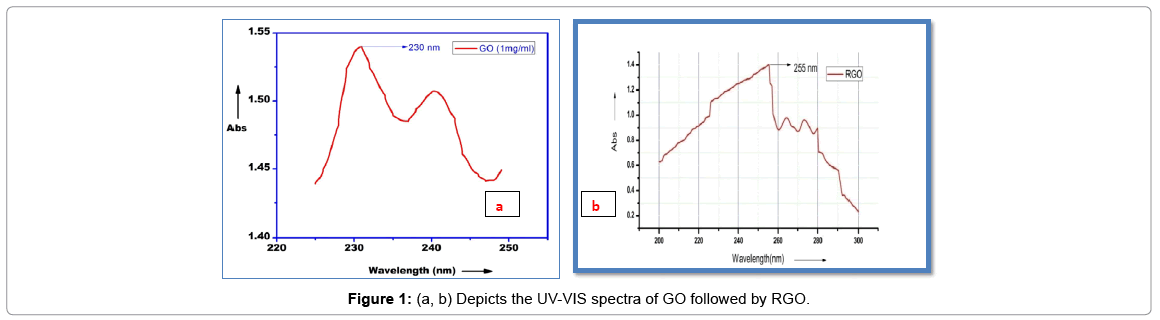
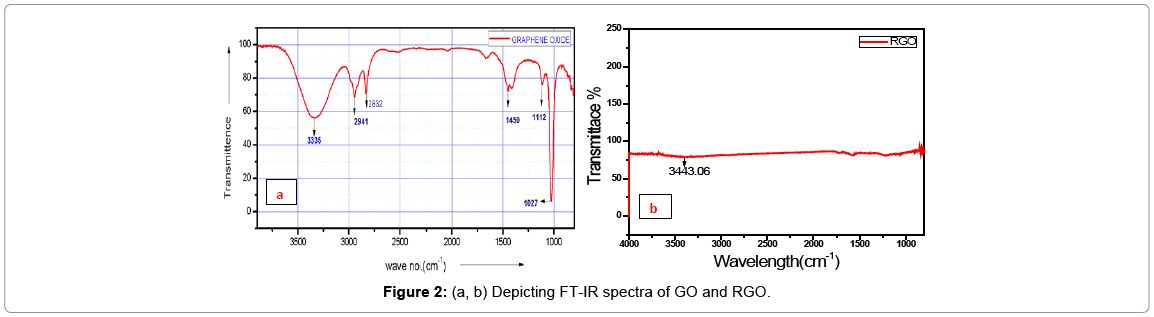
The ultraviolet absorption spectroscopy deals with the measurement of energy absorbed when electron is promoted at higher energy levels. “In each possible case, an electron is excited from a full orbital into an empty anti-bonding orbital. Each jump takes energy from the light. “The ultraviolet spectrum is simply a plot of wavelength of light absorbed versus absorption intensity (absorbance or transmittance) and is conveniently recorded by plotting molar absorptivity (ε) against wavelength (nm).” The optical properties such as absorption maxima and absorption intensity are dependent on the“particle size. Observed that we can get different peaks for thick layer graphene and different peak for few layer graphene by observing their absorbance peaks. The few layer graphene as in graphene oxide gives an intense peak at 230 nm while RGO give intense peak at 255 nm (Figures 1a and 1b). Here the wavelength of maximum absorbance of graphene oxide was found to be at 230 nm, attributed to π-π* transition of aromatic C-C ring and 255 nm for reduced graphene oxide due to decrease in oxygen functional groups and an increase in aromatic rings, causing electrons to be easily excited at lower energy. FT-IR Analysis (Figure 2a) shows FT-IR spectra of graphene oxide. “The spectra showing presence of hydroxyl group at 3335 cm-1, the C=O carbonyl stretching at 1027 cm-1, the CO epoxide group stretching at 1112 cm-1, and C=C peak at 1450.” The high intensity of main peaks in graphene oxide “confirms the presence of large amount of oxygen functional group after the oxidation process.” While the Figure 2b shows the reduced graphene oxide peaks where the hydroxyl and alkoxy groups were significantly decreased and the phenol”C=C ring stretching. For the elimination of oxygen functional groups on carbon planes however, strongly acidic environment might be required.
Thermo gravimetric analysis (TGA)
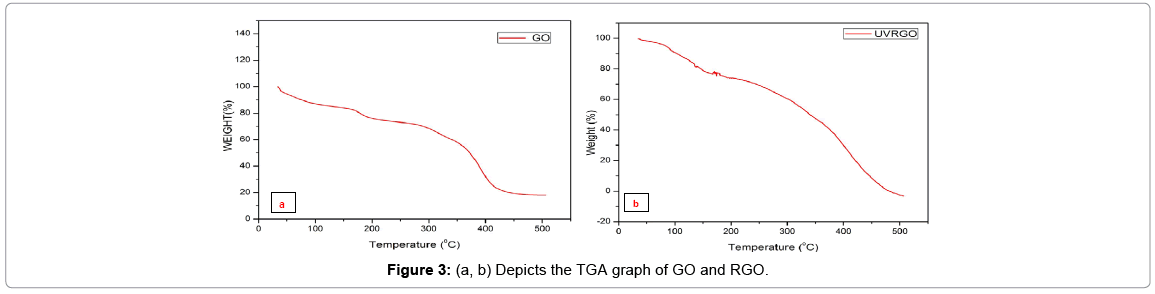
The thermal stability of “graphene oxide and reduced graphene oxide”was examined by TGA analysis. The graphene oxide decomposes in three steps. The first weight loss at 50°C-120°C depicts the loss of water molecule. The second step observed from 120°C to 440°C in GO is due to the loss of oxygen-containing groups, and the third step above 440°C relates to an unstable carbon remaining in the structure and pyrolysis of oxygen functional group in the main structure to yield CO and CO2. The reduced graphene oxide shows similar characteristics but with lower amount of weight loss, compared to GO (Figures 3a and 3b). This is due to the smaller amount of oxygen functional groups in the structure.”
Scanning electron microscopy (SEM)
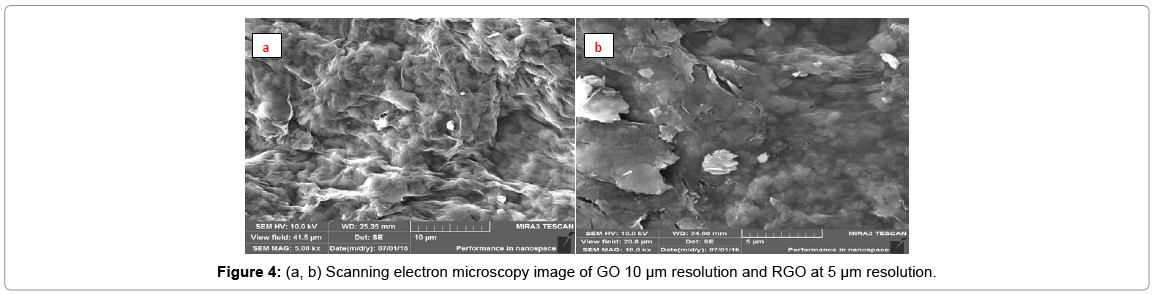
After acid oxidation and ultrasonication, the graphene oxide sheets became smaller and transparent; the sheets are so thin that electron beam can be passed through sample. The reduced graphene oxide exhibits typical wrinkled structure that causes sheet folding (Figures 4a and 4b).
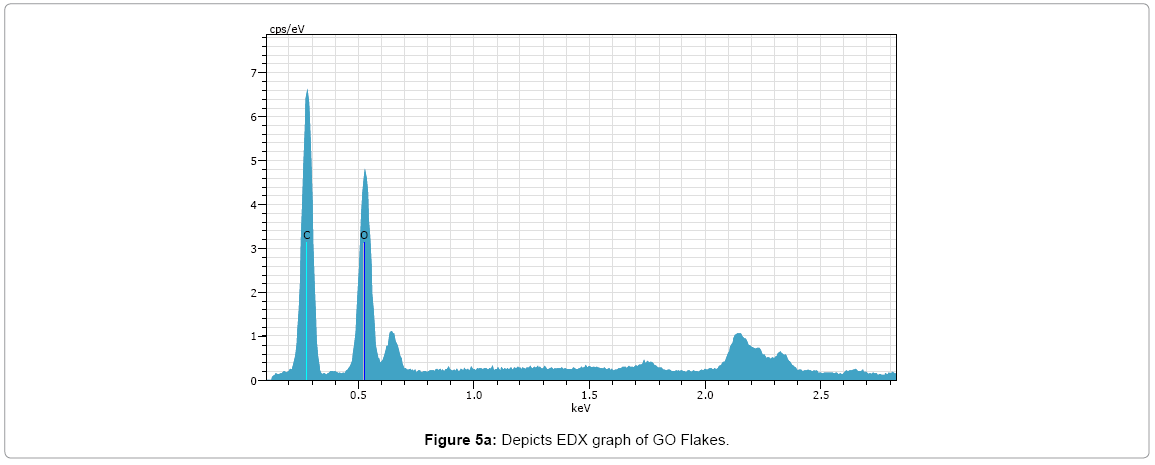
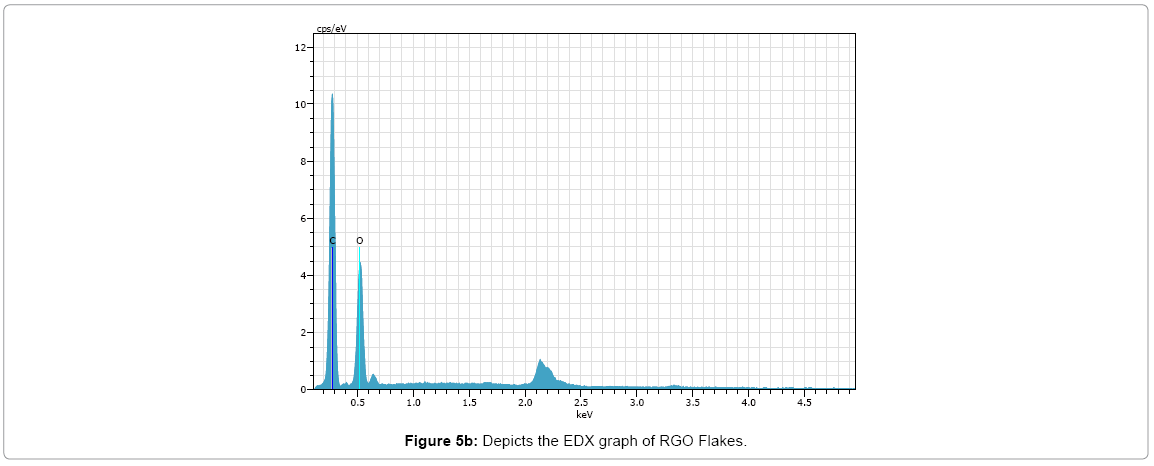
EDX
The results of the EDX elemental microanalysis of C and O elements are listed in Figures 5a and 5b (Tables 1 and 2). The carbon contents of the graphene oxide and reduced graphene oxide are 52.67 and 77.39, respectively. It can be expected that the C contents of reduced graphene oxide increases due to reduction treatment of partial oxygen containing functional groups. The presence of oxygen in RGO depicts some oxygen containing functional group are still present.
| Element | Normal. C Wt% |
Atomic. C Wt% |
|---|---|---|
| C | 52.67 | 54.48 |
| O | 47.33 | 45.52 |
| Total | 100 | 100 |
Table 1: Elemental composition of GO flakes.
| Element | Normal. C Wt% | Atomic. C Wt% |
|---|---|---|
| C | 77.39 | 63.27 |
| O | 13.61 | 36.73 |
| Total | 100 | 100 |
Table 2: Elemental composition of RGO flakes.
Steps Involved in Modification of Crystal
Cleaning of the crystal surface
The FET crystal was cleaned by methanol solution and then air dried under stream of nitrogen. After this treatment crystal converts into a bare crystal. The observed current flow in this bare crystal is almost constant across source and drain.
Fabrication of graphene on crystal surface
We fabricate the R-GO FET biosensor by simply drop-casting 100 μL of graphene oxide (GO) aqueous solution of concentration 0.5 mg mL-1 in between a pair of 3 μm apart micro fabricated gold electrodes over a hot plate at a temperature of 70°C. The current shift of dried FET crystal was monitored and it was found that decrease in current arises. This decrease in the current corresponds to the attachment of graphene.
Activation of crystal
The GFET channel is modified with bilinker 1-Pyrenebutyric acid N-hydroxysuccinimide ester in methanol for 2 h at room temperature, washed with methanol, and dried under N2 flow. “Pyrenes are hydrophobic polycyclic aromatic that binds avidly to the hydrophobic graphene and they do not affect the electrical properties of graphene sp2 bonds.” “The hydrophobic pyrene anchors the reactive NHS ester to the nanomaterial and allows it to react with solution phase molecules i.e. antibody. The current of the dried crystal was monitored and it was found that again decrease in current occurs.
Blocking of Non-Specific Binding Sites
The gold microelectrodes surface of the device was then passivated by incubation with 6 mM 6-mercapto-1-hexanol (MCH) in methanol for 1 h to block the nonspecific binding sites. Again decrease in current is observed.
Antibody immobilization
The channel was covalently immobilized by 100 μg/mL-1 antibody of rotavirus in “phosphate buffer solution”(PBS; pH 7.4). The device was then repeatedly washed with PBS to remove the unbound protein molecules and dried under nitrogen flow.
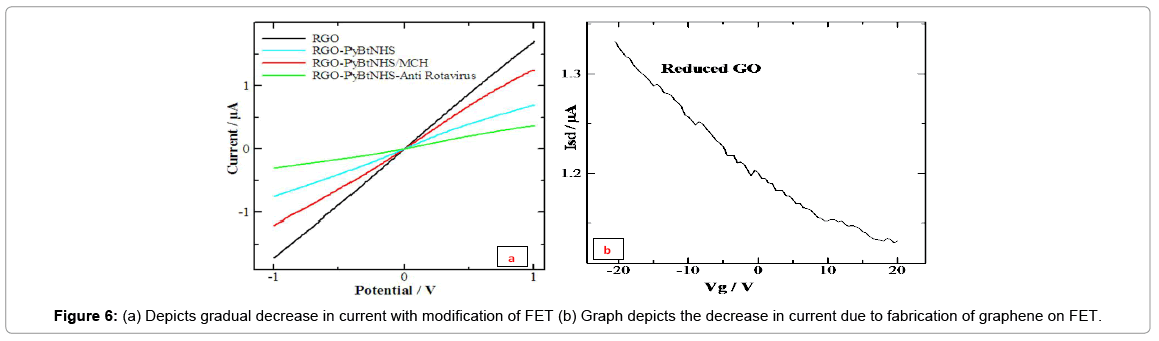
Current Voltage Characterization after Different Modification Step
The fabrication of the graphene chemiresistive device at each step of surface modification was monitored by recording the currentvoltage (I-V) characteristics from -1 Volt to +1 Volt measured on PGSTAT302N, AUTOLAB instrument from Eco Chemie, “The Netherlands connected to a Micromanipulator model 450PM-B, probe station that makes electrical contact to the source and drain electrodes.” A decrease in the current as shown in Figures 6a and 6b was obtained by the functionalization of the graphene with a cross linker, 1-Pyrenebutyric acid N-hydroxysuccinimide ester, due to a π-π stacking interaction of the graphene with pyrene ring moiety. A subsequent decrease in the current was seen after the passivation of gold microelectrodes with MCH. This is because Pyrene-NHS and MCH are electron donor molecules which donate electron to P type graphene, therefore decrease the total current. The depicts the typical gate voltage dependence on the normalized source-drain current (Isd) at Vd of 0.1 V before and after annealing graphene channel measured on a Keithley semiconductor characterization system 2420.
To investigate the sensitivity of the chemiresistive device, it was exposed to varying concentrations of rotavirus in the PBS. The resistance was calculated as the inverse of the slope of the (I-V) plot between -1 V and +1 V (linear range).
Antigen attachment
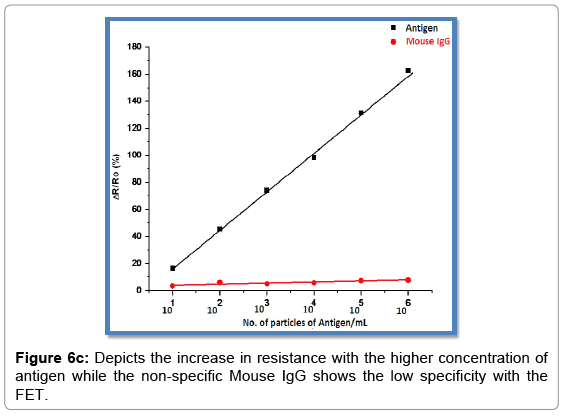
The device was exposed to 2 μl aliquot of each concentration of antigen of rotavirus in PBS at room temperature and the I-V measurements was taken after a gap of 10-minute exposure to different antigen rotavirus concentrations. It was observed that the conductance of the graphene FET device continued to decrease i.e. the resistance increased with increasing concentrations of rotavirus. The Figure 6c shows the normalized response of the Anti rotavirus/G device (R-R0)/R0, where R0 and R is the resistance of the device measured before and after exposure to rotavirus in PBS, respectively as a function of rotavirus concentration in PBS.”A concentration-dependent change in the source-drain current of the hybrid device was observed in the range of 101 particles per ml to 106 particles per ml.”The device exhibited a linear response (normalized resistance change) to target rotavirus from 101 particles per ml to 106 particles per ml. Since specificity of a device is a critical factor in sensing, it was investigated by checking the response of the graphene device to a varying concentration of a non-specific protein, mouse IgG, in PBS (Figure 6c). Mouse IgG shows very low specificity towards graphene FET device. “The resistance of the device was found to increase with increasing concentration of IgG.” This change in resistance was significantly smaller in comparison to a change in resistance for the same concentration range of Rota-virus sample in PBS. This demonstrated the specificity of the graphene FET device to rotavirus causing a change in resistance due to antibodyantigen immunoreactions only.
Summary
Many efforts are being made to enhance the efficiency, efficacy and sensitivity of FET by modern and latest technique which uses appropriate carbon based nanomaterial and other nanomaterial like silicon based nano-wires etc. It was further characterized by“Fourier Transform Infrared (FT-IR) spectroscopy, UV-Vis spectroscopy, Thermo gravimetric Analysis (TGA), Energy-dispersive X-ray (EDX), and Scanning electron microscopy (SEM).”The graphene used in the fabrication of FET crystal was provided by Biosensor laboratory of NPL Delhi. We fabricated the graphene on the FET crystal by simple drop casting method. The 100 μl of the graphene aqueous solution of concentration 0.5 mg/ml was drop casted in between a pair of 3 μm apart micro fabricated gold electrodes. Linker pyrene-NHS was used to activate the FET crystal. Pyrene are hydrophobic polycyclic and aromatic that binds avidly to the hydrophobic graphene. “The hydrophobic pyrene anchors the reactive NHS ester and NHS allows to react with solution phase molecule i.e. antibody.” The antibody attached to the graphene coated FET Crystal was further subjected to BSA to block the remaining attaching sites, so that the antibody only attaches to the antigen. To investigate the sensitivity of the fabricated device it was exposed to varying concentration of the target antigen (Rota-virus) in the PBS buffer (pH 7.4). The FET based chemiresistive biosensor is used to detect the various modifications in the crystal. It was observed that the conductance of the graphene hybrid device continued to decrease i.e. the resistance increases with increasing concentration of Rota-virus. The device exhibited a linear response normalized resistance change to the target Rota-virus from 101 to 106 particle/ml concentration. Since specificity of the device is the critical or we can say important factor in sensing, thus it was investigated by checking the response with the Rota-virus-graphene hybrid device to varying concentration of nonspecific protein, mouse IgG in PBS. The resistance of the device was seen to increase with increasing concentration of IgG. This change in the resistance was significantly smaller in comparison with change in resistance for the same concentration range of Rota-virus in PBS.
Conclusion
In conclusion, we demonstrated a facile method of detection of Rota-virus using graphene based device. The linker pyrene NHS is used as a linker where pyrene help in attachment with hydrophobic graphene and NHS attaches the solution phase which is Rota-virus specific antibody. The device helps in the label free detection of the virus. The specificity of the device was determined by exposing it to the mouse IgG and was found to be specific only to Rota-virus based on antigen-antibody interaction. The device exhibited a liner response to Rota-virus in the range of 101 to 106 particle/ml. This label free, specific and less time-consuming detection technique makes it a better method than other techniques.
Acknowledgement
The present investigation work was conducted in the Biosensor Research Laboratory at National Physical Laboratory of Council of Scientific and Industrial Research (CSIR). While preparation of graphene and reduced graphene was carried out in the “Membrane Biophysics and Nano-biosensor Research Laboratory” of Biophysics Unit of College of Basic Sciences and Humanities at G. B. Pant University of Agriculture and Technology Pantnagar Uttarakhand, India. Presented work was partly performed at the Material Evaluation Laboratory of CSIR-CBRI, Roorkee (IIT Roorkee)
References
- Sarkar D, Liu W, Xie X, Anselmo CA, Mitragotri S, et al. (2014) MoS2 field effect transistor for next generation label-free biosensor. ACSNANO 8: 3992-4003.
- Schoning MJ, Poghossian A (2002) Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst 127: 1137-1151.
- Joshi HC, Singh KP, Tomar A, Singh P (2017) Application of nanopore of solid membrane for recognition of fluorescent Pseudomonas. Int J Biochem Biophy 5: 53-64.
- Liu J,Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rew 109: 1948-1958.
- Baselt DR, Leea GU, Natesan M, Metzger SW, Colton RJ (1998) A biosensor based on magnetoresistance technology. Biosens Bioelectron 13: 731-739.
- Zhang Z, Kermekchiew BM, Barnes MW (2010) Direct DNA amplification from crude clinical samples using PCR enhancer cocktail and noval mutant of Taq. J Mol Diagn 12: 152-161.
- Arlett JL, Myers EB, Roukes ML(2011) Comparative advantages of mechanical biosensors. Nature Nanotechnology 6: 203-215.
- Malmquist M (1993) Biospecific interaction analysis using biosensor technology. Nature 14: 186-187.
- Chen KI, Li RB, Chen TY (2011) Silicon nanowire field effect transistor-based biosensor for biomedical diagnosis and cellular recording investigation. Nano Today 6: 131-154.
- Artiles MS, Rout CS, Fisher TS (2011)Graphene-based hybrid materials and devices for biosensing. Adv Drug Deliv Rev 63: 14-15.
- Zhang W, Zhou M, Zhu H, Tian Y, Wang K, et al. (2011) Tribological properties of oleic acid-modified grapheme as lubricant oil additives. J Physics D: Appl Phy 44: 20.
- Balasubramanian K, Burghard M (2006) Biosensors based on carbon nanotubes. Anal Bioanal Chem 385: 452-468.
- Holzinger M, Goff AL, Cosnier S (2014) Nanomaterial for biosensing applications. Europe PMC 2: 63.
- Geim AK, Novoselov KS (2007) The rise of graphene. Nature Materials 6: 183-191.
- Luz RAS, Iost RM, Crespilho FN (2013) Nanomaterials for biosensors and implantable biodevices. Nano Bioelectrochemistry 27-48.
- Yuan W, Chen J, Shi G (2014) Nanoporous graphene material. Materials today 17: 77-85.
- Schoning JM, Poghossian A (2002) Recent advances in biologically sensitive field effect transistors (BioFETs). The Analyst 127: 1137-1151.
- Nehra A, Singh KP (2015) Current trends in nanomaterial embedded field effecttransistor-based biosensor. Biosensor Bioelectr 74: 731-743.
Citation: Pant M, Kharkwal H, Singh KP, Joshi HC (2017) Detection of Rota Virus with the Help of Nanomaterial Based Field Effect Transistor (BIO-FET). Biosens J 6: 149. DOI: 10.4172/2090-4967.1000149
Copyright: ©2017 Pant M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17438
- [From(publication date): 0-2017 - Apr 18, 2025]
- Breakdown by view type
- HTML page views: 16235
- PDF downloads: 1203