Detection of Mycoplasma Infection in Small Ruminants by Polymerase Chain Reaction
Received: 21-Dec-2017 / Accepted Date: 11-Jan-2018 / Published Date: 15-Jan-2018
Abstract
Mycoplasma is a major respiratory pathogen of domestic sheep, goat and other animals causes primary atypical pneumonia and also predispose to secondary pneumonia with other agents. Species of Mycoplasma also causes conjunctivitis, polyarthritis and mastitis. For any disease, rapid and accurate diagnosis is essential to prevent the spread of infection. In the recent years, molecular genetic techniques with better sensitivity, specificity and less time consuming are available to detect Mycoplasma species. The present study was undertaken to detect the presence of Mycoplasma in various clinical samples by using 16s rRNA gene primers. Out of 20 samples tested, 2 samples showed PCR product at 280 bp level indicative of positive for Mycoplasma species. The results indicated that the PCR can be used for diagnosis of Mycoplasma infection at field level as it is sensitive and rapid compared to other conventional serological and isolation methods.
Keywords: Polymerase Chain Reaction (PCR); Phosphate Buffered Saline (PBS); Base pairs; Ribosomal ribonucleic acid
Introduction
Mycoplasma is an important pathogen of veterinary importance as it causes heavy economic losses by infecting many species of livestock. The major syndrome associated with Mycoplasma infection in small ruminants is pneumonia. The study indicated that PCR can be adapted to screen the field samples for detection of Mycoplasma as it is a rapid and efficient method compared to other methods.
Materials and Methods
A total number of 20 samples including milk, synovial fluid, conjunctival and nasal swabs from the affected sheep showing symptoms of conjunctivitis, arthritis and mastitis were collected from Nizamabad, Karimnagar and Mahaboobnagar districts of Andhra Pradesh and swabs were immediately placed in PBS and transported to the laboratory on ice.
The Mycoplasma group specific primers which amplify 280 bp fragment of 16S rRNA gene for detection all the species of Mycoplasma genus were used. The sequence of the forward primer was 5’- GGG AGC AAA CAG GAT TAG ATA CCCT-3’ and the reverse primer was 5’-TGC ACC ATC TGT CAC TCT GTT AAC CTC-3’.
The DNA extraction, amplification of DNA and analysis of amplified DNA were performed as per the method described by Van Kuppeveld et al. [1] with slight modifications. Briefly, DNA was isolated from 500 μl of the sample with 10% sodium dodecyl sulfate and 2.5 mg/ml of proteinase K, extracted with Phenol:Chloroform:Isoamyl alcohol mixture (25:24:1), then with chloroform:Isoamyl alcohol mixture (24:1) and precipitated with absolute alcohol in the presence of 3 M sodium acetate. The pellets were washed with 80% alcohol and resuspended in 20 μl of nuclease free water. Positive (Mycoplasma positive cell cultures) and negative controls (Distilled water) were incorporated in the test to rule out false positive and false negative results.
Amplification of DNA was performed using 10 μl of nucleic acid in a 50 μl reaction mixture containing 10X PCR buffer, 25 mM MgCl2, 25 picomoles of each primer, 10 mM dNTP mix and 1.5 U of TaqDNA polymerase. The thermal profile consisted of initial denaturation at 94°C for 5 min, 40 cycles of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min and extension at 72°C for 2 min and final extension was done at 72°C for 7 min.
Post PCR analysis was carried out by agarose gel electrophoresis using 1.2% agarose gel stained with ethidium bromide loaded with 15 μl of amplified product using 6X gel loading buffer and 100 bp DNA ladder (GeNei) for comparison.
Results and Discussion
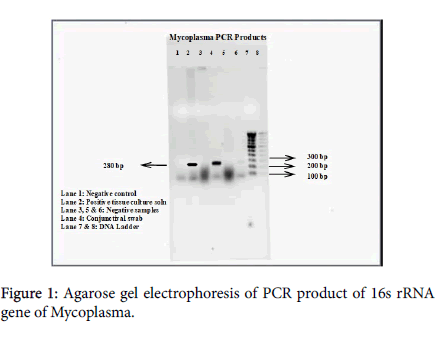
The positive control and conjunctiva swab samples has shown the band at 280 bp level (Figure 1) indicative of positive for Mycoplasma species [2] whereas the other samples did not show any amplification. The results indicated that the organism may belong to the members of M. conjunctivae , as the DNA from the eye swabs was amplified [3], M. agalactiae when it is correlated with the clinical symptoms [4] and M. capricolum subspecies capripneumoniae as reported by the Cetinkaya et al. [2].
The diagnosis of Mycoplasma infection has not been paid enough attention because of the inadequate laboratory facilities and the causative agent is rather difficult to cultivate in vitro . In the present study, the positive results paved a way to adopt PCR as a diagnostic tool to screen the field samples for Mycoplasma detection, as it is a rapid, specific, and sensitive test when compared to serological and cultural methods which lack specificity and may require several days to establish the presence of the organism. In future, the Mycoplasma species specific primers can be procured and species specific diagnosis can be made.
However, further studies are to be taken up to standardize PCR technique for all the field samples including milk, nasal swabs, vaginal swabs and fecal samples etc. and for all the species of livestock, susceptible for Mycoplasma infection. Moreover, it is necessary to evaluate the best suitable sample for conducting PCR to detect Mycoplasma by testing a number of samples [5].
Conclusion
In this study, an attempt was made to Mycoplasma infection from various field samples such as milk, synovial fluid, swabs from conjunctiva and nasal mucosa. Among the entire samples tested conjunctival swab sample showed positive reaction while rest of others are negative for Mycoplasma species in PCR. The study indicated that PCR can be adapted to screen the field samples for detection of Mycoplasma as it is a rapid and efficient method compared to other methods.
References
- Kuppeveld FJ, Johansson KE, Galama JM, Kissing J, Bölske G, et al. (1994) Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl Environ Microbiol 60: 149-152.
- Cetinkaya B, Kalin R, Karachan M, Atil E, Manso-Silvan L, et al. (2009) Detection of contagious caprine pleuropneumonia in East Turkey. Revue scientifique et Technique-Office International des Epizooties 28: 1037-1044
- Giacometti M, Nicolet J, Johansson KE, Naglic T, Degiorgis MP, et al. (1999) Detection and Identification of Mycoplasma conjunctivae in infectious keratoconjunctivitis by PCR. Based on the 16S. rRNA. Gene. Zoonoses Public Health 46: 173-180.
- Buxton A, Fraser G (1977) Animal microbiology: Immunology, bacteriology, mycology, diseases of fish and laboratory methods. Blackwell Scientific Publications 1: 267-283.
- Dussurget OL, Roulland-Dussoix DA (1994) Rapid, sensitive PCR-based detection of mycoplasmas in simulated samples of animal sera. Appl Environ Microbiol 60: 953-959.
Citation: Kandimalla K, Jyothi YK (2018) Detection of Mycoplasma Infection in Small Ruminants by Polymerase Chain Reaction. J Vet Pathol Res 2: 101.
Copyright: © 2018 Kandimalla K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 3933
- [From(publication date): 0-2018 - Mar 28, 2025]
- Breakdown by view type
- HTML page views: 3001
- PDF downloads: 932