Research Article Open Access
Detection of BRCA1 Founder Mutation 185DELAG in Breast Cancer Patients using Pyrosequencing Technique
Marwa H Saied1*, Salsabil El Boraie1, Tarek Elfayoumy2 and Dalal El Guizery1
1Clinical Pathology Department, Faculty of Medicine, Alexandria University, Egypt
2Surgery Department, Faculty of Medicine, Alexandria University, Egypt
- *Corresponding Author:
- Marwa H Saied
Clinical Pathology Department
Faculty of Medicine, Alexandria University
Egypt
Tel: 00201278804546
E-mail: marwahanafi@yahoo.co.uk
Received date: August 24, 2016; Accepted date: November 28, 2016; Published date: December 27, 2016
Citation: Saied MH, El Boraie S, Elfayoumy T, El Guizery D (2017) Detection of BRCA1 Founder Mutation 185DELAG in Breast Cancer Patients using Pyrosequencing Technique. Breast Can Curr Res 2:117. doi:10.4172/2572-4118.1000117
Copyright: © 2016 Saied MH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Breast Cancer: Current Research
Abstract
Background: Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2 is the most common cause of hereditary forms of both breast and ovarian cancer and occurs in all ethnic and racial populations. Till now, no assessments of the BRCA1 founder mutation have been performed by sequencing in Egyptian population. The aim of this pilot study was to detect the prevalence BRCA1 founder mutation 185DELAG in familial and sporadic breast cancer patients. Blood samples of 100 Egyptian female including 40 patients who had no significant family history of BC in their families (sporadic BC), 40 patients had at least 2 positive family history in their first degree relatives (familial BC), 20 control patients with no BC or history of breast cancer in their families. All subjects went for detection for 185DELAG mutation using Pyrosequencing technique. There were significant differences between familial and sporadic BC as regards their age (P=0.004) and in the premenopausal patients in familial BC than sporadic BC (P=0.02). Moreover, sporadic BC showed a significant increase in the ER and PR +ve, HER2/neu -ve (luminal A) than familial BC patients (P=0.012). As regards the mutation, we found a carrier frequency of 2.5% (95% confidence interval 1.1-2.4). There was no significant relation between mutation and type of BC, or between the hormonal profile of BC tumor and 185DELAG carriers.
Conclusion: The prevalence of BRCA1 185AG deletion mutation is significantly lower than previously reported using other molecular techniques.
Keywords
Breast cancer; BRCA1 mutation; Pyrosequencer
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women worldwide [1]. It was reported as the most common cancer among women in 140 of 184 countries worldwide [2]. In Egypt, incidence rates are alarmingly higher than global ones, Incidence rate of newly diagnosed Female BC 180/100.000 population in 2014 [3]. Overall, BC represent around 34.4% of total diagnosed cancer in Egyptian females [3]. BC is responsible for 15% of cancer deaths in Egyptian women [4]. The more devastating truth is about 50% of Egyptian women diagnosed with BC present in the late stages of the disease rendering their cure a challenge and much worsening of their prognosis [5]. Optimal clinical management of patients and their families relies on the identification of BRCA founder mutations in the laboratory [6]. Using pyrosequencing, allows for the detection of the frequent mutations reliably and rapidly [6]. BRCA1 and BRCA2 (breast cancer predisposition gene 1/2) gene mutation is the most significant which has been described so far. BRCA1 and BRCA2 are normally expressed in the cells of many tissues including breast and ovary, where they help repair damaged DNA, or destroy cells if DNA cannot be repaired. However, There are three founder germline mutation implicated in hereditary and familial BC are two in BRCA1 (BRCA1*185delAG, BRCA1*5382insC) and only one in BRCA 2 (BRCA2*6174delT). The three together account for 95% of the detectable BRCA mutations in breast cancer [6]. BRCA1*185delAG this is a frameshift mutation in which there lead to production of truncated premature nonfunctioning protein. The BRCA1*185delAG mutation is the most common mutation with a role in hereditary breast ovarian cancer syndrome (HBOC). This mutation increase the lifetime risk in individuals of breast cancer, ovarian cancer, male breast cancer, less likely prostate cancer and pancreatic cancer [7].
It was believed that that 185delAG mutation in BRCA1 is detected in Ashkenazi Jews both in familial breast and ovarian cancer and in the general population. However, many studies points out to that 185delAG BRCA1 mutation is not limited to Ashkenazim [8]. It has been found in several populations example Spain, USA, Malaysia, Iraq, England, Brazil, South Africa, Greece [9-15].
Material and Methods
The study was carried on 100 female patients, Eighty female patients with breast cancer presented to surgery department or medical oncology department at Alexandria Main University Hospital: Forty patients had sporadic type of breast cancer and reported no family history of breast cancer in their first degree relatives and Forty of these patients had familial type of breast cancer and reported history of breast cancer in one or more first degree relative by previous documentation for these relatives. All patients were diagnosed by mammography and fine needle aspiration cytology (FNAC). Twenty healthy females matched with age enrolled as control group. They didn’t have any breast complain. They have negative family history of breast cancer in their first degree relatives.
DNA extraction from blood
DNA was extracted from blood using following protocol using the PureLink® Genomic DNA Mini Kits. Then DNA concentration was measured by Nanodrop 2000 Spectrophotomer.
DNA amplification
The reaction composition and cycling conditions are optimized for amplifying template DNA for pyrosequencing analysis. Target: to get a clean clear single band on the gel, e.g., No secondary product, No primer dimer to be suitable for Pyrosequencing. Starting from annealing temperature of 55°C, it yields a weak single band on the agarose gel (2%, TBE 1x, 5 cm/v). By increasing annealing temperature to 56°C, the band disappeared. By decreasing annealing temperature to 54°C, the band reappeared and relatively stronger than the first one. Further decreasing of annealing temperature to 52ºC gave a strong single band on the gel as desired. DNA from case samples was analyzed for the founder mutation in BRCA1 (185delAG) using the following oligonucleotide primers flanking the mutation loci: BRCA1, 185delAG forward (5'GAAGTTGTCATTTTATAAACCTTT3') and 185delAG reverse (5'TGTCTTTTCTTCCCTAGTATGT3').
The PCR product was purified and subjected to DNA sequencing. PCR conditions was consisted of initial denaturing at 95°C for 15 minutes; 50 cycles of 94°C for 30 seconds, 52°C for 30 seconds, and 72°C for 30 seconds; and a final extension at 72°C for 10 minutes. The PCR products were separated by agarose gel electrophoresis to confirm successful amplification.
DNA mutation analysis by pyrosequencing
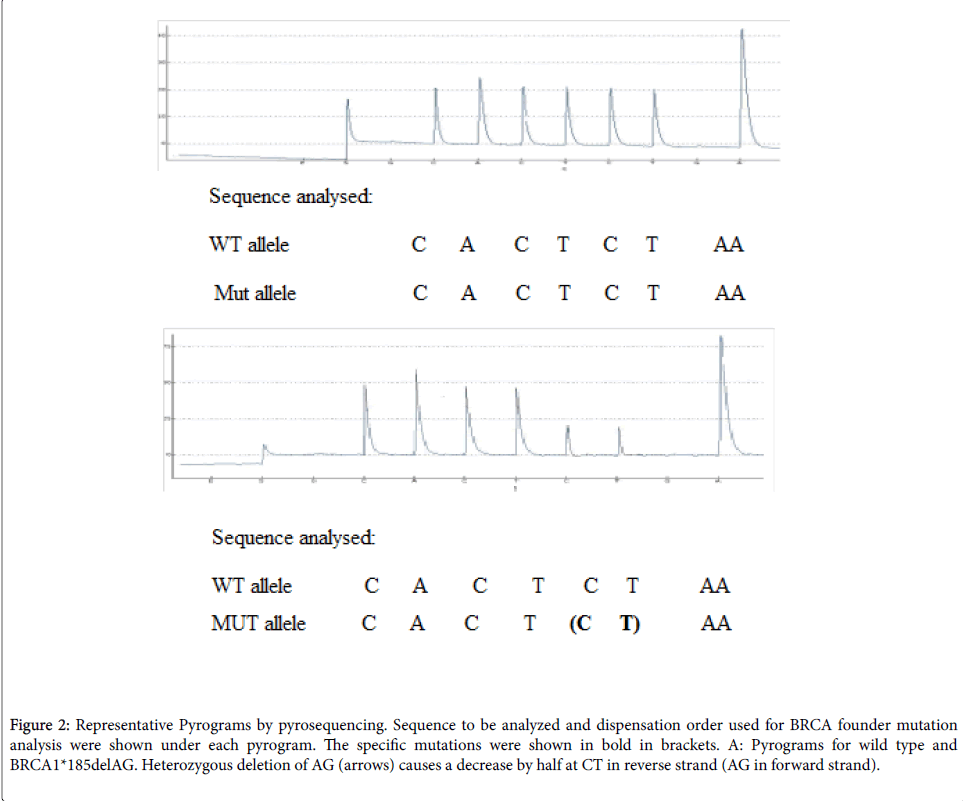
The PCR products (each 20 μl) were sequenced by pyrosequencing on a Pyromark Q24 following the manufacturer’s instructions pyrosequencing primers (Table 1). The PCR primers and sequencing primers are indicated in Figure 1. Sequences to be analyzed and nucleotide dispensation order are shown in Figure 2 under each pyrogram.
Figure 1: Assay design for BRCA1 Ashkenazi Jewish founder mutation analysis using pyrosequencing. BRCA1*185delAG were amplified using the PCR primers indicated. The pyrosequencing primers are shown by dotted lines as they overlap with PCR primers. Nucleotide positions of the founder mutations are boxed and in bold.
Figure 2: Representative Pyrograms by pyrosequencing. Sequence to be analyzed and dispensation order used for BRCA founder mutation analysis were shown under each pyrogram. The specific mutations were shown in bold in brackets. A: Pyrograms for wild type and BRCA1*185delAG. Heterozygous deletion of AG (arrows) causes a decrease by half at CT in reverse strand (AG in forward strand).
| Patients | Control | p-value | Mann-Whitney test | |
| Min-Max | 32-75 | 32-70 | 0.7248 | 0.3531 |
| Median | 52 | 52 |
Table1: Relation between age and type of breast cancer.
Design and interpretation of the pyrosequencing assay
The pyrosequencing assay was designed to begin sequence analysis right at the mutation site BRCA1 two nucleotides 3' of the mutation site (BRCA1*185delAG). Peak heights are proportional to the number of nucleotides that are incorporated with each dispensation (Figure 2). For the wild-type sequence for BRCA1*185delAG the generated peak heights for nucleotide dispensations at positions 2, 3, 4, 5, 6, and 7 (CACTCT in the reverse strand) are approximately equivalent and represent incorporation of a single nucleotide in both alleles (2 light units). Note that the signal intensity of the A at position 9 is two times greater (4 light units) and reflects the incorporation of two as in tandem in both alleles (four as in total). Using the peaks at positions 2, 3, 4, 5, 6, or 7 as an internal reference, a peak equivalent to 1 light unit within the variable site represents the incorporation of a nucleotide into only one of two alleles and the detection of a heterozygous deletion mutant. The intensities of C and T peaks at positions 6 and 7 are approximately half of those in the wild-type control (1 light unit), which indicates a deletion of CT in the reverse strand in one allele (heterozygous delAG in the forward strand).
Statistical analysis of data:
Data were fed to the computer and analyzed using Graphic PAD Prism software package version 6.0.
Qualitative data were described using number and percent. Quantitative data were described using range (minimum and maximum), mean, standard deviation and median.
Comparison between different groups was tested using Fisher’s Exact test was used. For normally distributed data, comparison between two independent populations was done using independent ttest. For abnormally distributed data, comparison between two independent populations was done using Mann-Whitney test, Significance of the obtained results was judged by p-value <0.05.
Results
We found a carrier frequency of 2.5% (1/40, 95% Confidence Interval (CI=1.1-2.4) for heterozygous mutation of BRCA1 AG185del. BRCA1 185 DEL AG mutation was found in 1 patient out of 80 patients; the mutation was detected in 55 years old female with positive family history of BC with an older sister (died) and ovarian cancer in her aunt (died at young age). Her first presentation was at 42 years with an axillary lump and she underwent a total mastectomy and pre and post-surgical radiotherapy. A year after it had both lung and bone metastasis (stage 4). She was triple positive as regard hormonal receptor profile and died within 8 month of her recurrence.
There was no significant age difference between the cancer patients and the controls (Table 1). However, our present study revealed a significant age difference between FBC and SBC patients (student ttest= 2.9, p=0.004) (Table 2). We found that the mean age of patients with FBC was 47.73 ± 12.27 years old and SBC is 55.7 ± 8.78 years old. FBC patients acquire BC almost a decade earlier than SBC patients (Table 1).
| Familial patients | Sporadic patients | t-test | p-value | |
| Age (years ) | ||||
| Min-Max | 32-72 | 45-75 | 2.943 | 0.004 |
| Mean ± SD | 47.73 ± 12.27 | 55.7±8.78 | ||
| Median | 46.5 | 55 | ||
Table 2: Significant age difference between FBC and SBC.
Various reproductive factors were studied e.g. age of menarche, age of menopause, BF, marital status, parity and use of OCP and none of those different reproductive factors enrolled in the present study was significantly associated with occurrence of FBC or SBC except the menopausal status. There was a significant difference between FBC and SBC according to menopausal status. There was a significant increase of the premenopausal patients with FBC patients than SBC patients (pvalue= 0.02) (Table 3).
| Reproductive Factors | FBC | SBC | Fisher exact test | |||
| No | % | No | % | p-value 0.02 |
||
| Menopausal state | Premenopausal | 24 | 55% | 13 | 33.5 % | |
| Postmenopausal | 16 | 45% | 27 | 67.5% | ||
Table 3: Relation between menopausal state and type of breast cancer.
Hormonal profile
Immunohistochemistry examination of the tumor tissues showed that about 75% of total breast cancer patients were ER and PR positive (ER, PR +ve), while Her2/neu gene was in 23% of the patients. Remarkably, there was a significant difference between familial breast cancer and sporadic breast cancer as regards the hormonal profile of the tumor. Sporadic breast cancer showed a significant increase in the ER, PR+ve, Her2/neu -ve (Luminal A) than familial breast cancer (Table 4).
| FBC | SBC | Fisher exact test, p-value | ||||
| NO | % | NO | % | 0.012 | ||
| ER&PR +ve , HER2 -ve | 20 | 50% | 31 | 77.50% | ||
| Not ER and PR+ve, HER2 -ve | 20 | 50% | 9 | 22.50% | ||
Table 4: Relation between hormonal profile and type of breast cancer.
Notably, there was no significant difference between FBC patients and SBC patients as regards the breast cancer stage (Fisher exact test p=1.33).
Both FBC and SBC patients were mainly presented in stage II; 50% of familial breast cancer and 47.5% of sporadic breast cancer (Table 5).
| Familial | Sporadic | Fisher exact test, p-value | |||
| No. | % | No. | % | ||
| Stage I | 2 | 5 | 1 | 2.5 | |
| Stage II | 20 | 50 | 19 | 47.5 | 1.334 |
| Stage III | 12 | 30 | 16 | 40 | |
| Stage IV | 6 | 15 | 4 | 10 | |
Table 5: Stages of the studied breast cancer patients.
Discussions
BC is the most common diagnosed cancer in women in both Egypt and worldwide [16]. BC is considered the leading cause of female related mortality in Egypt [17]. The incidence rates are rising all over the world increasing the disease burden and necessity to increase awareness of such devastating disease.
There are three types of BC: hereditary BC (5-10%), familial BC (10-15 %) and sporadic BC (75-85%).
Familial BC and hereditary BC are linked to germline mutations in the BRCA gene and usually occur early in life [6]. However, sporadic BC has somatic mutation in BRCA gene, and tends to occur later in life in combination with other genetic alterations and/or environmental exposure [6,14].
Basically, there are three founder mutations in BRCA genes, two in BRCA1 ((BRCA1 185delAG, BRCA1 5382insC) and only one in BRCA2 (BRCA2 6174delT). The three mutations account for 95% of the detectable BRCA mutations in breast cancer [14].
BRCA1 185delAG mutation is considered one of the most common mutations of BRCA1 gene and the most devastating mutation of BRCA genes. This mutation has linked greatly to Ashkenazi Jews [8]. However, other populations including Brazilian, Asian, Iranian, southern African, Greek, Spanish, English [9-13] and many others reported this mutation also.
Certainly, the most precise method for detection of gene mutations is sequencing since sequencing depends on visualization of the gene sequence with a resolution down to a single base nucleotide.
Sequencing can be performed by different methods. The first published was by chain termination i.e. Sanger sequencing, that is based on the selective incorporation of a complementary dideoxynucleotides by DNA polymerase.
Later, sequencing was performed during synthesis of complementary nucleotide to the target DNA template i.e. sequencing by synthesis of DNA template, one of which is pyrosequencing.
The idea of Pyrosequencing was first published in 1986. Pyrosequencing has the potential advantages of flexibility, accuracy, multiple processing, and can be easily automated [18]. Pyrosequencing has the advantage over Sanger sequencing as it is cost effective and ideal for short fragments sequencing. Therefore, pyrosequencing is ideal for SNP and small genetic deletions in which PCR target amplification product not exceeding 50-60 bp [19].
To date, in Egypt, there isn’t any published study to detect BRCA1 185delAG mutation using pyrosequencing technique. So, we aimed to study BRCA1 185delAG mutation in Egyptian BC patients; forty familial BC patients and forty BC sporadic patients. That is in addition to twenty healthy control using pyrosequencing techniques.
In the current study, in comparison between FBC and SBC, there were three main significant differences between FBC and SBC. The first one was the age difference. We found that the mean age of patients with FBC was 47.73 years while for SBC the mean age was 55.7 years.
In line with the present study, Abulkhair et al. [20] who studied the prevalence of BC reported a mean age as 45 years in FBC. Obviously FBC starts earlier than SBC because of the presence of germline mutations that lead to occurrence of BC almost a decade earlier in life [17]. Similarly, Runnak et al. [21] conducted a case control analysis of parity and family risk of BC in Kurdish women and reported a mean age of women with FBC as 47 years, while the mean age of women with SBC patients was 53 years. Also, Chen et al. [22], who studied the incidence and mortality of major cancers in China, stated in his study that the mean age for SBC was 53 years. In addition in a recent cohort study which conducted to highlight the age difference between SBC and FBC, showed the effect of BRCA mutations was more profound with each successive generation [23]. The average age of BC diagnosis was declined by 6.8 years in BRCA1 carriers in each generation [23].
Similarly, several epidemiological studies consistently indicate the women’s risk of BC is increased by the presence of BC in their mothers and sisters. BC could even occur in a younger age when compared to others with no history of the disease in their families [20-23]. That implies a continuous and regular breast examination and mammogram for females in families with one or more first degree relative with BC to ensure a proper detection of the disease in its early stages [24-26].
The second significant difference in the current study was the menopausal status. It was found the percent of menopausal females with SBC was significantly greater than the percent of menopausal females with FBC. This difference of pre and post-menopausal females maybe attributed to relatively older age of females with SBC than FBC females.
Interestingly, the third significant difference was concerning the hormonal status (ER, PR, and Her2/neu). As previously mentioned, SBC showed a significant increase in ER/PR +ve, HER2 -ve (luminal A). That was demonstrated before by G’Eredita et al. [27]. In his study, a total of 849 patients treated for BC were included. The patients were stratified into 2 groups: FBC (160 patients) and SBC (689 patients). He linked SBC with ER/PR +ve, HER2 -ve rather than FBC [27] and stated that reason of that was not clear. However, such hormonal status in SBC could explain the better outcome of SBC patients than FBC patients.
Conversely, Sanford et al. [28], who studied 314 patients with known BRCA status, revealed that more triple negative BC patients in FBC.
Remarkably, in the present study, the triple negative BC (ER and PR and Her2 negative) was found in only 8 patients out of 40 of FBC patients ( 8/40, 20%), while it was found in 3 patients out of 40 of SBC patients (3/40, 7%). None of the triple negative BC patients was a carrier for BRCA 185delAG mutation.
The correlation between the triple negative BC and BRCA 1 mutation is controversial. Until recently, it was believed that there was a positive correlation between triple negative BC patients and BRCA mutations. Lips et al. [29], who studied 377 BC patients stated that among BC patients with a familial BRCA1 mutations, over 80% was a triple negative BC [30,31]. Similarly, Couch et al. [30], who studied 1824 triple negative BC patients for BRCA mutations found a significant correlation of BRCA mutations and triple negative BC. Yet, genetic testing should be discussed with patients with triple negative BC, however the link between BRCA mutation and triple negative BC isn’t fully established [32-37]. Correspondingly, Gonzalez-Angulo et al. [37], who studied 77 cases of triple negative BC reported only 19.5% incidence of BRCA mutations among them.
Moreover some studies showed the combination of BRCA and triple negativity was connected to both poor outcome and lower survivability of the patients [32-34].
In the present study, other aspects were also compared between FBC and SBC but showed no significant difference. Our results are in line with other studies since Alford et al. [38] stated that as far as clinicpathological presentations, SBC and FBC were the same [38].
Moreover, the current study showed no difference between SBC and FBC as regards BC stage, distant metastasis and survivability of the patients. On the contrary, Alford et al. [38] reported an increased risk for distant metastasis in patients with early stages of FBC rather than SBC, also a decreased survivability among BRCA carriers [38]. All of those factors (late stage of presentation and earlier distant metastasis) contribute to the relatively bad prognosis of FBC than SBC [39].
Furthermore, the present study reported the prevalence of BRCA1 185delAG mutation among 80 Egyptian female breast cancer patients using Pyrosequencing technique. Out of 40 FBC patients studied, there was one patient only where the mutation was detected (1/40=2.5% [95% confidence interval CI 1.1-2.4]). Additionally, we did not find 185delAG mutation among SBC patients (0/40=0%). Our carrier frequency in FBC is in concordance with Ashkenazi Jewish population (8/800=0.9%, 95% CI [0.4-1.9]) [40]. That was also reported in non- Ashkenazi populations. For example, the percentage of the mutation in southern Brazil was 0.78%, in south eastern Iran was 4.9%, in India was 2.5%, Spanish was 3% and Greece was 1.1 % [9-15,41].
In the current study, the only patient identified with BRCA1 185 delAG mutation was a 55 years old female. She was presented with recurrent BC and a positive family history of BC in her older sister (died) and an ovarian cancer in her aunt (both died at young age). Her first presentation was at 42 years and she had total mastectomy, chemotherapy, post-surgical radiotherapy and hormonal therapy. During our study, she presented with both lung and bone metastasis (stage 4). As regard hormonal receptor profile, she was triple positive. Unfortunately, she died within 8 month of her recurrence. We have informed the result to her daughter warning her of the devastating effects of the mutation in her family and the possibility of all the three daughters could have BC at much younger age and encouraged her to do a screening mammogram every year to help diagnose BC at its early stages.
Additionally, histone methyl transferases (HMTs) G9a/GLP were found to recruit DNA methyltransferase to maintain the genomic status in cancer patients [15]. Therefore, it is suggested to investigate whether BRCA1 185delAG carrier patient show an ectopic expression of HMTs e.g. G9a/GLP. Moreover, BRCA1 185delAG truncation protein might block the ability of the ectopic expression of BRCA1 to induce chemosensitivity and inhibit estrogen receptor transcriptional activity. Using epitope-tagged truncated protein showed the ability of their expression, nuclear localization, and functionality [16].
Notably, the only 2 available article on PubMed to address this topic in Egypt are Fattouh et al. [42] and Ibrahim et al. [43]. Fatouh’s study used PCR-SSCP method on 62 patients with invasive BC belonging to 60 families and their asymptomatic female first-degree relatives. They reported mutations in both BRCA1 and BRCA2 genes (86.7% of the families). 60% of these families were attributable to BRCA1 mutations, while 26.7% of them were attributable to BRCA2 mutations. The other study Ibrahim et al. applied his work not on actual BC patients but on 120 healthy first degree female relatives of the patients, either sisters and/or daughters. That was for early detection of presymptomatic breast cancer mutation carriers. He reported 10% prevalence of the BRCA mutations.
The difference of our results in relation to results obtained by other groups may be attributed to different methodology, difference in number of studied cases and different studied populations.
The similarity of the prevalence of BRCA1 185delAG between the international literature and our study points to a necessity for further and wider screening of this mutation in FBC patients in Egypt. That will assist in a better decisive medical and surgical preventive options and certainly a better outcome for the patients.
References
- Hery C, Ferlay J, Boniol M, Autier P (2008) Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann Oncol 19: 1009-1018.
- Azim HA, Ibrahim AS (2014) Breast cancer in Egypt, China and Chinese: statistics and beyond. J Thorac Dis 6: 864-866.
- Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H (2014) Cancer incidence in egypt: Results of the national population-based cancer registry program. J Cancer Epidemiol p: 437971.
- Ibrahim SS, Hafez EE, Hashishe MM (2010) Presymptomatic breast cancer in Egypt: role of BRCA1 and BRCA2 tumor suppressor genes mutations detection. J ExpClin Cancer Res 29:82.
- Stapleton JM, Mullan PB, Dey S, Hablas A, Gaafar R, et al. (2011) Patient-mediated factors predicting early and late stage presentation of breast cancer in Egypt. Psycho-oncology 20: 532-537.
- Zhang L, Kirchhoff T, Yee CJ, Offit K (2009) A rapid and reliable test for BRCA1 and BRCA2 founder mutation analysis in paraffin tissue using pyrosequencing. J MolDiagn 11:176-181.
- Petrucelli N, Daly MB, Pal T (1998) BRCA1 and BRCA2 Hereditary Breast and Ovarian Cancer. GeneReviews(R). Seattle (WA).
- Bar-Sade RB, Kruglikova A, Modan B, Gak E, Hirsh-Yechezkel G, et al. (1998) The 185delAG BRCA1 mutation originated before the dispersion of Jews in the diaspora and is not limited to Ashkenazim. Hum Mol Genet 7:801-805.
- Laitman Y, Feng BJ, Zamir IM, Weitzel JN, Duncan P, et al. (2013) Haplotype analysis of the 185delAG BRCA1 mutation in ethnically diverse populations. Eur J Hum Genet 21:212-216.
- Diez O, Domenech M, Alonso MC, Brunet J, Sanz J, et al. (1998) Identification of the 185delAG BRCA1 mutation in a Spanish Gypsy population. Hum Genet103:707-708.
- DillenburgCV, Bandeira IC, Tubino TV, Rossato LG, Dias ES, et al. (2012) Prevalence of 185delAG and 5382insC mutations in BRCA1, and 6174delT in BRCA2 in women of Ashkenazi Jewish origin in southern Brazil. Genet MolBiol35:599-602.
- Makriyianni I, Hamel N, Ward S, Foulkes WD, Graw S (2005) BRCA1:185delAG found in the San Luis Valley probably originated in a Jewish founder. J Med Genet 42:e27.
- Kataki A, Gomatos IP, Pararas N, Armakolas A, Panousopoulos D, et al. (2005) Identification of germline BRCA1 and BRCA2 genetic alterations in Greek breast cancer moderate-risk and low-risk individuals-correlation with clinicopathological data. Clin Genet 67:322-329.
- Berliner JL, Fay AM (2007) Practice Issues Subcommittee of the National Society of Genetic Counselors' Familial Cancer Risk Counseling Special Interest G. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns16:241-260.
- Shu XZ, Zhang LN, Zhang R, Zhang CJ, He HP, et al. (2015) Histone acetyltransferase p300 promotes MRTF-A-mediates transactivation of VE-cadherin gene in human umbilical vein endothelial cells. Gene 563:17-23.
- Fan S, Yuan R, Ma YX, Meng Q, Goldberg ID, et al. (2001) Mutant BRCA1 genes antagonize phenotype of wild-type BRCA1. Oncogene20:8215-8235.
- Hery C, Ferlay J, Boniol M, Autier P (2008)Quantification of Changes in breast cancer countries with Caucasian majority populations. Ann Oncol 19:1009-1018.
- Ronaghi M, Uhlen M, Nyren P (1998) A sequencing method based on real-time pyrophosphate. Science281: 363
- Siqueira JF, Fouad AF, Rocas IN(2012)Pyrosequencing as a tool for better understanding of human microbiomes. J Oral Microbiol4.
- Abulkhair OA, AlTahan FM, Young SE, Musaad SM, Jazieh AR (2010) The first national public breast cancer screening program in Saudi Arabia. Ann Saudi Med 30:350-357.
- Runnak MA, Hazha MA, Hemin HA, Wasan AA, Rekawt RM, etal. (2012) A population-based study of Kurdish breast cancer in northern Iraq: hormone receptor and HER2 status. A comparison with Arabic women and United States SEER data. BMC Womens Health 12:16.
- Chen P, Li M, Gu X, Liu Y, Li X, et al. (2013) Higher blood 25(OH)D level may reduce the breast cancer risk: evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS One 8:e49312.
- Narod SA (2011) Earlier age of onset in BRCA carriers-anticipation or cohort effect? A Countercurrents Series. CurrOncol18:257-258.
- Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, et al. (2015) Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J ClinOncol33:1128-1135.
- Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, et al. (2013) Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer119:1652-1659.
- Oktay K, Kim JY, Barad D, Babayev SN (2010) Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J ClinOncol28:240-244.
- D'Eredita G, Giardina C, Napoli A, Troilo VL, Fischetti F, et al. (2011) Familial and sporadic breast cancers: differences in clinical, histopathological, and immunohistochemical features. Int J SurgPathol19:724-732.
- Sanford RA, Song J, Gutierrez-Barrera AM, Profato J, Woodson A, et al. (2015) High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer 121:3422-3427.
- Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, et al. (2013) Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer 108:2172-2177.
- Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, et al. (2015) Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J ClinOncol33:304-311.
- Narod SA, Dent RA, Foulkes WD (2015) CCR 20th Anniversary Commentary: Triple-Negative Breast Cancer in 2015-Still in the Ballpark. Clin Cancer Res 21:3813-3814.
- Lakhani SR, Gusterson BA, Jacquemier J, Sloane JP, Anderson TJ, et al. (2000) The pathology of familial breast cancer: histological features of cancers in families not attributable to mutations in BRCA1 or BRCA2. Clin Cancer Res6:782-789.
- Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. (2007) BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene26:2126-2132.
- Buckley NE, Haddock P, De Matos Simoes R, Parkes E, Irwin G, et al. (2016) A BRCA1 deficient, NFkB driven immune signal predicts good outcome in triple negative breast cancer. Oncotarget7:19884-19896.
- Asleh-Aburaya K, Fried G (2015) Clinical and molecular characteristics of triple-negative breast cancer patients in Northern Israel: single center experience. Springerplus4:132.
- Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S (2011) Triple negative breast cancer - prognostic factors and survival. RadiolOncol45:46-52.
- Gonzalez-AnguloAM, Timms KM, Liu S, Chen H, Litton JK, et al. (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res17:1082-1089.
- Alford SH, Toy K, Merajver SD, Kleer CG (2012) Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat 132:429-437.
- Easton DF, Ford D, Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56:265-271.
- Struewing JP, Abeliovich D, Peretz T, Avishai N, Kaback MM, et al. (1995) The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet11:198-200.
- Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, et al. (1997) The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet60:505-542.
- Fattouh M, Ahmed H, Hafez Eel S (2011) Detection of BRCA1 and BRCA2 gene mutation in Egyptian females with breast cancer and their relatives by PCR-SSCP method. Egypt J Immunol18:9-16.
- Ibrahim SS, Hafez EE, Hashishe MM (2010) Presymptomatic breast cancer in Egypt: role of BRCA1 and BRCA2 tumorsuppressor genes mutations detection. J ExpClin Cancer Res29:82.
Relevant Topics
- Advances in Breast Cancer Treatment
- Alternative Treatments for Breast Cancer
- Breast Cancer Biology
- Breast Cancer Cure
- Breast Cancer Grading
- Breast Cancer Prevention
- Breast Cancer Radiotherapy
- Breast Cancer Research
- Breast Cancer Therapeutic & Market Analysis
- Breast Screening
- Cancer stem cells
- Fibrocystic Breast
- Hereditary Breast Cancer
- Inflammatory Breast Cancer
- Invasive Ductal Carcinoma
- Making Strides in Breast Cancer
- Mastectomy
- Metastatic Breast Cancer
- Molecular profiling
- Radiotherapy for Breast Cancer
- Smoking in Breast Cancer
- Terminal Breast Cancer
- Tumor biomarkers
Recommended Journals
Article Tools
Article Usage
- Total views: 11062
- [From(publication date):
March-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 9645
- PDF downloads : 1417